The Characterization and Study of Antibacterial, Free Radical Scavenging, and Anticancer Potential of Livistona chinensis-Mediated Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Preparation of L. chinensis Leaf Extracts
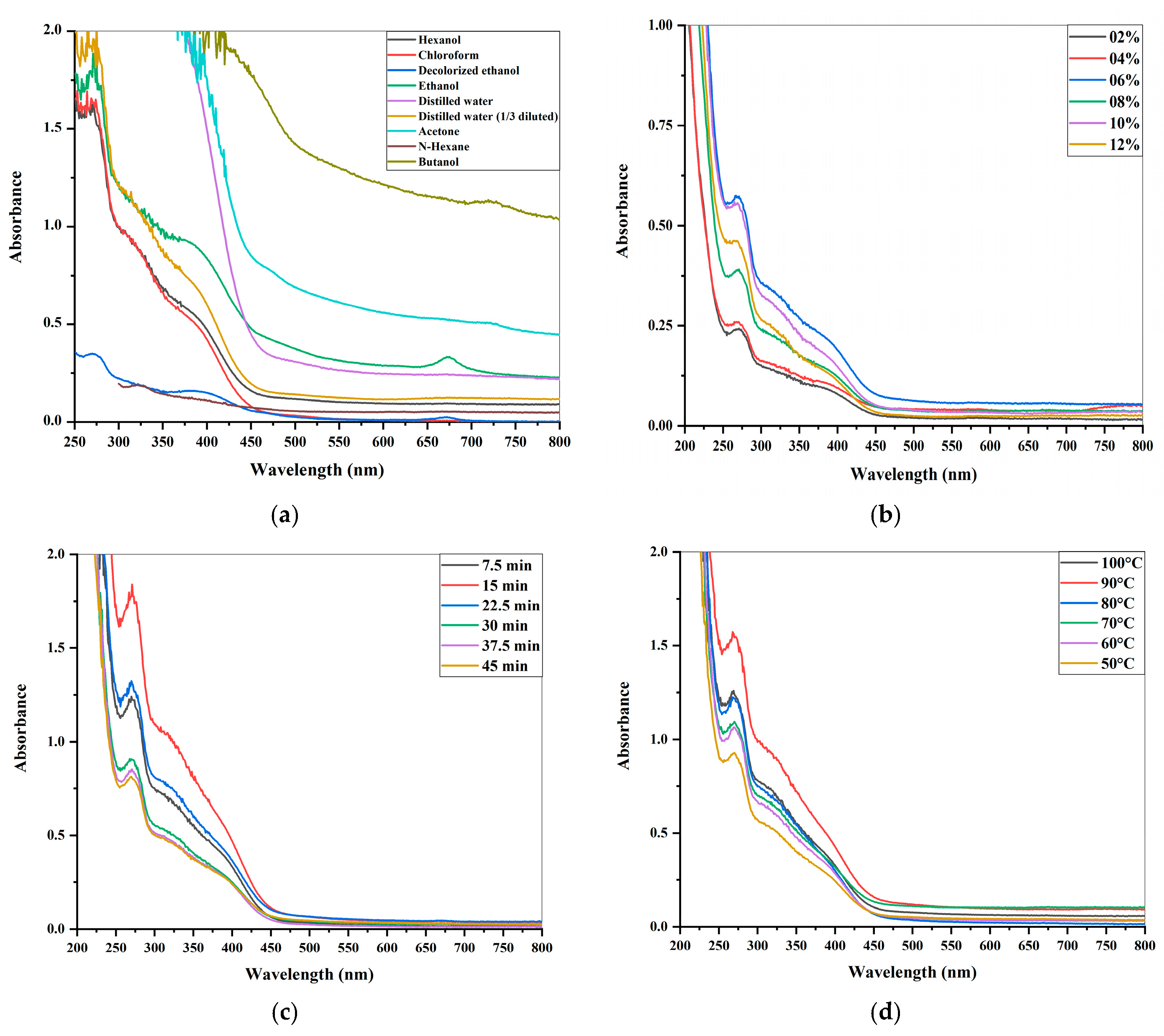
2.1.1. The Evaluation of Different Solvent-Based Leaf Extracts
2.1.2. The Estimation of Variations in Leaf Extract Concentrations
2.1.3. Dynamic Extraction’s Effect on Leaf Extract
2.1.4. The Influence of Temperature on Leaf Extract
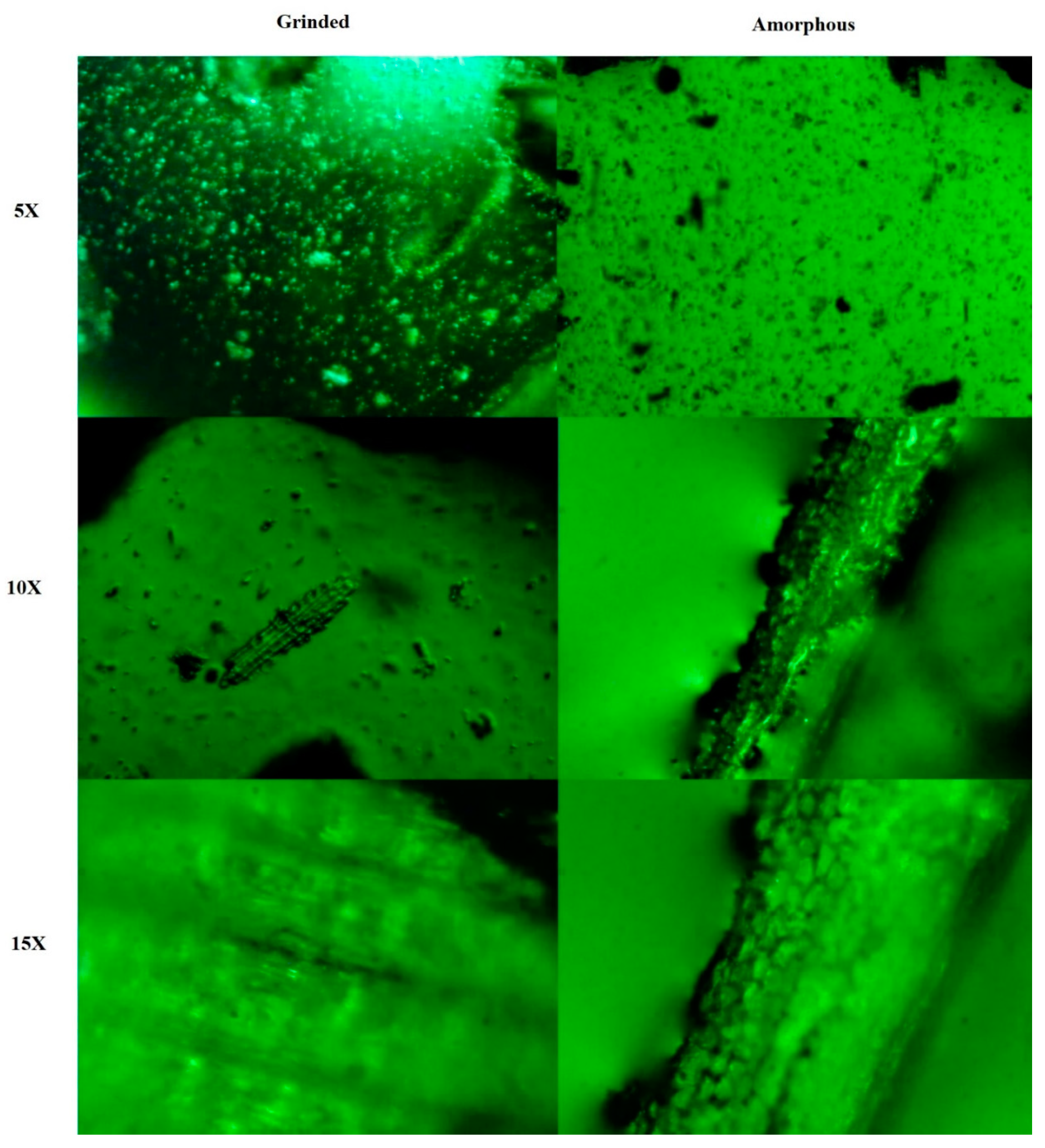
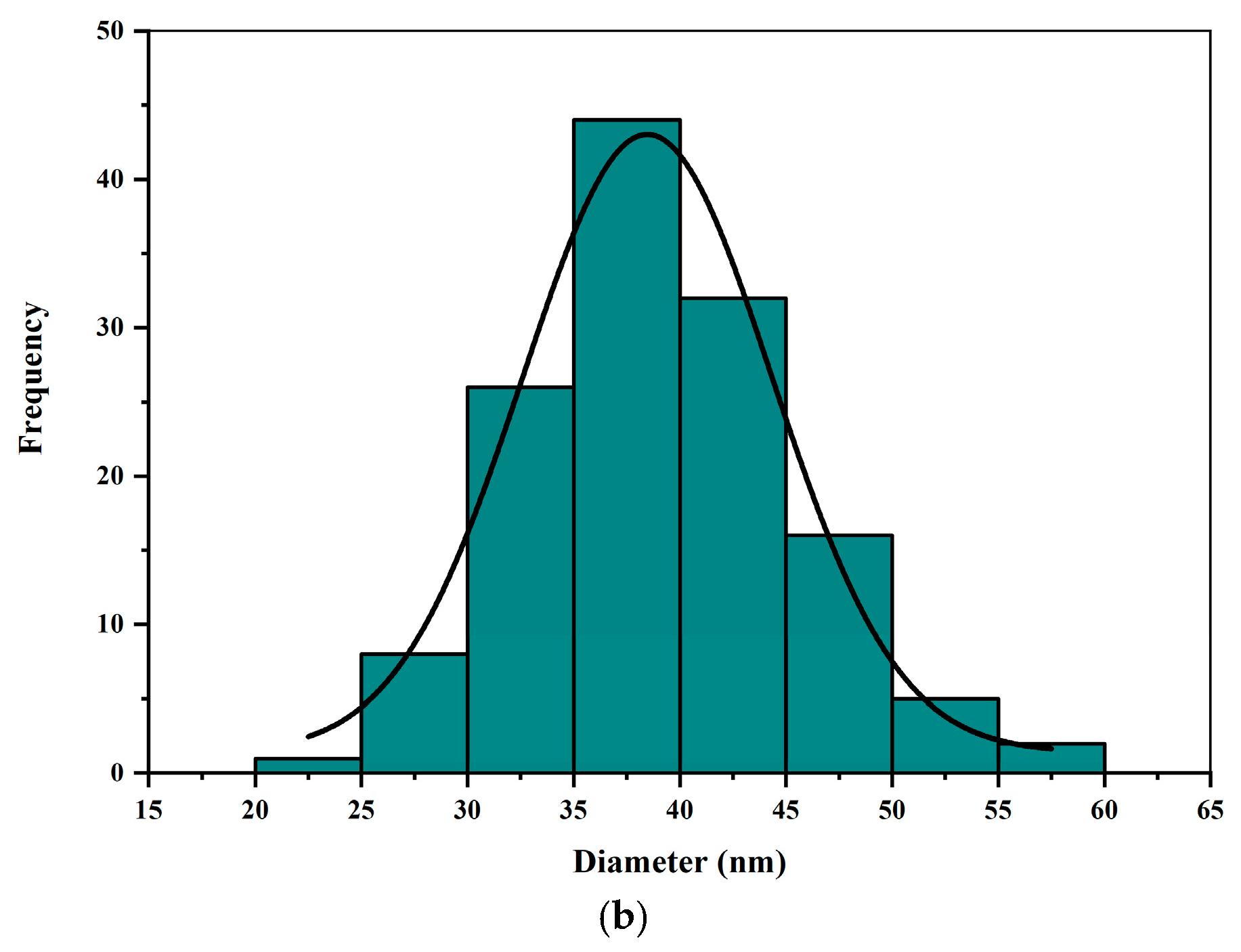
2.2. The Metallurgical Microscopy of L. chinensis Leaf Particles
2.3. The UV/VIS Analysis of Plant Extract and LC-AgNPs
2.4. FTIR Analysis
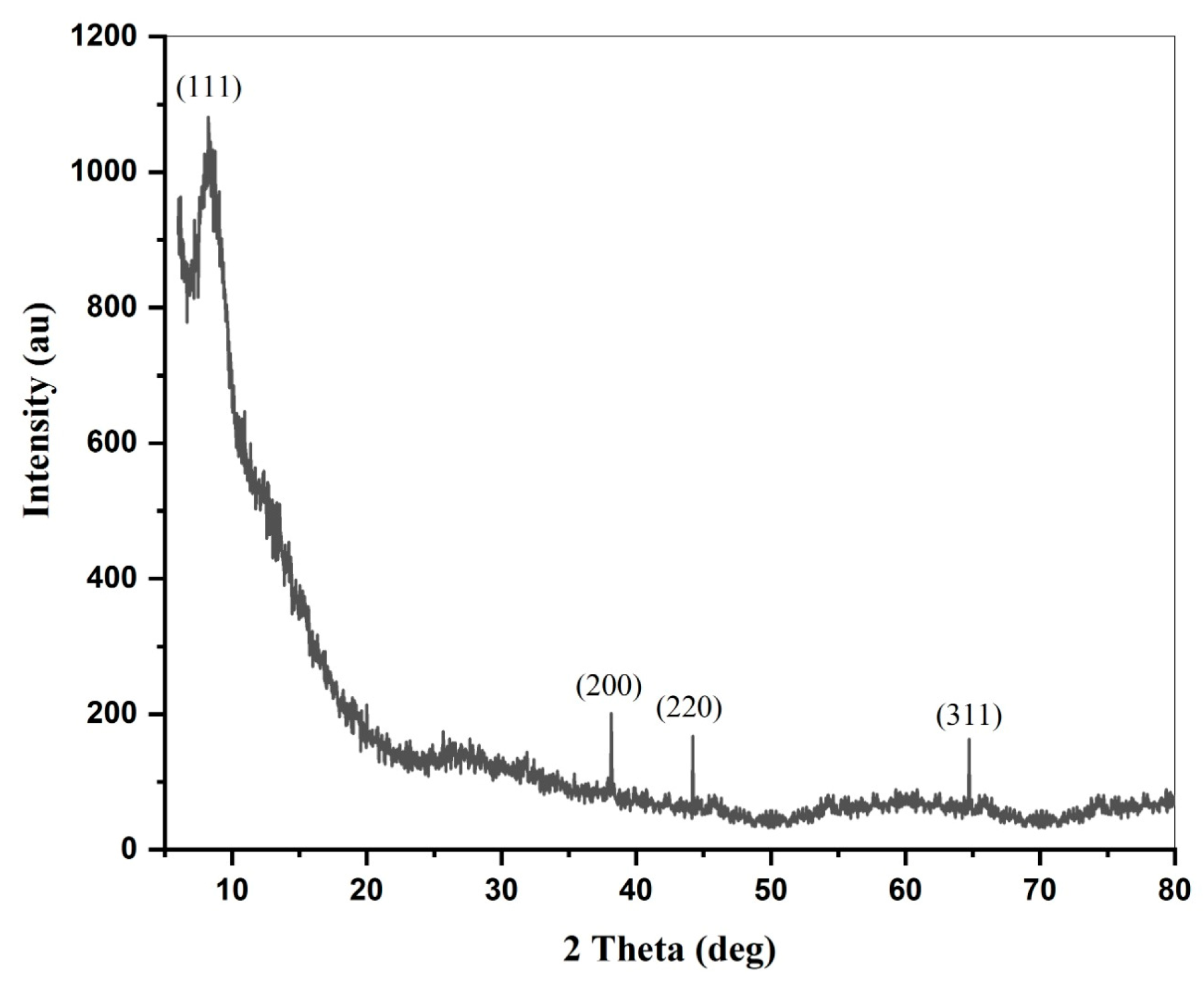
2.5. XRD
2.6. Zeta Potential
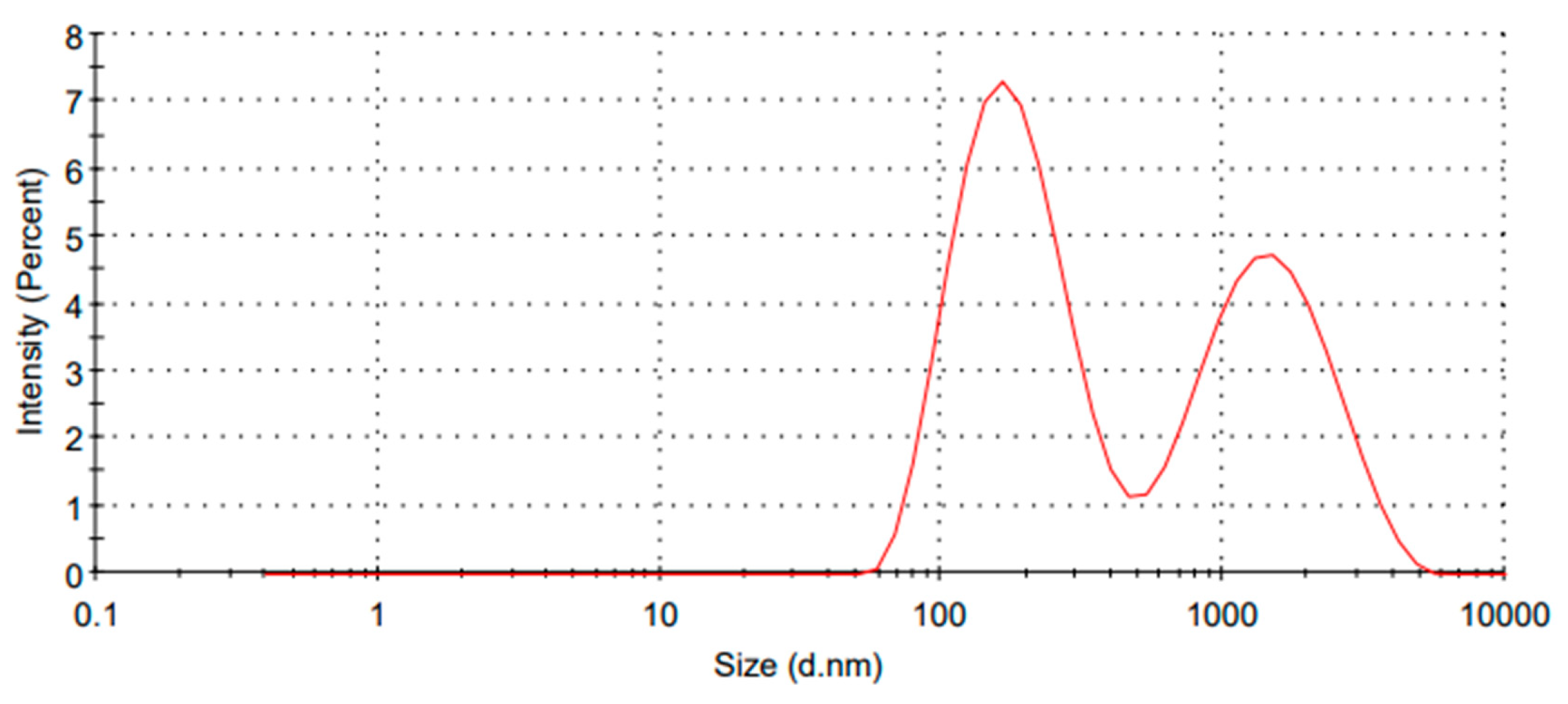
2.7. LC-AgNPs Size Distribution by Intensity
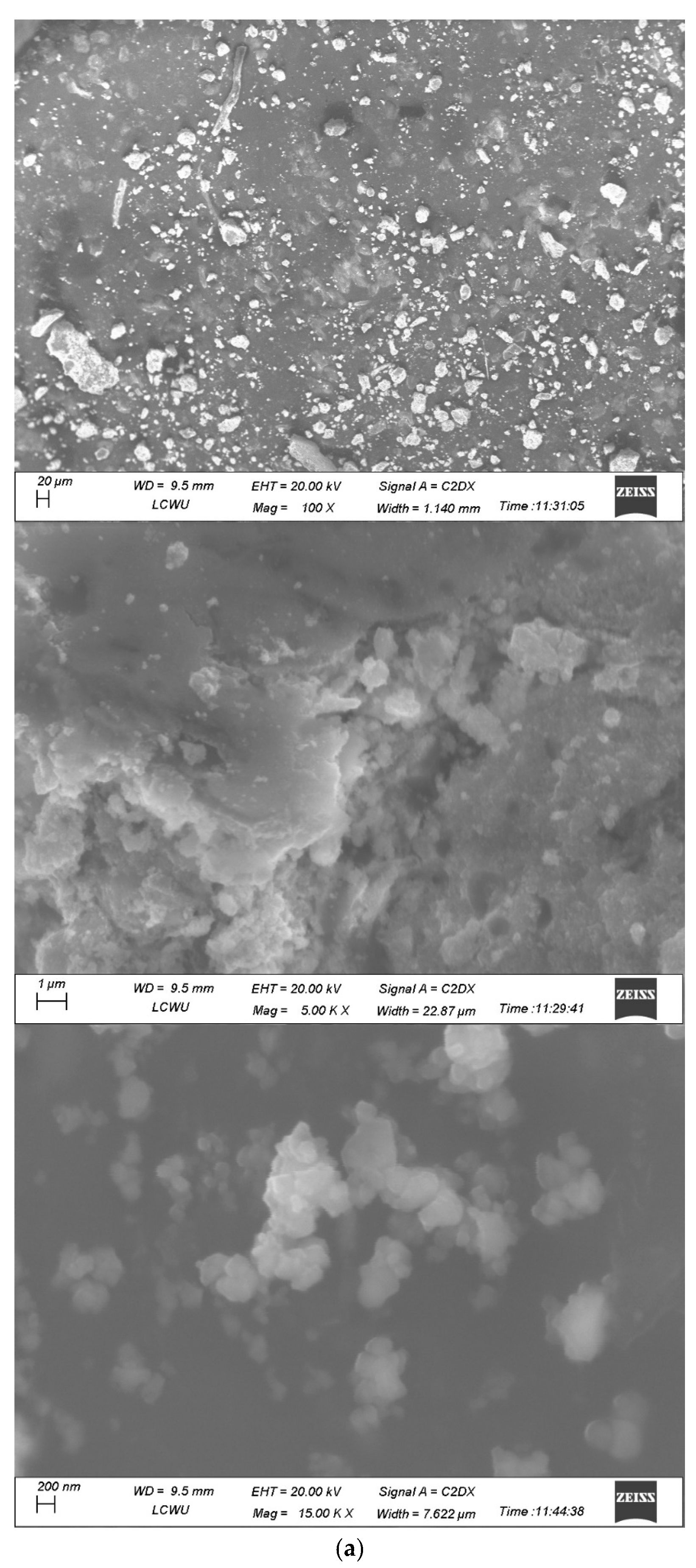
2.8. SEM
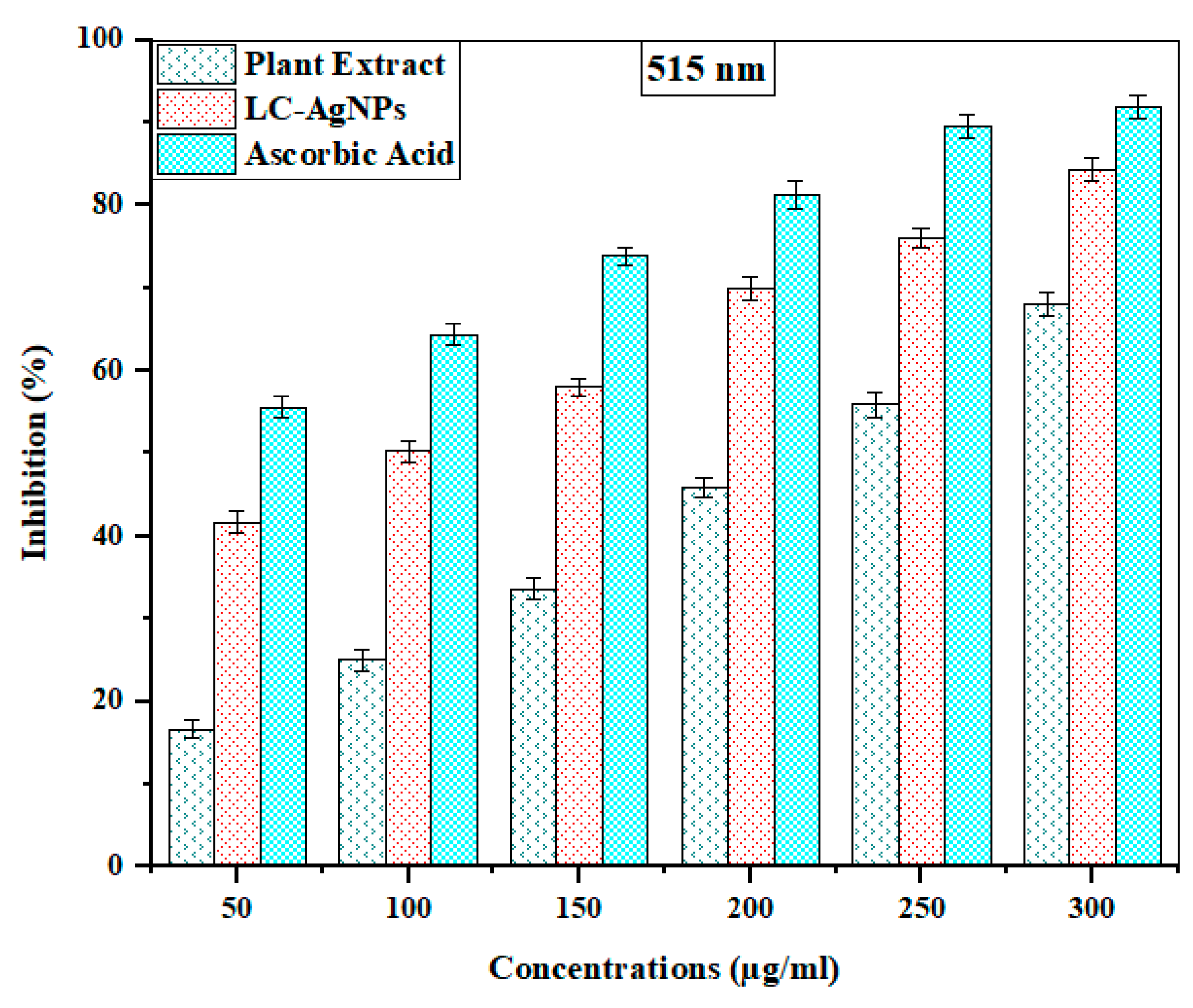
2.9. Antioxidant Activity
2.10. Antibacterial Activity
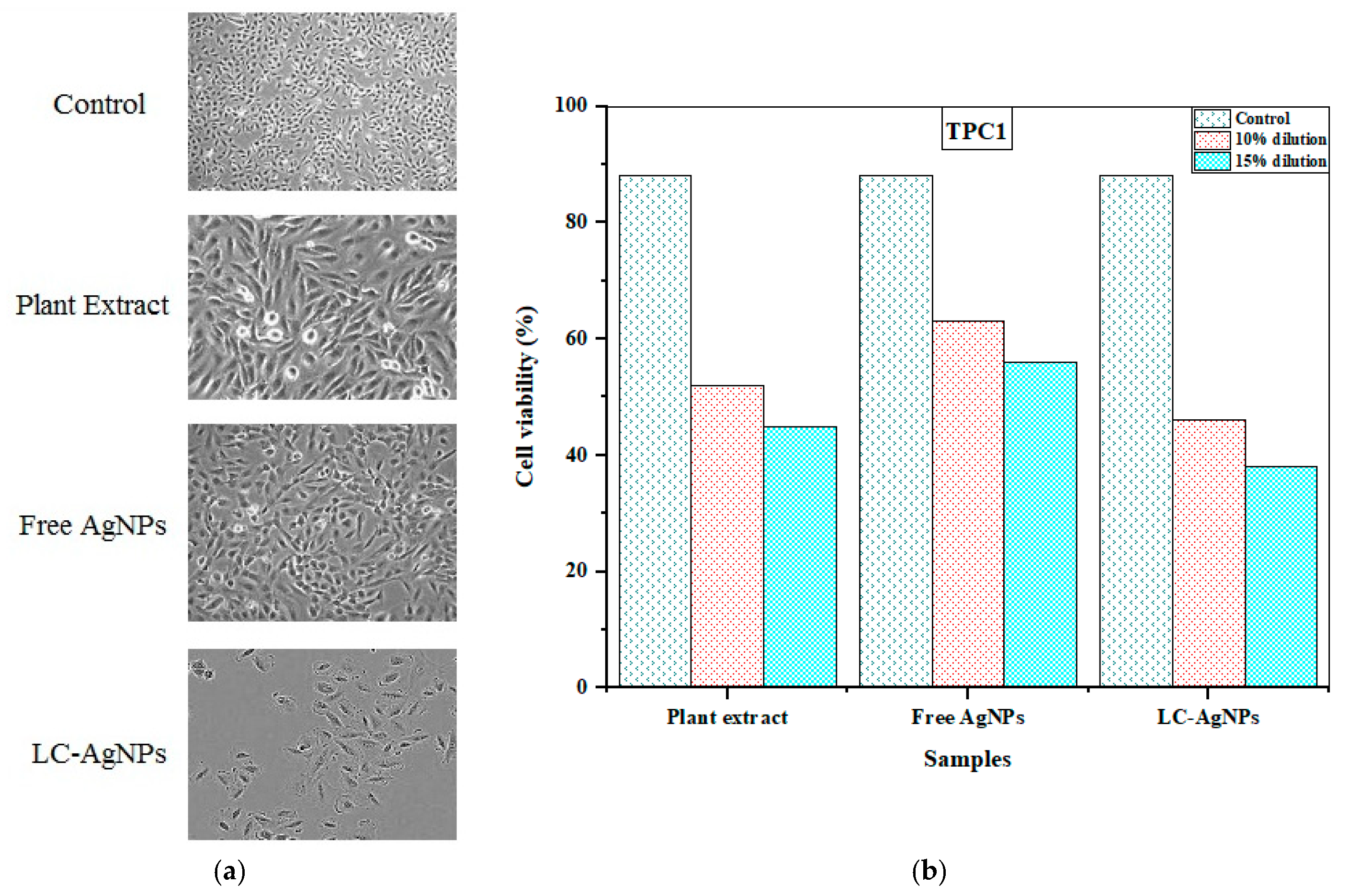
2.11. Anticancer Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. The Collection and Pre-Treatment of Livistona Chinensis Leaves
3.3. Preparation and Parameter Optimization for Efficient Leaf Extracts
3.4. The Lyophilization of Organic Filtrates
3.5. The Metallurgical Microscopy of L. chinensis
3.6. The Green Biosynthesis of AgNPs
3.7. Pellet Formation
3.8. The Production of Free AgNPs
3.9. The Characterization of LC-AgNPs via UV/VIS, FTIR and XRD Analysis
3.10. Zeta Potential Measurement
3.11. SEM
3.12. Biomedical Applications
3.12.1. Antioxidant Activity via DPPH Assay
3.12.2. Antibacterial Activity
3.12.3. Anticancer Activity
