Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Contents
2.2. Determination of Trypanocidal Activity in Epimastigote Form of Y Strain of Trypanosoma cruzi
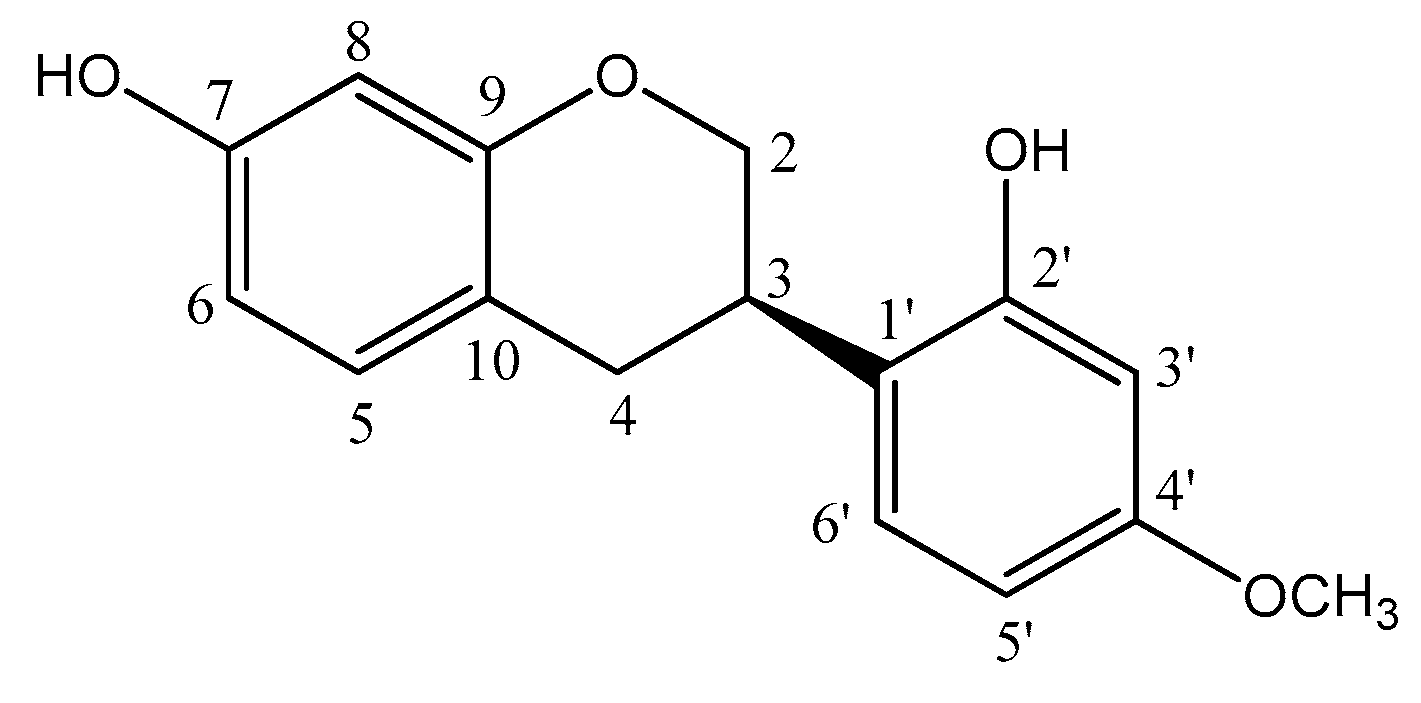
2.3. Nuclear Magnetic Resonance (NMR) and HRMS Analysis of Vestitol (2′,7-Dihydroxy-4′-methoxyisoflavan)
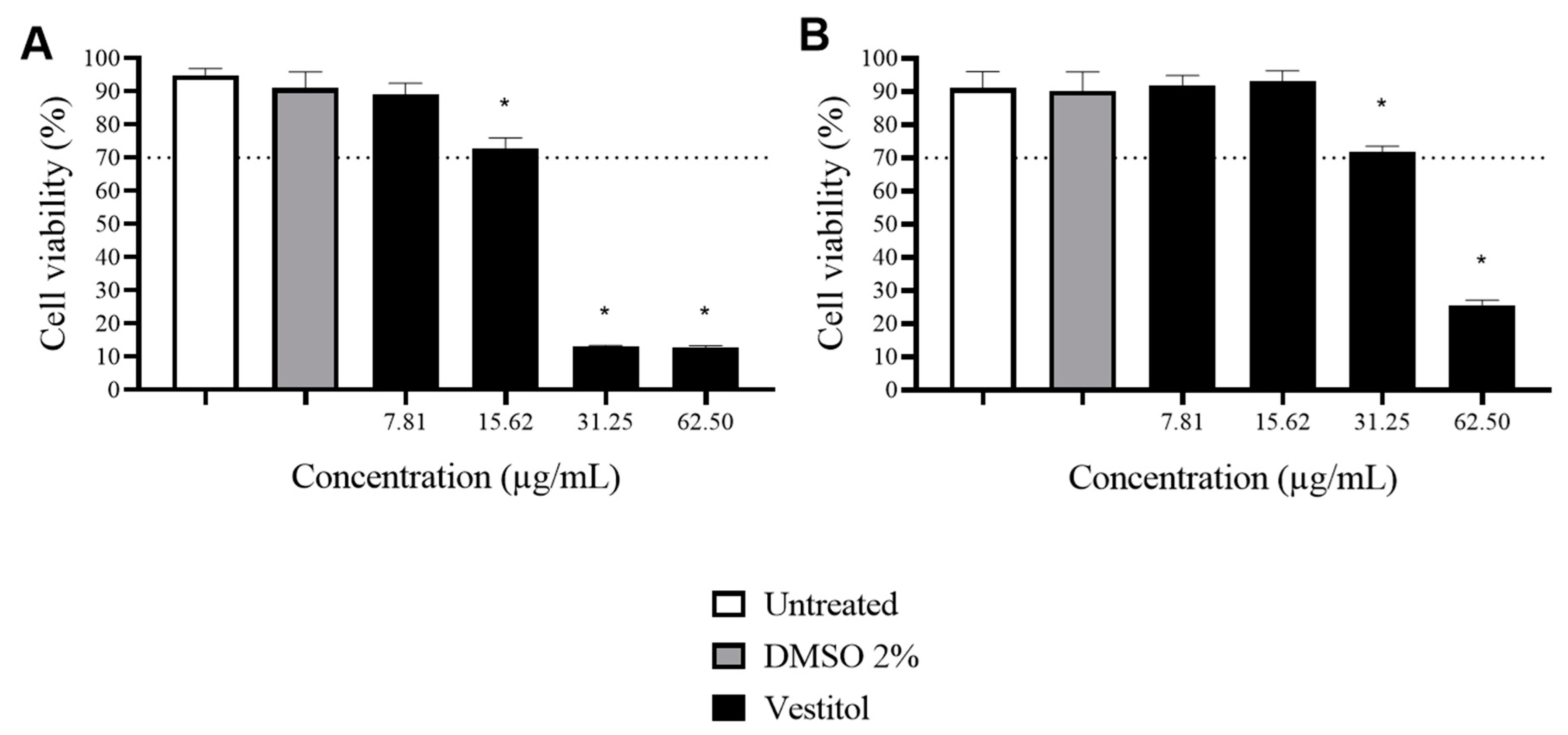
2.4. Cytotoxicity Assay
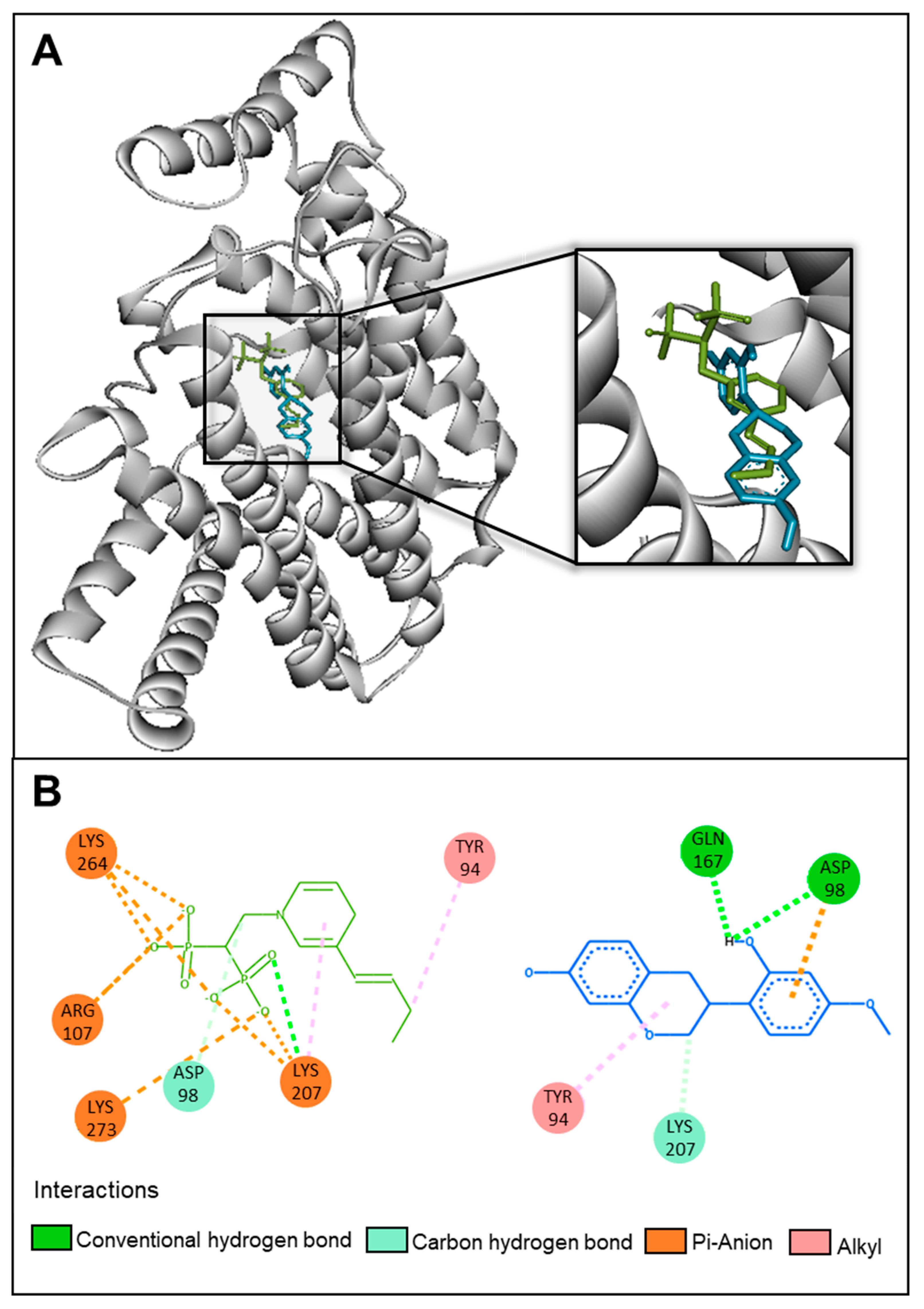
2.5. In Silico and In Vitro Investigations of the Mechanism of Action
3. Material and Methods
3.1. General Experimental Procedures
3.2. Red Propolis Samples
3.3. The Ethanolic Extract Preparation of Red Propolis (EEP 75% Heated)
3.4. Determination of Total Phenolics Content
3.5. Determination of Total Flavonoids Content
3.6. Liquid–Liquid Partition with EEP-75% Heated
3.7. Isolation of Active Compound
3.8. Determination of Trypanocidal Activity in Epimastigotes Forms of the Y Strain Trypanosoma cruzi
3.9. Nuclear Magnetic Resonance (NMR) and HRMS Analyses of Vestitol
3.10. Cytotoxicity Assay
3.11. Prediction of the Biological Activity Spectra
3.12. In Vitro Cytoplasmic Membrane Permeability Assay
3.13. Molecular Docking
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases. Second WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2013; pp. 57–59. [Google Scholar]
- Morel, C.M. Chagas disease, from discovery to control—And beyond: History, myths and lessons to take home. Mem. Inst. Oswaldo Cruz 1999, 94, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.R.D.; Azevedo, M.L.S.; Rocha, D.F.; Andrade, A.L.; Amparo, T.R.; Dos Santos, O.D.H.; Seibert, J.B.; Pereira, L.R.; Vieira, P.M.A.; Carneiro, C.M.; et al. Trypanocidal activity and increased solubility of benznidazole incorporated in PEG 4000 and its derivatives. J. Braz. Chem. Soc. 2021, 6, 1162–1172. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Factsheet: Chagas Disease in the Americas for Public Health Workers. In Chagas in the Americas for Public Health Workers; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Dias, J.C.P. Natural history of Chagas disease. Arq. Bras. Cardiol. 1995, 65, 359–366. [Google Scholar]
