Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Powder X-ray Diffraction (PXRD)
2.2. Single Crystal X-ray Diffraction
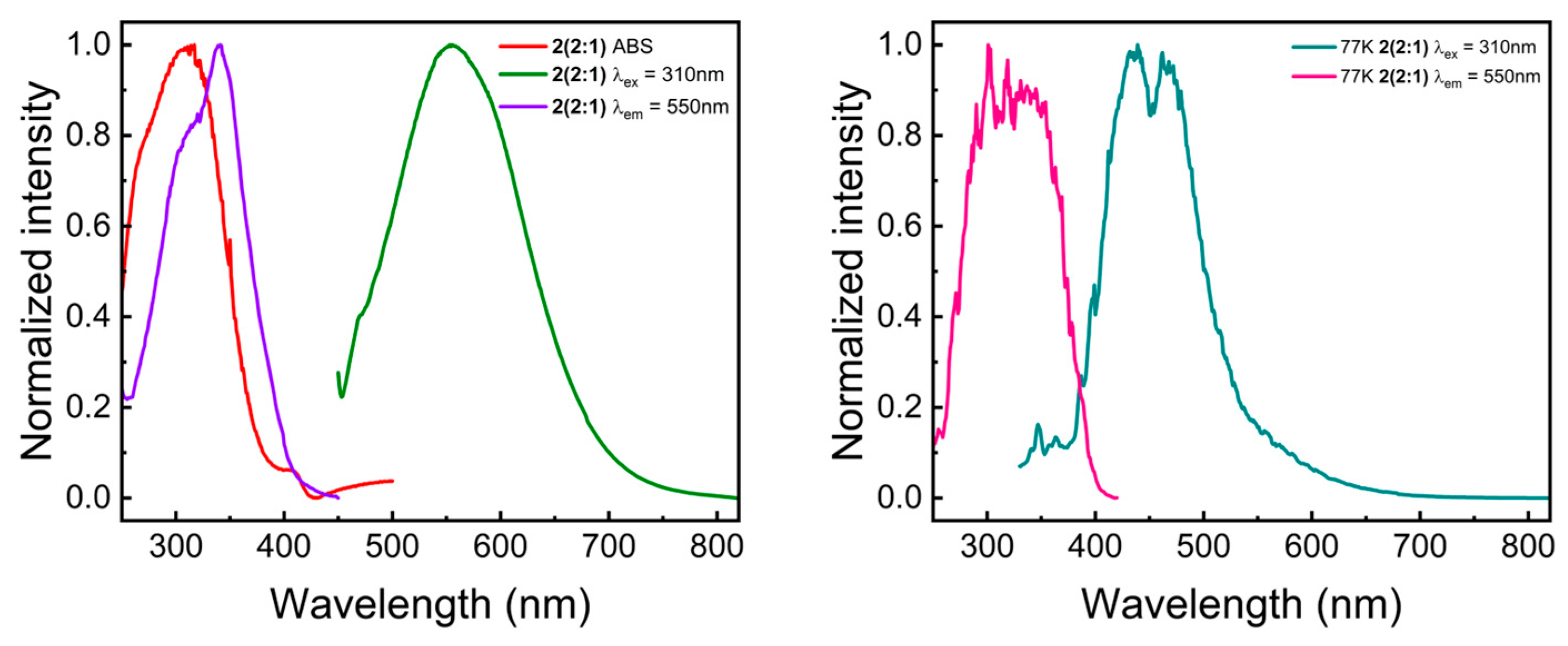
2.3. Steady-State UV-Vis Spectroscopy
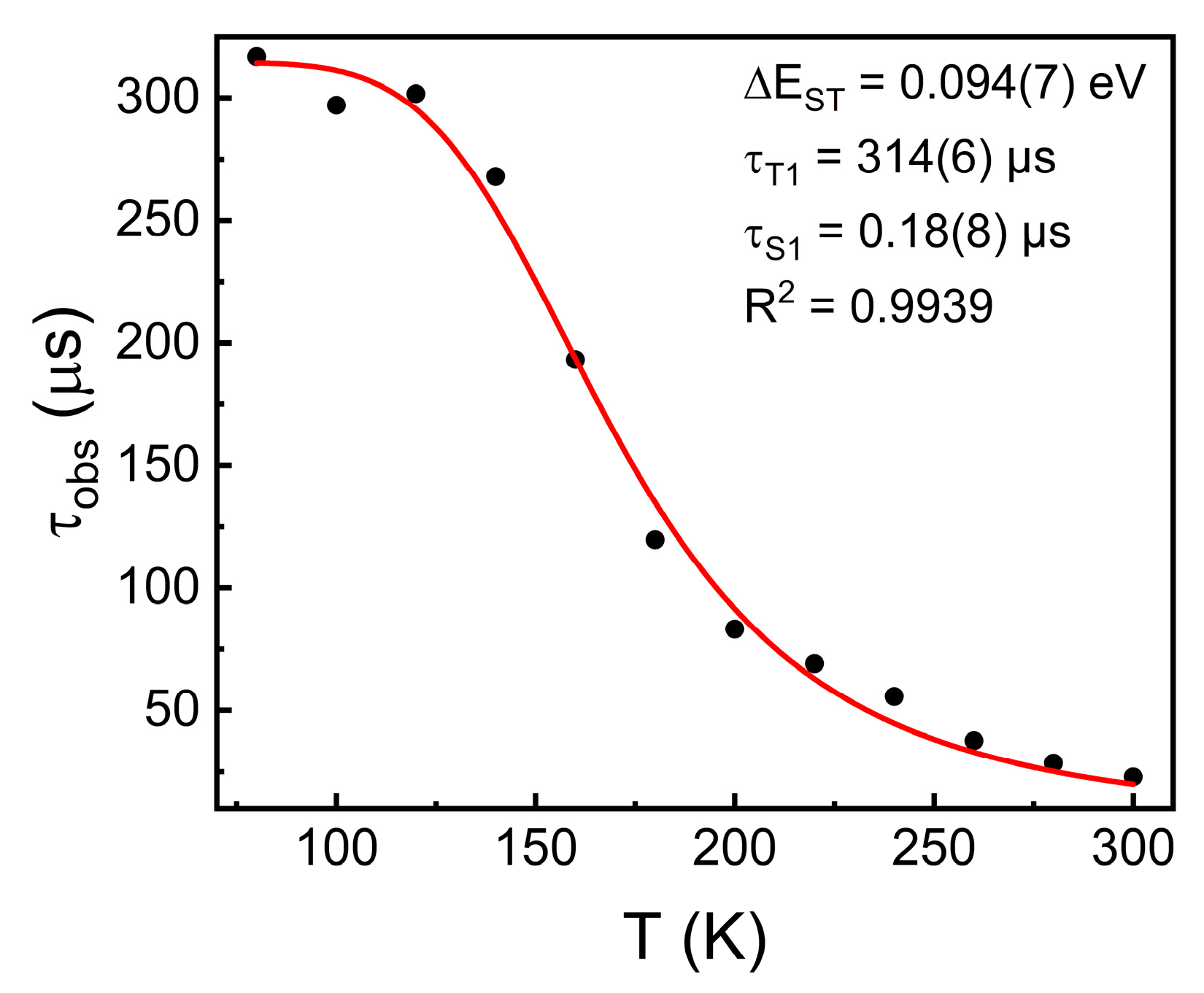
2.4. Time-Resolved UV-Vis Spectroscopy and Thermally Activated Delayed Fluorescence (TADF)
3. Discussion
3.1. Statistical Structural Predictability
3.2. Photophysical Trends
4. Materials and Methods
4.1. Synthesis
4.1.1. Materials
4.1.2. Procedure for the Synthesis of 1,7-Bis(Diphenylphosphino)Heptane (L7)
4.1.3. General Procedure for the Synthesis of Complexes
4.1.4. Crystalization
4.2. Instrumentation
4.2.1. Powder X-ray Diffraction Measurements
4.2.2. Single Crystal X-ray Stucture Analyses
4.2.3. Solid-State UV-Vis Absorption Spectra
4.2.4. Solid-State UV-Vis Photoluminescence Spectra
4.2.5. Solid-State UV-Vis Photoluminescence Lifetime Measurements
4.2.6. Quantum Yield Measurements
4.2.7. Temperature-Dependent Photoluminescence Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlachter, A.; Tanner, K.; Harvey, P.D. Copper Halide-Chalcogenoether and -Chalcogenone Networks: Chain and Cluster Motifs, Polymer Dimensionality and Photophysical Properties. Coord. Chem. Rev. 2021, 448, 214176. [Google Scholar] [CrossRef]
- Schlachter, A.; Harvey, P.D. Properties and Applications of Copper Halide-Chalcogenoether and -Chalcogenone Networks and Functional Materials. J. Mater. Chem. C Mater. 2021, 9, 6648–6685. [Google Scholar] [CrossRef]
- Marsich, N.; Nardin, G.; Randaccio, L. Tetranuclear Copper(I) Complex. Crystal and Molecular Structure of the 2:1 Derivative of Copper(I) Iodide and Bis(Diphenylphosphino)Methane. J. Am. Chem. Soc. 1973, 95, 4053–4054. [Google Scholar] [CrossRef]
- Camus, A.; Nardin, G.; Randaccio, L. Structure Determination of the 2:1 Derivatives of Copper(I) Bromide and Iodide with Bis(Diphenylphosphino)Methane. A Simple Structural Scheme for the Formation of (CuX)NLm Species. Inorganica Chim. Acta 1975, 12, 23–32. [Google Scholar] [CrossRef]