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, B.A.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–371. [Google Scholar]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Mobin, M.; Ahmad, I.; Shoeb, M. Investigation into the highly efficient Artemisia absinthium-silver nanoparticles composite as a novel environmentally benign corrosion inhibitor for mild steel in 1M HCl. J. Adhes. Sci. Technol. 2022, 36, 2562–2587. [Google Scholar] [CrossRef]
- Basik, M.; Mobin, M.; Shoeb, M. Cysteine-silver-gold Nanocomposite as potential stable green corrosion inhibitor for mild steel under acidic condition. Sci. Rep. 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, C.R.; da Silva, D.R.C.; McIlroy, E.; Calvert, N.D.; Shuhendler, A.J.; Scaiano, J.C. Silver nanoparticles with exceptional near-infrared absorbance for photoenhanced antimicrobial applications. J. Mater. Chem. B 2023, 11, 6114–6122. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Hull, R.; Meyer, M.; Dlamini, Z. Reports of Plant-Derived Nanoparticles for Prostate Cancer Therapy. Plants 2023, 12, 1870. [Google Scholar] [CrossRef]
- Shoeb, M.; Mashkoor, F.; Khan, M.N.; Anwer, A.H.; Ahmad, S.; Yi, H.; Jeong, C. Unraveling the electrochemical properties and charge storage mechanisms of lactobacillus-mediated synthesized RGO-titanium silver nanocomposite as a promising binder-free electrode for asymmetric supercapacitor device. J. Alloys Compd. 2023, 964, 171188. [Google Scholar] [CrossRef]
- Khan, R.; Assad, N.; Naeem, M.; Sher, M.; Alatawi, S.; Alatawi, M.S.; Omran, A.M.E.; Jame, R.M.A.; Adnan, M.; Khan, M.N.; et al. Aconitum lycoctonum L. (Ranunculaceae) mediated biogenic synthesis of silver nanoparticles as potential antioxidant, anti -inflammatory, antimicrobial and antidiabetic agents. BMC Chem. 2023, 17, 128. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, B.; Al-Sadoon, M.K.; Al-Otaibi, H.S.; Ali, A. LC-MS/MS and GC-MS Identification of Metabolites from the Selected Herbs and Spices, Their Antioxidant, Anti-Diabetic Potential, and Chemometric Analysis. Processes 2023, 11, 2721. [Google Scholar] [CrossRef]