- Teixeira, A.R.L.; Gomes, C.; Lozzi, S.P.; Hecht, M.M.; Rosa, A.C.; Monteiro, P.S. Evaluation of in vitro anti-Trypanosoma cruzi activity of medications benznidazole, amiodarone hydrochloride, and their combination. Cad. Saúde Pública 2009, 25, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.L.; Fagúndes, M.; Klein, S.; Escanilla, S.; Torres, G.; Lee-Liu, D.; Ferreira, J.; Orellana, U.K.M.; Morello, A.; Ferreira, A.; et al. Trypanosoma cruzi: In vitro effect of aspirin with nifurtimox and benznidazole. Exp. Parasitol. 2010, 124, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.P.; Coura, J.R. Clínica e Terapêutica da doenFça de Chagas: Uma Abordagem Prática Para o Clínico Geral, 1st ed.; Edição Fiocruz: Rio de Janeiro, Brazil, 1997; pp. 11–486. [Google Scholar]
- Coura, J.R.; De Castro, S.L. A critical review on chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2022, 97, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Werbovetz, K. Natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis. Curr. Med. Chem. 2006, 13, 2571–2598. [Google Scholar] [CrossRef]
- Lustosa, S.R.; Galindo, A.B.; Nunes, L.C.C.; Randau, K.P.; Neto, P.J.R. Própolis: Atualizações sobre a química e a farmacologia. Rev. Bras. Farmacogn. 2008, 18, 447–454. [Google Scholar] [CrossRef]
- Almeida, W.A.D.S.; Sousa, L.R.D.; Antunes, A.D.S.; De Azevedo, A.S.; Do Nascimento, A.M.; Amparo, T.R.; De Souza, G.H.B.; Dos Santos, O.D.H.; Andrade, A.L.; Cazati, T.; et al. Green propolis: In vitro photoprotective and photostability studies of single and incorporated extracts in a sunscreen formulation. Rev. Bras. Farmacogn. 2020, 30, 436–443. [Google Scholar] [CrossRef]