- Nardin, G.; Randaccio, L.; Zangrando, E. Stereochemistry of Copper(I) Complexes. Part II. The Molecular Structure of the 3: 2 Reaction Product between Copper Iodide and Bis(Diphenylphosphino)Methane: Di-µ-[Bis(Diphenylphosphino)Methane]-µ-Iodo-Di-Μ3-Iodo-Triangulo-Tricopper(I)–0.5 Dichloromethane. J. Chem. Soc. Dalton Trans. 1975, 2566. [Google Scholar] [CrossRef]
- Comba, P.; Katsichtis, C.; Nuber, B.; Pritzkow, H. Solid-State and Solution Structural Properties of Copper(I) Compounds with Bidentate Phosphane Ligands. Eur. J. Inorg. Chem. 1999, 1999, 777–783. [Google Scholar] [CrossRef]
- Di Nicola, C.; Pettinari, C.; Pizzabiocca, A.; Skelton, B.W.; Somers, N.; White, A.H. The Structural Definition of Adducts of Stoichiometry MX:Dpex (2:3)(∞), M = CuI, AgI, X = simple Anion, Dpex = Ph2E(CH2)xEPh2, E = P, As. Inorganica Chim. Acta 2006, 359, 64–80. [Google Scholar] [CrossRef]
- Benito, Q.; Le Goff, X.F.; Nocton, G.; Fargues, A.; Garcia, A.; Berhault, A.; Kahlal, S.; Saillard, J.-Y.; Martineau, C.; Trébosc, J.; et al. Geometry Flexibility of Copper Iodide Clusters: Variability in Luminescence Thermochromism. Inorg. Chem. 2015, 54, 4483–4494. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Hong, M.; Shi, H.; Xu, K.; Lin, Q.; Zhao, Y.; Tian, Y.; Sun, J.; Shu, K.; et al. Luminescent Dinuclear Copper(I) Halide Complexes Double Bridged by Diphosphine Ligands: Synthesis, Structure Characterization, Properties and TD-DFT Calculations. Polyhedron 2014, 81, 687–694. [Google Scholar] [CrossRef]
- Aslanidis, P.; Cox, P.J.; Divanidis, S.; Tsipis, A.C. Copper(I) Halide Complexes with 1,3-Propanebis(Diphenylphosphine) and Heterocyclic Thione Ligands: Crystal and Electronic Structures (DFT) of [CuCl(PymtH)(Dppp)], [CuBr(PymtH)(Dppp)], and [Cu(μ-I)(Dppp)] 2. Inorg. Chem. 2002, 41, 6875–6886. [Google Scholar] [CrossRef]