- Ullah, B.; Hassan, S.; Khan, M.N.; Razzaq, A.; Al-Sadoon, M.K.; Wahab, S.; Kaplan, A.; Celikoglu, U.; Razak, S.A.; Ozdemir, F.A.; et al. Phytochemical screening, antimicrobial, antipellicle and antibiofilm activities of the root of alpine medicinal herb (Arnebia euchroma (Royle) I.M.Johnst.). Pol. J. Environ. Stud. 2024, 33, 1–18. [Google Scholar] [CrossRef]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized Silver Nanoparticles Using Morus alba (White Mulberry) Leaf Extract as Potential Antibacterial and Anticancer Agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqbal, A.; Badshah, S.L.; Ahmad, I.; Shami, A.; Ali, B.; Alatawi, F.S.; Alatawi, M.S.; Mostafa, Y.S.; Alamri, S.A. Exploring the hidden treasures of Nitella hyalina: A comprehensive study on its biological compounds, nutritional profile, and unveiling its antimicrobial, antioxidative, and hypoglycemic properties. World J. Microbiol. Biotechnol. 2023, 39, 345. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Gergeroglu, H. Synergistic effects of plant extracts and polymers on structural and antibacterial properties for wound healing. Polym. Bull. 2019, 76, 3709–3731. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Zhu, H.; Ahmad, T.; Zheng, Y.; Moosa, A.; Nie, C.; Liu, Y. First report of leaf blight disease of Livistona chinensis caused by Alternaria alternata in Pakistan. Plant Dis. 2022, 106, 2997. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ali, S.; Rashid, U.; Kamran, M.; Malik, M.F.; Sughra, K.; Zeeshan, N.; Afroz, A.; Saleem, J.; Saghir, M. Biosynthesis of silver nanoparticles from leaf extract of Litchi chinensis and its dynamic biological impact on microbial cells and human cancer cell lines. Cell Mol. Biol. 2018, 64, 42–47. [Google Scholar] [CrossRef]