- Almeida, W.A.D.S.; Antunes, A.D.S.; Penido, R.G.; Correa, H.S.D.G.; Do Nascimento, A.M.; Andrade, A.L.; Santos, V.R.; Cazati, T.; Amparo, T.R.; De Souza, G.H.B.; et al. Photoprotective activity and increase of SPF in sunscreen formulation using lyophilized red propolis extracts from Alagoas. Rev. Bras. Farmacogn. 2019, 29, 373–380. [Google Scholar] [CrossRef]
- De Castro, S.L. Propolis: Biological and pharmacological activities therapeutic uses of this bee-product. Annu. Rev. Biomed. Sci. 2001, 3, 49–83. [Google Scholar] [CrossRef]
- Salomão, K.; Dantas, A.P.; Borba, C.M.; Campos, L.C.; Machado, D.G.; Aquino Neto, F.R.; De Castro, S.L. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Appl. Microbiol. 2004, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Prytzyk, E.; Dantas, A.P.; Salomão, K.; Pereira, A.S.; Bankova, V.S.; De Castro, S.L.; Aquino Neto, F.R. Flavonoids and trypanocidal activity of Bulgarian propolis. J. Ethnopharmacol. 2003, 88, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.P.; Salomão, K.; Barbosa, H.S.; Castro, S.L. The effect of Bulgarian propolis against Trypanosoma cruzi and during its interaction with host cells. Mem. Inst. Oswaldo Cruz 2006, 101, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S.; Salomão, K.; Dantas, A.P.; Santa-Rita, R.M.; Soares, M.J.; Barbosa, H.S.; De Castro, S.L. Different cell death pathways induced by drugs in Trypanosoma cruzi: An ultrastructural study. Micron 2009, 40, 157–168. [Google Scholar] [CrossRef]
- Aldana-Mejia, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Rodrigo, C.S.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef] [PubMed]
- De Mendonça, I.C.G.; Porto, I.C.C.D.M.; Do Nascimento, T.G.; De Souza, N.S.; Oliveira, J.M.D.S.; Arruda, R.E.D.S.; Mousinho, K.C.; Dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Alencar, S.M.; Oldoni, T.L.C.; Castro, M.L.; Cabral, I.S.R.; Costa-Neto, C.M.; Cury, J.A.; Rosalen, P.L.; Ikegaki, M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007, 13, 278–283. [Google Scholar] [CrossRef]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; De Castro, R.D.; Fernandez, L.G. Metabolite profiling, antioxidant and antibacterial activities of Brazilian propolis: Use of correlation and multivariate analyses to identify potential bioactive compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef]