- Tan, X.; Chen, J.; Cao, X.; Chen, X.; Zhang, F.; Wen, H.; Liao, J. Design Synthesis and Crystalline Structure of Copper(I) Halide Complexes. Nonferrous Met. Sci. Eng. 2013, 4, 31–36. [Google Scholar]
- Miao, H.; Pan, X.; Li, M.; Zhaxi, W.; Wu, J.; Huang, Z.; Liu, L.; Ma, X.; Jiang, S.; Huang, W.; et al. A Copper Iodide Cluster-Based Coordination Polymer as an Unconventional Zero-Thermal-Quenching Phosphor. Inorg. Chem. 2022, 61, 18779–18788. [Google Scholar] [CrossRef]
- Di Nicola, C.; Fianchini, M.; Pettinari, C.; Skelton, B.W.; Somers, N.; White, A.H. The Structural Definition of Adducts of Stoichiometry MX:Dppx (1:1) M = CuI, AgI, X = simple Anion, Dppx = Ph2P(CH2)xPPh2, X = 3–6. Inorganica Chim. Acta 2005, 358, 763–795. [Google Scholar] [CrossRef]
- Qian, H.; Chen, Y.; Ren, Y.; Zhang, Y.; Lang, J. Solvothermal Synthesis and Crystal Structure of Cu2 (µ-Dppb)2 (µ-I)2 (Dppb = 1,4-Di(Diphenylphosphine)Butane). J. Suzhou Univ. (Nat. Sci. Ed.) 2008, 24, 83–86. [Google Scholar]
- Utrera-Melero, R.; Cordier, M.; Massuyeau, F.; Mevellec, J.-Y.; Latouche, C.; Martineau-Corcos, C.; Perruchas, S. How Subtle Molecular Changes Can Strongly Impact Luminescence Properties of Copper Iodide Complexes. J. Lumin. 2023, 263, 120104. [Google Scholar] [CrossRef]
- Utrera-Melero, R.; Massuyeau, F.; Latouche, C.; Camerel, F.; Perruchas, S. Copper Iodide Clusters Coordinated by Emissive Cyanobiphenyl-Based Ligands. Inorg. Chem. 2022, 61, 4080–4091. [Google Scholar] [CrossRef] [PubMed]
- Stal, S.; Huitorel, B.; Coustham, T.; Stephant, N.; Massuyeau, F.; Gacoin, T.; Bouteiller, L.; Perruchas, S. Photoactive CuI-Cross-Linked Polyurethane Materials. ACS Appl. Mater. Interfaces 2022, 14, 47931–47940. [Google Scholar] [CrossRef]