- Essien, E.E.; Antia, B.S.; Etuk, E.I. Phytoconstituents, antioxidant and antimicrobial activities of Livistona chinensis (Jacquin), Saribus rotundifolius (Lam.) Blume and Areca catechu Linnaeus Nuts. Pharm. Biosci. J. 2017, 5, 59–67. [Google Scholar] [CrossRef]
- Jabeen, S.; Ali, M.F.; Mohi ud Din, A.; Javed, T.; Mohammed, N.S.; Chaudhari, S.K.; Javed, M.A.; Ali, B.; Zhang, L.; Rahimi, M. Phytochemical screening and allelopathic potential of phytoextracts of three invasive grass species. Sci. Rep. 2023, 13, 8080. [Google Scholar] [CrossRef]
- Hafeez, A.; Ali, B.; Javed, M.A.; Saleem, A.; Fatima, M.; Fathi, A.; Afridi, M.S.; Aydin, V.; Oral, M.A.; Soudy, F.A. Plant breeding for harmony between sustainable agriculture, the environment, and global food security: An era of genomics-assisted breeding. Planta 2023, 258, 97. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; AL-Huqail, A.A.; Albalawi, S.; Alghamdi, S.A.; Ali, B.; Alghanem, S.M.S.; Al-Haithloul, H.A.S.; Amro, A.; Tammam, S.A.; El-Mahdy, M.T. Calcium nanoparticles mitigate severe salt stress in Solanum lycopersicon by instigating the antioxidant defense system and renovating the protein profile. S. Afr. J. Bot. 2023, 161, 36–52. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; Saleem, M.H.; Asghar, M.A.; Mumtaz, S.; Ameer, A.; Ali, B.; Alwahibi, M.S.; Elshikh, M.S.; Ercisli, S.; Elsharkawy, M.M. Ameliorative Effects of Exogenous Potassium Nitrate on Antioxidant Defense System and Mineral Nutrient Uptake in Radish (Raphanus sativus L.) under Salinity Stress. ACS Omega 2023, 8, 22575–22588. [Google Scholar] [CrossRef]
- Alshegaihi, R.M.; Mfarrej, M.F.B.; Saleem, M.H.; Parveen, A.; Ahmad, K.S.; Ali, B.; Abeed, A.H.A.; Alshehri, D.; Alghamdi, S.A.; Alghanem, S.M.S.; et al. Effective citric acid and EDTA treatments in cadmium stress tolerance in pepper (Capsicum annuum L.) seedlings by regulating specific gene expression. S. Afr. J. Bot. 2023, 159, 367–380. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Ali, B.; Usman, F.; Jabeen, Q.; Sajid-ur-Rehman, M.; Ahmed, M.; Zubair, H.M.; Alkazmi, L.; Batiha, G.E.-S. Chemical profiling and evaluation of toxicological, antioxidant, anti-inflammatory, anti-nociceptive and tyrosinase inhibitory potential of Portulacaria afra using in-vitro, in-vivo and in-silico studies. Arab. J. Chem. 2023, 16, 104784. [Google Scholar] [CrossRef]
- Ali, Q.; Shabaan, M.; Ashraf, S.; Kamran, M.; Zulfiqar, U.; Ahmad, M.; Zahir, Z.A.; Sarwar, M.J.; Iqbal, R.; Ali, B. Comparative efficacy of different salt tolerant rhizobial inoculants in improving growth and productivity of Vigna radiata L. under salt stress. Sci. Rep. 2023, 13, 17442. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Afridi, M.S.; Javed, M.A.; Sumaira; Suleman, F.; Nadeem, M.; Ali, S.; Alwahibi, M.S.; Elshikh, M.S.; et al. Bacterial-Mediated Salinity Stress Tolerance in Maize (Zea mays L.): A Fortunate Way toward Sustainable Agriculture. ACS Omega 2023, 8, 20471–20487. [Google Scholar] [CrossRef]
- Batool, S.; Batool, S.; Batool, T.; Iram, F.; Almas, T.; Faizan, M.; Assad, N.; Al-Sadoon, M.K.; Khan, M.N.; Ullah, B. Delving the Role of the Ameliorative Effects of Caralluma tuberculata NE Br.(Apocynaceae) on Diabetes and Its Effect on the Organs Weight of Alloxan-Induced Adult Male Mice. Polish J. Environ. Stud. 2023, 33, 1. [Google Scholar]
- Khan, S.; Ullah, H.; Rahim, F.; Hussain, R.; Khan, Y.; Khan, M.S.; Iqbal, R.; Ali, B.; Albeshr, M.F. Synthesis, biological evaluation and molecular docking study of pyrimidine based thiazolidinone derivatives as potential anti-urease and anti-cancer agents. J. Saudi Chem. Soc. 2023, 27, 101688. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, S.; Singh, N.; Ali, B.; Hafeez, A.; Vodnar, D.C.; Marc, R.A. Nutritional evaluation, phytochemical makeup, antibacterial and antioxidant properties of wild plants utilized as food by the Gaddis-a tribal tribe in the Western Himalayas. Front. Agron. 2022, 4, 1010309. [Google Scholar] [CrossRef]