- Gulçin, I.; Bursal, E.; Şehitoglu, M.H.; Bilsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, R.B.; Zengin, G.; Sinan, K.I.; Montesano, D.; Zheleva-Dimitrova, D.; Gevrenova, R.; Uba, A.I.; Çakılcıoğlu, U.; Kaplan, A.; Jugreet, S.; et al. Which Extraction Solvents and Methods Are More Effective in Terms of Chemical Composition and Biological Activity of Alceafasciculiflora from Turkey? Molecules 2022, 15, 6–27. [Google Scholar]
- Coelho, G.S.; Andrade, J.S.; Xavier, V.F.; Sales Junior, P.A.; De Araujo, B.C.R.; Fonseca, K.D.S.; Caetano, M.S.; Murta, S.M.F.; Vieira, P.M.; Carneiro, C.M.; et al. Design, synthesis, molecular modelling, and in vitro evaluation of tricyclic coumarins against Trypanosoma cruzi. Chem. Biol. Drug Des. 2019, 93, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Fresno, M.; Gironès, N. Comparative proteomic analysis of trypomastigotes from Trypanosoma cruzi strains with different pathogenicity. Infect. Genet. Evol. 2019, 76, 104041. [Google Scholar] [CrossRef] [PubMed]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Dantas, S.R.P.; Machado, B.A.S.; Barreto, G.D.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Romanha, A.J.; De Castro, S.L.; Soeiro, M.N.C.; LannesVieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.A.; Costa, S.S.; Silva, D.F.; Brandão, H.N. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Lotti, C.; Fernandez, M.C.; Piccinelli, A.L.; Cuesta-Rubio, O.; Hernandez, I.M.; Rastrelli, L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef]