- Kyle, K.R.; Palke, W.E.; Ford, P.C. The Photoluminescence Properties of the Copper(I) Clusters Cu4I4A4 (A = Aromatic Amine) in Solution. Coord. Chem. Rev. 1990, 97, 35–46. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-Responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Piché, D.; Harvey, P.D. The Lowest Energy Excited States of the Binuclear Silver(I) Halide Complexes, Ag2 (Dmb)2 X2. Metal-Centered or Charge Transfer States? Can. J. Chem. 1994, 72, 705–713. [Google Scholar] [CrossRef]
- Chen, B.-L.; Liu, L.; Zhong, X.-X.; Asiri, A.M.; Alamry, K.A.; Li, G.-H.; Li, F.-B.; Zhu, N.-Y.; Wong, W.-Y.; Qin, H.-M. Synthesis, Characterization and Luminescent Properties of Copper(I) Halide Complexes Containing Biphenyl Bidentate Phosphine Ligand. J. Coord. Chem. 2017, 70, 3907–3919. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhao, Z.; Yu, X.; Li, P.; Yao, Y.; Liu, Z.; Jin, Q.; Bian, Z.; Lu, Z.; et al. Bluish-Green Cu(I) Dimers Chelated with Thiophene Ring-Introduced Diphosphine Ligands for Both Singlet and Triplet Harvesting in OLEDs. ACS Appl. Mater. Interfaces 2019, 11, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Y.-J.; Zhang, T.-T.; Han, J.; Cui, G. QM/MM Studies on Luminescence Mechanism of Dinuclear Copper Iodide Complexes with Thermally Activated Delayed Fluorescence. RSC Adv. 2019, 9, 20786–20795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Huang, C.-H.; Yang, M.; Chen, X.-L.; Lu, C.-Z. Rotational Isomerization: Spontaneous Structural Transformation of a Thermally Activated Delayed Fluorescence Binuclear Copper(I) Complex. Dalton Trans. 2023, 52, 9893–9898. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, J.; Faizullin, B.; Dayanova, I.; Strelnik, I.; Strelnik, A.; Gerasimova, T.; Fayzullin, R.; Babaev, V.; Khrizanforov, M.; Budnikova, Y.; et al. Reversible Temperature-Responsible Emission in Solutions within 293–333 K Produced by Dissociative Behavior of Multinuclear Cu(I) Complexes with Aminomethylphosphines. Inorganica Chim. Acta 2019, 498, 119125. [Google Scholar] [CrossRef]