- Farrukh, A.; Khattak, S.H.; Kaleem, I.; Basheer, S.; Bangash, S.A.K.; Ali, G.M.; Khan, M.N.; Abul Farah, M.; Al-Anazi, K.M.; Kaplan, A.; et al. Evaluation of Counteraction Potential of ZnO-NPs and/or Piperacillin-Tazobactam against Multi-Drug Resistant Pseudomonas aeruginosa and MCF-7 and HepG2 Cell Lines. Polish J. Environ. Stud. 2023, 33, 1–11. [Google Scholar] [CrossRef]
- Moradi, M.T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.S. Anti-influenza virus activity and phenolic content of pomegranate (Punica granatum L.) peel extract and fractions. Avicenna J. Med. Biotechnol. 2019, 11, 285–291. [Google Scholar] [PubMed]
- Gali, L.; Bedjou, F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. 2019, 120, 163–169. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Rajan, K.S. Microwave assisted extraction of flavonoids from Terminalia bellerica: Study of kinetics and thermodynamics. Sep. Purif. Technol. 2016, 157, 169–178. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Zhou, X.; Deng, Q.; Hu, E.; Zhao, C.; Gong, X. Optimized microwave-assistant extraction combined ultrasonic pretreatment of flavonoids from Periploca forrestii Schltr. and evaluation of its anti-allergic activity. Electrophoresis 2017, 38, 1113–1121. [Google Scholar] [CrossRef]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; García-Alvarado, M.Á.; Palma, M.; Rodríguez-Jimenes, G.d.C.; Barbero, G.F. Optimization of an ultrasound-assisted extraction method applied to the extraction of flavonoids from Moringa leaves (Moringa oleífera Lam.). Agronomy 2022, 12, 261. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Erşan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; Yrjas, P.; Lindberg, D.; Trikkel, A. Characterization of ash melting of reed and wheat straw blend. ACS omega 2022, 7, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Das, C.M.; Yang, F.; Yang, Z.; Liu, X.; Hoang, Q.T.; Xu, Z.; Neermunda, S.; Kong, K.V.; Ho, H.; Ju, L.A. Computational Modeling for Intelligent Surface Plasmon Resonance Sensor Design and Experimental Schemes for Real-Time Plasmonic Biosensing: A Review. Adv. Theory Simul. 2023, 6, 2200886. [Google Scholar] [CrossRef]
- Nagime, P.V.; Singh, S.; Shaikh, N.M.; Gomare, K.S.; Chitme, H.; Abdel-Wahab, B.A.; Alqahtany, Y.S.; Khateeb, M.M.; Habeeb, M.S.; Bakir, M.B. Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes. Materials 2023, 16, 4058. [Google Scholar] [CrossRef] [PubMed]
- Nduati, T.W.; Wagara, I.N.; Walyambillah, W.; Were, B.; Matasyoh, J.C. Bioactive compounds from Juniperus procera (Cupressaceae) with activity against common bean bacterial pathogens. Afr. J. Biotechnol. 2023, 22, 106–113. [Google Scholar]
- Taha, M.; Elazab, S.T.; Abdelbagi, O.; Saati, A.A.; Babateen, O.; Baokbah, T.A.S.; Qusty, N.F.; Mahmoud, M.E.; Ibrahim, M.M.; Badawy, A.M. Phytochemical analysis of Origanum majorana L. extract and investigation of its antioxidant, anti-inflammatory and immunomodulatory effects against experimentally induced colitis downregulating Th17 cells. J. Ethnopharmacol. 2023, 14, 116826. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef]
- Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials 2019, 12, 189. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Manikandan, E.; Krishnan, V. Green Synthesis of Silver Nanoparticles Using Piper nigrum Concoction and its Anticancer Activity against MCF-7 and Hep-2 Cell Lines. J. Antimicrob. Agents 2016, 2, 3–7. [Google Scholar] [CrossRef]