- Da Rocha, C.Q.; Queiroz, E.F.; Meira, C.S.; Moreira, D.R.M.; Soares, M.B.P.; Marcourt, L.; Wolfender, J.L. Dimeric flavonoids from Arrabdaea brachypoda and assessment of their anti-Trypanosoma cruzi activity. J. Nat. Prod. 2014, 77, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Florencio, M.; Nery, E.T.; Rosa, D.; Ribeiro, T.A.N.; Moraes, J.B.B.; Zuma, A.A.; Fampa, P. The effect of the biflavonoid 2″,3″-dihydroochnaflavone on Trypanosoma cruzi Y strain. Parasitol. Int. 2020, 102180. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.M.K.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Edrada, E.R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem. Anal. 2016, 27, 107–115. [Google Scholar] [CrossRef]
- De Souza, W.; Rodrigues, J.C.F. Sterol Biosynthesis Pathway as Target for Anti-trypanosomatid Drugs. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 642502. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Baudhuin, P.; Opperdoes, F.R.; Courtoy, P.J. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: Purification and involvement in the growth of the parasite. Proc. Natl. Acad. Sci. USA 1988, 85, 6753–6757. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, G.R.; De Lemos, T.L.G.; Dornelas, C.A.; Da Silva, A.M.; De Almeida, M.C.S.; Ferreira, D.A.; Monte, F.J.Q.; Braz-Filho, R.; De Oliveira, I.R.; Do Nascimento, P.G.G. Estudo químico e avaliação biológica da própolis vermelha de Alagoas. Rev. Virtual Quim. 2018, 10, 2–12. [Google Scholar]
- Alenezi, S.S.; Alenezi, N.A.; Ebiloma, G.U.; Natto, M.J.; Ungogo, M.A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Fearnley, J.; Koning, H.P.; et al. The activity of red Nigerian propolis and some of its components against Trypanosoma brucei and Trypanosoma congolense. Molecules 2023, 28, 622. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktara, C.; Govender, T. Transforming linoleic acid into a nanoemulsion for enhanced activity against methicillin susceptible and resistant Staphylococcus aureus. RSC Adv. 2015, 5, 90482–90492. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical devices-Part 5: Test Dor In Vitro Cytotoxicity. International Standard Organization: Geneva, Switzerland, 2009.