- Demyanov, Y.V.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Artem’ev, A.V. 1D CuI Coordination Polymers Based on Triphenylarsine and N,N’-Ditopic Co-Ligands: Synthesis, Crystal Structure and TADF Properties. Mendeleev Commun. 2022, 32, 649–651. [Google Scholar] [CrossRef]
- Pollak, P.; Romeder, G.; Hagedorn, F.; Gelbke, H. Nitriles. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The Triplet State of Organo-Transition Metal Compounds. Triplet Harvesting and Singlet Harvesting for Efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
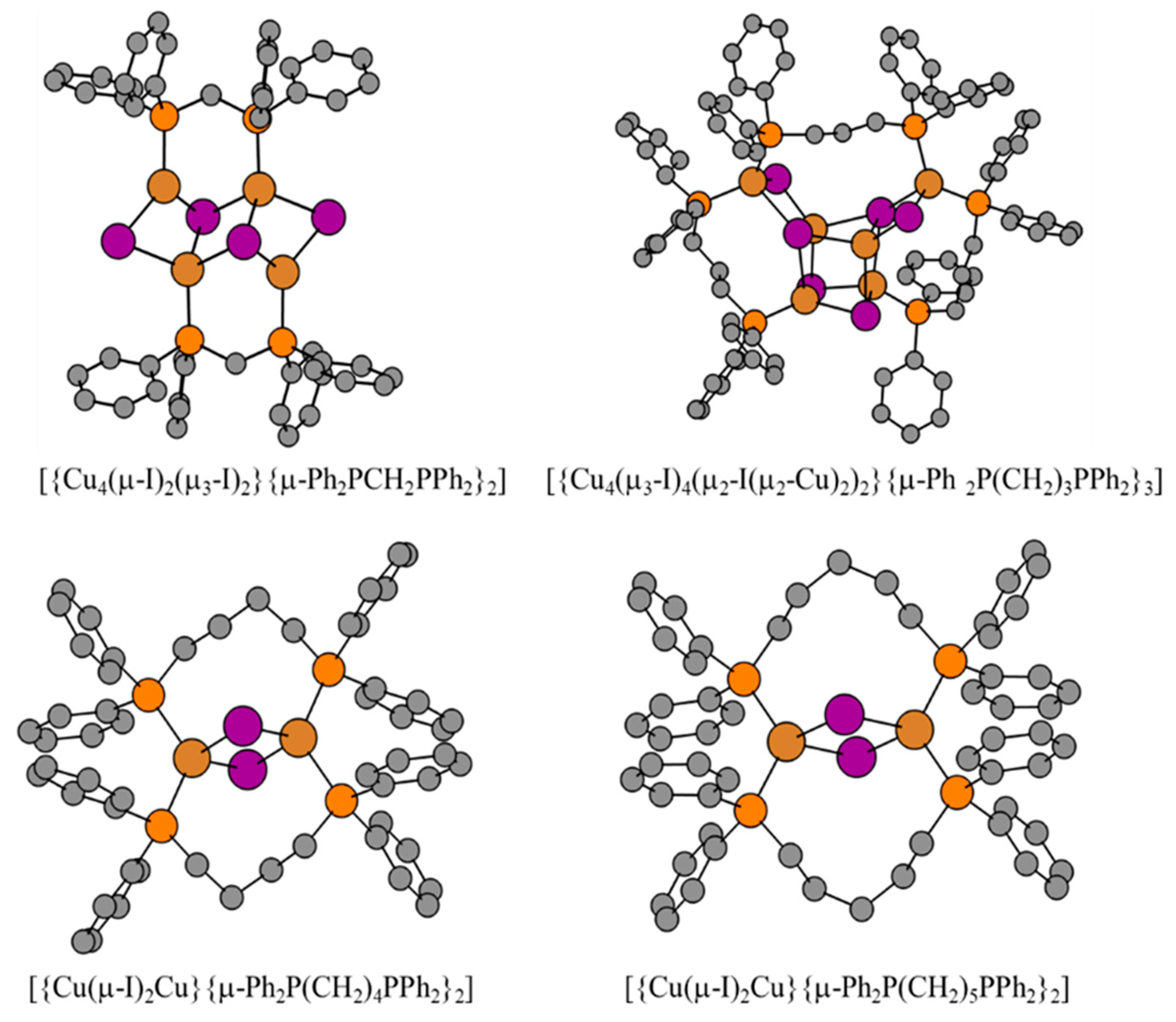

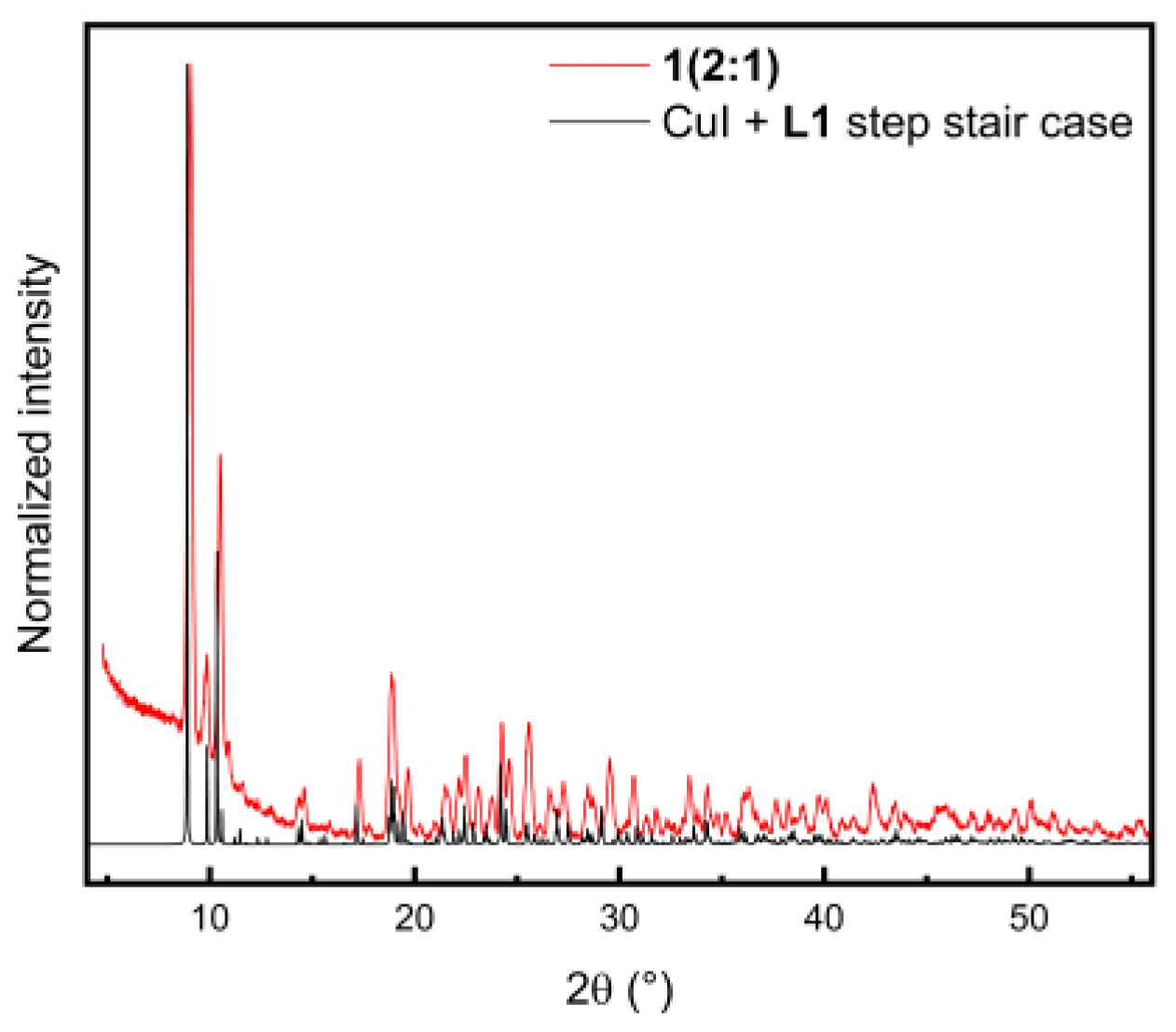

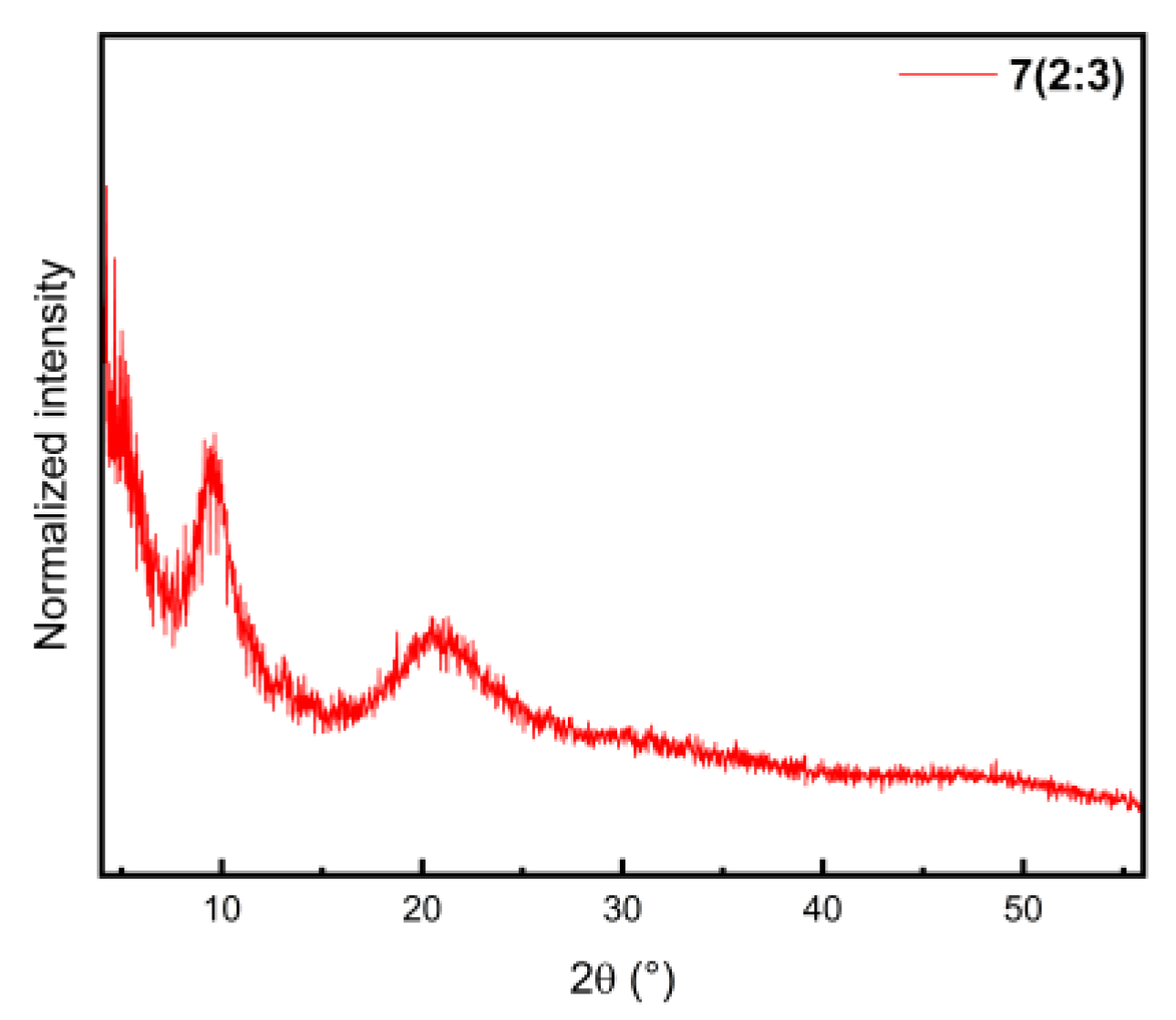

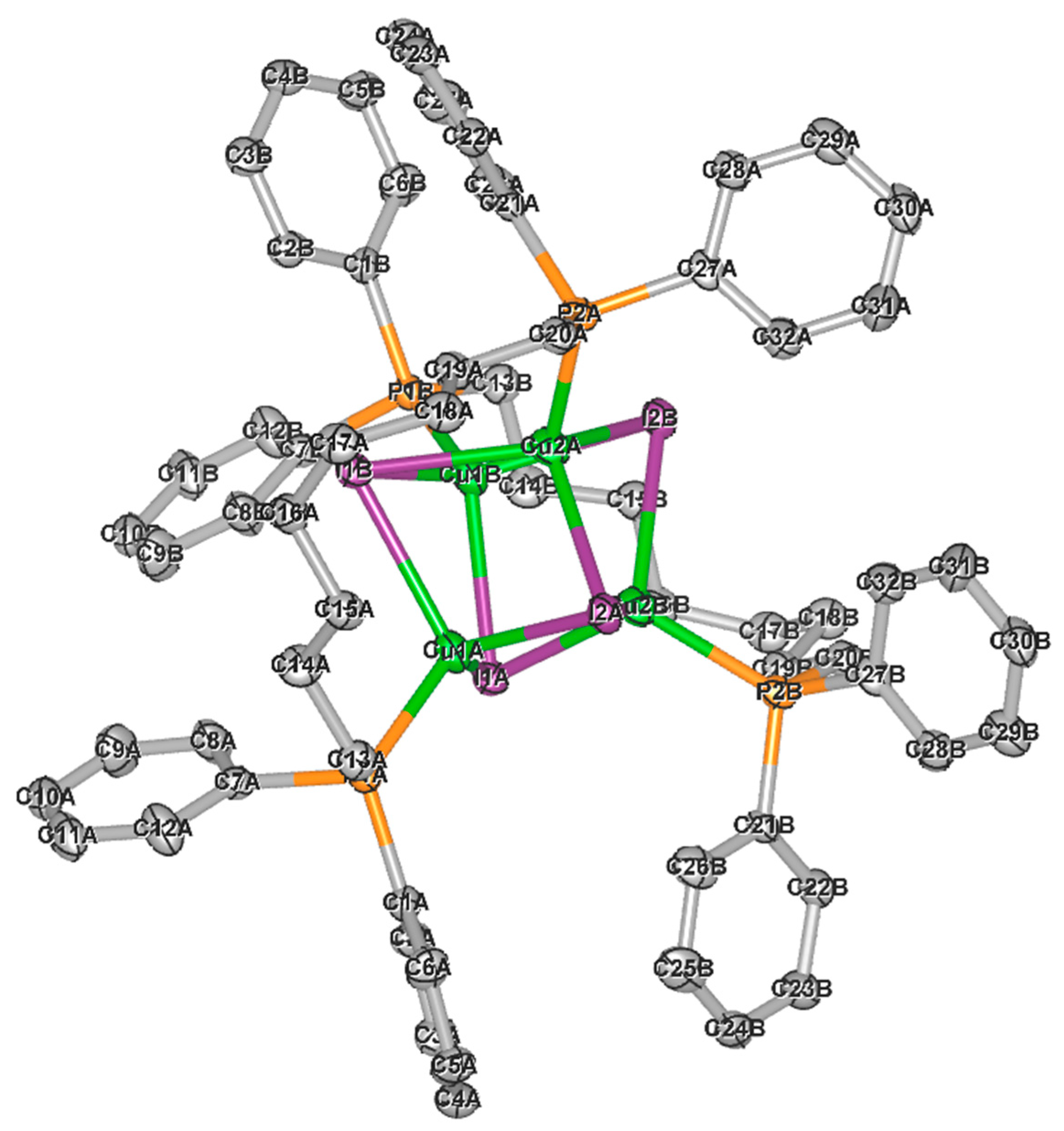

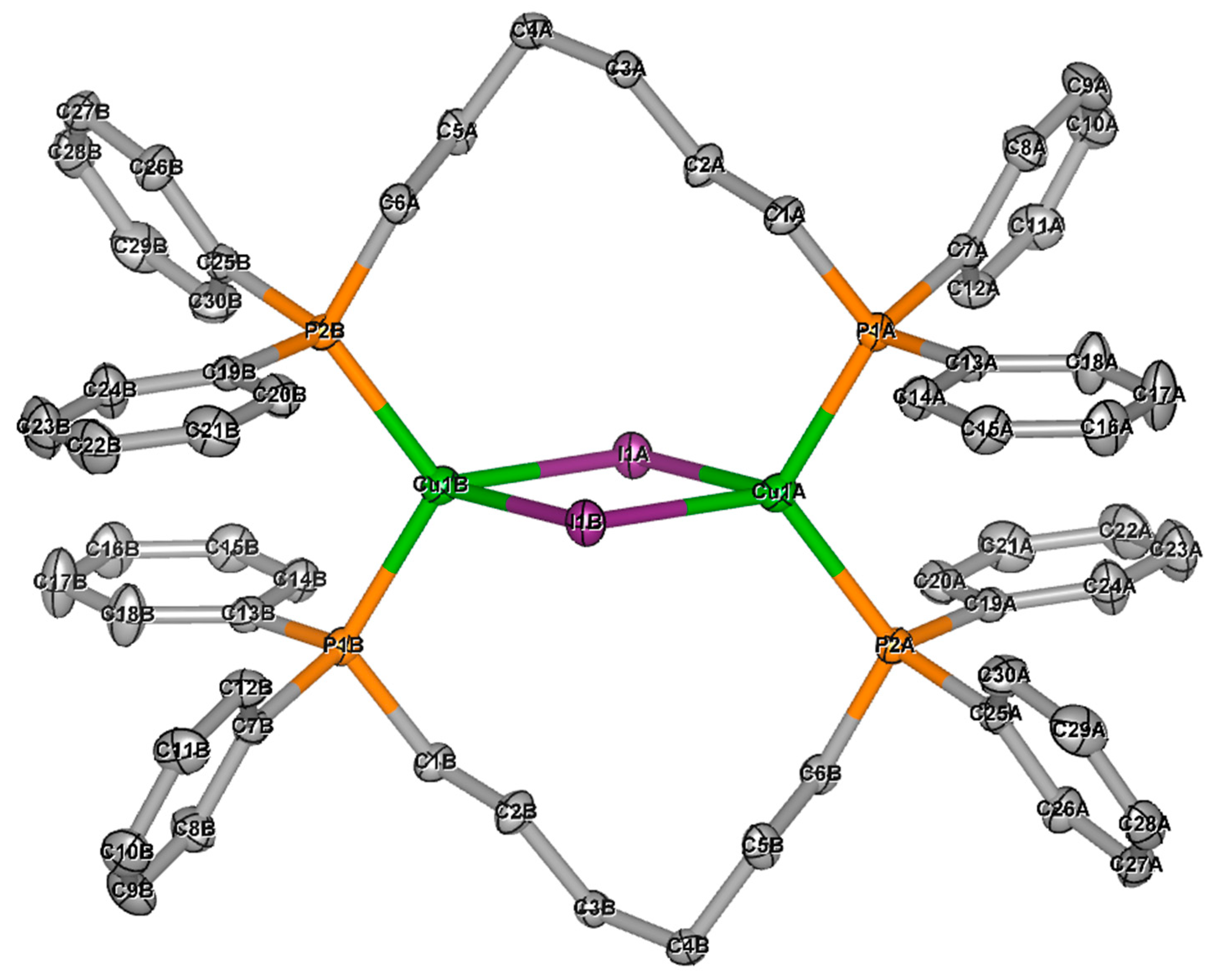

| Compound | 2(2:1) ∙ 2 PrCN | 8(2:1) | 1(1:1) ∙ 4CHCl3 | 6(1:1) |
|---|---|---|---|---|
| Formula | C60H62Cu3I3N2P4 | C64H72Cu4I4P4 | C79H70Cl12Cu3I3P6 | C60H64Cu2I2P4 |
| Formula weight/g·mol−1 | 1506.35 | 1726.85 | 2201.89 | 1289.87 |
| Temperature/K | 173(2) | 173(2) | 173(2) | 173(2) |
| Wavelength/Å | 1.54178 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic |
| Space group | P21/c | C2/c | P21/c | P -1 |
| a/Å | 17.9896(5) | 21.253(6) | 23.405(8) | 9.9268(13) |
| b/Å | 12.3774(4) | 15.418(4) | 18.989(7) | 12.1348(16) |
| c/Å | 26.7592(8) | 21.004(5) | 19.594(7) | 12.5080(16) |
| α/° | 90 | 90 | 90 | 103.0640(10) |
| β/° | 96.468(1) | 110.805(2) | 93.927(5) | 92.427(2) |
| γ/° | 90 | 90 | 90 | 101.5630(10) |
| Volume/Å3 | 5910.5(3) | 6434.0(3) | 8688.0(5) | 1431.8(3) |
| Z | 4 | 4 | 4 | 1 |
| Density (calc.)/g·cm−3 | 1.693 | 1.783 | 1.683 | 1.496 |
| Absorption coefficient/mm−1 | 14.876 | 3.361 | 2.315 | 1.969 |
| F(000) | 2968 | 3376 | 4336 | 648 |
| θ range for data collection/° | 2.48 to 71.08 | 1.67 to 26.41 | 1.50 to 26.49 | 1.68 to 26.44 |
| Index ranges | −19 ≤ h ≤ 21 −15 ≤ k ≤ 15 −31 ≤ l ≤ 32 | −26 ≤ h ≤ 26 −19 ≤ k ≤ 19 −26 ≤ l ≤ 26 | −29 ≤ h ≤ 29 −23 ≤ k ≤ 23 −24 ≤ l ≤ 24 | −12 ≤ h ≤ 12 −15 ≤ k ≤ 15 −15 ≤ l ≤ 15 |
| Reflections collected | 75,549 | 54,820 | 68,189 | 19,954 |