- Antunes, A.S.; Gouveia, A.P.; Diogo, G.M.; Taylor, J.G.; Sousa, L.R.; Amparo, T.R.; Perasoli, F.B.; dos Santos, O.D.; Cazati, T.; Vieira, P.; et al. In vitro Photoprotective Evaluation and Development of Novel Nanoemulsion with Chromone Derivative. J. Braz. Chem. Soc. 2021, 32, 1813–1821. [Google Scholar] [CrossRef]
- Pinto, I.C.; Seibert, J.B.; Pinto, L.S.; Santos, V.R.; de Sousa, R.F.; Sousa, L.R.D.; Amparo, T.R.; Dos Santos, V.M.R.; do Nascimento, A.M.; de Souza, G.H.B.; et al. Preparation of glass-ionomer cement containing ethanolic Brazilian pepper extract (Schinus terebinthifolius Raddi) fruits: Chemical and biological assays. Sci. Rep. 2020, 10, 22312. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Farani, P.S.G.; Ferreira, B.I.S.; Gibaldi, D.; Lannes-Vieira, J.; Moreira, O.C. Modulation of miR-145-5p and miR-146b-5p levels is linked to reduced parasite load in H9C2 Trypanosoma cruzi infected cardiomyoblasts. Sci. Rep. 2022, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Do, L.T.M.; Huynh, T.T.N.; Tran, Q.H.N.; Nguyen, H.T.M.; Nguyen, T.T.A.; Nguyen, T.T.N.; Nguyen, P.H.H.; Sichaem, J. Placoisoflavones A and B, two new cytotoxic isoflavonoids from Placolobium vietnamense N.D.Khôi & Yakovlev. Nat. Prod. Res. 2022, 9, 1–7. [Google Scholar]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Vestitol drives LPS-activated macrophages into M2 phenotype through modulation of NF-κB pathway. Int. Immunopharmacol. 2020, 27, 106329. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bhatia, V.; Wen, J.-J.; Wu, Y.; Huang, M.-H.; Garg, N.J. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic. Biol. Med. 2009, 47, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.; Viegas, J.S.R.; Almeida, T.C.; Amparo, T.R.; Rodrigues, I.V.; Lanza, J.S.; Frézard, F.J.G.; Soares, R.D.O.A.; Teixeira, L.F.M.; de Souza, G.H.B.; et al. Nanostructured Systems Improve the Antimicrobial Potential of the Essential Oil from Cymbopogon densiflorus Leaves. J. Nat. Prod. 2019, 82, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Ergosterol biosynthesis and drug development for Chagas Desease. Mem. Inst. Oswaldo Cruz 2009, 104, 311–331. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activity of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Huang, C.H.; Gabelli, S.B.; Oldfield, E.; Amzel, L.M. Binding of nitrogen-containing bisphosphonates (N-BPs) to the Trypanosoma cruzi farnesyl diphosphate synthase homodimer. Proteins 2010, 78, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Andriani, G.; Amata, E.; Beatty, J.; Clements, Z.; Coffey, B.J.; Courtemanche, G.; Devine, W.; Erath, J.; Juda, C.E.; Wawrzak, Z.; et al. Antitrypanosomal lead discovery: Identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. J. Med. Chem. 2013, 56, 2556–2567. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Li, Q.; Ko, T.P.; Chan, H.C.; Li, J.; Zheng, Y.; Huang, C.H.; Ren, F.; Chen, C.C.; Zhu, Z.; et al. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog. 2014, 10, e1004114. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.C.; Murray, C.W.; Nissink, J.W.; Taylor, R.D.; Taylor, R. Comparing protein-ligand docking programs is difficult. Proteins 2005, 60, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gabelli, S.B.; McLellan, J.S.; Montalvetti, A.; Oldfield, E.; Docampo, R.; Amzel, L.M. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: Implications for drug design. Proteins 2006, 62, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.C.; Da Silva, L.C.; De Alencar, S.M.; De Aguiar, C.L. Caracterização química de extratos etanólicos de própolis com atividade inibitória do crescimento de estafilococos isolados de mastite bovina. Rev. Bras. Tecnol. Agroindustr. 2014, 8, 1040–1153. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Valverde, T.M.; Soares, B.N.G.d.S.; do Nascimento, A.M.; Andrade, A.L.; Sousa, L.R.D.; Vieira, P.M.d.A.; Santos, V.R.; Seibert, J.B.; de Almeida, T.C.S.; Rodrigues, C.F.; et al. Anti-Inflammatory, antimicrobial, antioxidant and photoprotective investigation of red propolis extract as sunscreen formulation in polawax cream. Int. J. Mol. Sci. 2023, 24, 5112. [Google Scholar] [CrossRef]
- Dowd, L.E. Spectrophotometric Determination of Quercetin. Anal. Chem. 1959, 31, 1184–1187. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Chem. Biol. 2014, 1263, 243–250. [Google Scholar]

| Samples | Isolation Step | IC50 (μg/mL) |
|---|---|---|
| EEP 75% heated | 1st | 12.8 (±0.2) |
| Chloroformic partition—EEP 75% heated | 2nd | 8.1 (±0.3) |
| Hexanic partition—EEP 75% heated | 2nd | 31.3 (±0.4) |
| PV-C1 | 3rd | 24.4 (±0.3) |
| PV-C2 | 3rd | 7.2 (±0.3) |
| PV-C3 | 3rd | 15.9 (±0.5) |
| PV-C4 | 3rd | 9.6 (±1.3) |
| PV-C5 | 3rd | 34.5 (±0.4) |
| PV-C6 | 3rd | 25.8 (±0.6) |
| PV-C7 | 3rd | 24.9 (±0.3) |
| PV-C8 | 3rd | 22.9 (±2.6) |
| PV-C9 | 3rd | 45.3 (±0.5) |
| PV-C2-1 | 4th | 30.5 (±0.4) |
| PV-C2-2 | 4th | 35.8 (±0.3) |
| PV-C2-3 | 4th | 9.0 (±0.3) |
| PV-C2-4 | 4th | 5.9 (±0.2) |
| PV-C2-5 | 4th | 8.1 (±0.4) |
| PV-C2-6 | 4th | 37.4 (±0.3) |
| PV-C2-7 | 4th | 35.0 (±0.2) |
| Compound 1 | 5th | 5.2 (±0.2) |
| Benznidazole | 5th | 4.3 (±0.3) |
| Binding Energy (kcal/mol) | ||
|---|---|---|
| Target | Isoflavan | Crystallographic Ligand |
| Lanosterol C-14 demetilase (CYP51) a | −7.7 | −8.0 |
| Squalene synthase (SQS) b | −7.8 | −11.2 |
| Farnesyl diphosphate synthase (FPPS) c | −9.3 | −8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.R.D.; Amparo, T.R.; Souza, G.H.B.d.; Ferraz, A.T.; Fonseca, K.d.S.; Azevedo, A.S.d.; Nascimento, A.M.d.; Andrade, Â.L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules 2023, 28, 7812. https://doi.org/10.3390/molecules28237812
Sousa LRD, Amparo TR, Souza GHBd, Ferraz AT, Fonseca KdS, Azevedo ASd, Nascimento AMd, Andrade ÂL, Seibert JB, Valverde TM, et al. Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules. 2023; 28(23):7812. https://doi.org/10.3390/molecules28237812
Chicago/Turabian StyleSousa, Lucas Resende Dutra, Tatiane Roquete Amparo, Gustavo Henrique Bianco de Souza, Aline Tonhela Ferraz, Kátia da Silva Fonseca, Amanda Scofield de Azevedo, Andréa Mendes do Nascimento, Ângela Leão Andrade, Janaína Brandão Seibert, Thalita Marcolan Valverde, and et al. 2023. "Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis" Molecules 28, no. 23: 7812. https://doi.org/10.3390/molecules28237812
APA StyleSousa, L. R. D., Amparo, T. R., Souza, G. H. B. d., Ferraz, A. T., Fonseca, K. d. S., Azevedo, A. S. d., Nascimento, A. M. d., Andrade, Â. L., Seibert, J. B., Valverde, T. M., Braga, S. F. P., Vieira, P. M. d. A., & Santos, V. M. R. d. (2023). Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules, 28(23), 7812. https://doi.org/10.3390/molecules28237812