| Independent reflections | 11,215 [R(int) = 0.07] | 6588 [R(int) = 0.08] | 17,432 [R(int) = 0.15] | 5863 [R(int) = 0.02] |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/ parameters | 11,215/0/651 | 6588/0/343 | 17,432/40/812 | 5863/0/307 |
| Goodness-of-fit on F2 | 1.073 | 1.023 | 0.999 | 1.040 |
| Final R indices [I > 2σ(I)] | R1 = 0.0339 wR2 = 0.0819 | R1 = 0.0344 wR2 = 0.0581 | R1 = 0.0719 wR2 = 0.1416 | R1 = 0.0275 wR2 = 0.0598 |
| R indices (all data) | R1 = 0.0396 wR2 = 0.0849 | R1 = 0.0619 wR2 = 0.0650 | R1 = 0.1890 wR2 = 0.1830 | R1 = 0.0355 wR2 = 0.0628 |
| Largest diff. peak and hole/e·Å−3 | 0.939 and −0.766 | 0.712 and −0.593 | 1.562 and −1.378 | 1.106 and −0.754 |
| Sample | Cu · Cu distances (Å) |
|---|---|
| 2(2:1) | 2.5216(8) (Cu2-Cu3) 3.1667(8) (Cu1-Cu2) 3.1837(8) (Cu1-Cu3) |
| 8(2:1) | 2.767(1) (Cu1B-Cu2A) 2.834(1) (Cu2A-Cu2B) 3.0868(9) (Cu1A-Cu2A) 3.202(2) (Cu1A-Cu1B) |
| 1(1:1) | 3.074(2) (Cu1-Cu3) 3.098(2) (Cu2-Cu3) 3.148(2) (Cu1-Cu2) |
| 6(1:1) | 3.9822(8) (Cu1A-Cu1B) |
| m | 2:1 | 3:2 | 1:1 | 2:3 | 1:2 |
|---|---|---|---|---|---|
| 1 | step staircase [3,4] | diamond [5] | [triangular bipyramid]+ a | ─ | ─ |
| 2 | diamond a | ─ | chelate-rhomboid [6] | CuI [6,7] | CuI b |
| 3 | eared cubane [8] 1D-CP cubane [12] | ─ | chelate-rhomboid [10,13] | ─ | ─ |
| 4 | rhomboid b | rhomboid b | bridging-rhomboid [9,14] | CuI b | CuI [11] |
| 5 | ─ | rhomboid b,c | bridging-rhomboid [9,13] | ─ | ─ |
| 6 | ─ | rhomboid b,c | bridging-rhomboid a | ─ | ─ |
| 7 | ─ | ─ | ─ | ─ | ─ |
| 8 | cubane a,c | ─ | ─ | ─ | ─ |
| m | 2:1 | 3:2 | 1:1 | 2:3 | 1:2 |
|---|---|---|---|---|---|
| 1 | pure | mixture a + γ-CuI | mixture a | unknown but pure | mixture a |
| 2 | mixture a + γ-CuI | pure + γ-CuI | mixture a | pure | pure |
| 3 | mixture a | mixture a | mixture a | mixture a | mixture a |
| 4 | pure + γ-CuI | pure + γ-CuI | pure | pure | pure |
| 5 | mixture + γ-CuI | mixture γ-CuI | mixture | mixture a | mixture a |
| 6 | mixture a + γ-CuI | mixture a | pure | mixture a | mixture a |
| 7 | unknown but pure + γ-CuI | mixture a | mixture a | mixture a | mixture a |
| 8 | mixture a | mixture a | mixture a | mixture a | mixture a |
| Ligand | 2:1 | 3:2 | 1:1 | 2:3 | 1:2 |
|---|---|---|---|---|---|
| L1 | 597 nm (0.19) 622 nm | 720 nm (<0.01) 423, 667 nm | ─ a 443 nm | 644 nm (─ b) 441, 679 nm | ─ a 441 nm |
| L2 | 555 nm (0.03) 435, 471 nm | 542 nm (<0.01) 405, 500 nm | 546 nm (<0.01) 445, 761 nm | ─ a 430 nm | ─ a 443 nm |
| L3 | 560 nm (0.08) 430, 600 nm | 590 nm (0.06) 450, 600 nm | 483 nm (0.04) 430 nm | 476 nm (0.01) 445 nm | ─ a 459 nm |
| L4 | 510 nm (0.02) 470 nm | 576 nm (0.03) 439, 475 nm | ─ a 413 nm | 488 nm (0.02) 475 nm | ─ a 417 nm |
| L5 | 585 nm (0.31) 433, 599 nm | 586 nm (0.14) 435, 569 nm | 475 nm (0.01) 464 nm | 479 nm (<0.01) 441 nm | ─ a 434 nm |
| L6 | 584 nm (0.39) 428, 586 nm | 588 nm (0.17) 429, 589 nm | ─ a 431 nm | 488 nm (─ b) 438 nm | ─ a 433 nm |
| L7 | 544 nm (0.45) 457, 563 nm | 585 nm (0.37) 433, 588 nm | 560 nm (0.05) 430 nm | 520 nm (0.01) 474 nm | 513 nm (─ b) 433 nm |
| L8 | 566 nm (0.24) 430, 572 nm | 588 nm (0.08) 429, 579 nm | 470 nm (0.01) 435, 463 nm | 483 nm (<0.01) 430 nm | ─ a 440 nm |
| Sample | τ1 (f1,%) | τ2 (f2,%) | τ3 (f3,%) | τ4 (f4,%) | χ2 | Notes |
|---|---|---|---|---|---|---|
| 1(2:1) | 1.03 µs (1.64) | 6.86 µs (98.4) | n.a. | n.a. | 1.032 | 293 K |
| 366 ns (28.3) | 1.65 µs (48.0) | 6.38 µs (23.7) | n.a. | 1.156 | 77 K, short lifetimes | |
| 37.0 µs (100) | n.a. | n.a. | n.a. | 1.053 | 77 K, long lifetimes | |
| 2(3:2) | <100 ps (21.8) | 247 ps (28.5) | 1.30 ns (49.7) | n.a. | 1.070 | 293 K, short lifetimes |
| 139 ns (11.5) | 704 ns (27.5) | 3.25 µs (61.0) | n.a. | 1.076 | 293 K, long lifetimes | |
| 8.82 µs (72.7) | 41.0 µs (14.0) | 160 µs (13.3) | n.a. | 1.094 | 77 K | |
| 4(2:1) | 1.73 (49.9) | 5.87 µs (31.3) | 42.5 µs (18.8) | n.a. | 1.011 | 293 K |
| 7.85 µs (34.2) | 54.2 µs (33.4) | 250 µs (32.4) | n.a. | 1.024 | 77 K, short lifetimes | |
| 26.5 µs (1.25) | 1.60 ms (13.2) | 9.40 ms (85.6) | n.a. | 1.043 | 77 K, long lifetimes | |
| 4(3:2) | 6.54 µs (19.3) | 17.9 µs (56.7) | 91.3 µs (24.0) | n.a. | 1.011 | 293 K |
| 308 ps (16.1) | 1.23 ns (53.9) | 4.85 ns (30.0) | n.a. | 1.069 | 77 K, short lifetimes | |
| 120 µs (5.08) | 1.60 ms (8.03) | 8.70 ms (86.9) | n.a. | 1.045 | 77 K, long lifetimes | |
| 4(1:1) | 36.9 µs (22.2) | 110 µs (47.3) | 380 µs (30.6) | n.a. | 1.059 | 77 K |
| 4(2:3) | <100 ps (5.94) | 612 ps (16.2) | 2.24 ns (41.6) | 6.15 ns (36.3) | 1.075 | 293 K |
| 487 ps (20.3) | 1.61 ns (39.3) | 5.25 ns (40.4) | n.a. | 1.040 | 77 K, short lifetimes | |
| 190 µs (9.86) | 1.10 ms (11.4) | 10.0 ms (78.7) | n.a. | 1.003 | 77 K, long lifetimes | |
| 4(1:2) | 17.1 µs (19.9) | 68.1 µs (50.7) | 290 µs (29.5) | n.a. | 1.063 | 77 K |
| 6(1:1) | 294 ns (20.1) | 55.8 µs (49.6) | 110 µs (30.3) | n.a. | 1.036 | 77 K |
| 7(2:1) | 1.30 µs (3.79) | 5.00 µs (96.2) | n.a. | n.a. | 1.088 | 293 K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boivin, L.; Schlachter, A.; Fortin, D.; Lescop, C.; Harvey, P.D. Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers. Molecules 2023, 28, 7781. https://doi.org/10.3390/molecules28237781
Boivin L, Schlachter A, Fortin D, Lescop C, Harvey PD. Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers. Molecules. 2023; 28(23):7781. https://doi.org/10.3390/molecules28237781
Chicago/Turabian StyleBoivin, Léo, Adrien Schlachter, Daniel Fortin, Christophe Lescop, and Pierre D. Harvey. 2023. "Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers" Molecules 28, no. 23: 7781. https://doi.org/10.3390/molecules28237781
APA StyleBoivin, L., Schlachter, A., Fortin, D., Lescop, C., & Harvey, P. D. (2023). Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers. Molecules, 28(23), 7781. https://doi.org/10.3390/molecules28237781