- He, Y.; Wei, F.; Ma, Z.; Zhang, H.; Yang, Q.; Yao, B.; Huang, Z.; Li, J.; Zeng, C.; Zhang, Q. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017, 7, 39842–39851. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Ahmar Rauf, M.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Sarwar Zaman, G.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef]
- David, S.A.; Ponvel, K.M.; Fathima, M.A.; Anita, S.; Ashli, J.; Athilakshmi, A. Biosynthesis of silver nanoparticles by Momordica charantia leaf extract: Characterization and their antimicrobial activities. J. Nat. Prod. Plant Resour. 2014, 4, 1–8. [Google Scholar]
- Liu, Y.-S.; Chang, Y.-C.; Chen, H.-H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J. Food Drug Anal. 2018, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, D.B.; Sridhar, A.; Krishnasamy Sekar, R.; Perumalsamy, B.; Veeran, S.; Arumugam, M.; Karuppaiah, P.; Ramasamy, T. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng. 2021, 9, 104845. [Google Scholar] [CrossRef]
- Pungle, R.; Nile, S.H.; Makwana, N.; Singh, R.; Singh, R.P.; Kharat, A.S. Green Synthesis of Silver Nanoparticles Using the Tridax procumbens Plant Extract and Screening of Its Antimicrobial and Anticancer Activities. Oxidative Med. Cell. Longev. 2022, 2022, 9671594. [Google Scholar] [CrossRef]
- Shyamalagowri, S.; Charles, P.; Manjunathan, J.; Kamaraj, M.; Anitha, R.; Pugazhendhi, A. In vitro anticancer activity of silver nanoparticles phyto-fabricated by Hylocereus undatus peel extracts on human liver carcinoma (HepG2) cell lines. Process Biochem. 2022, 116, 17–25. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green synthesis of silver nanoparticles using Diospyros malabarica fruit extract and assessments of their antimicrobial, anticancer and catalytic reduction of 4-nitrophenol (4-NP). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Chandraker, S.K.; Lal, M.; Khanam, F.; Dhruve, P.; Singh, R.P.; Shukla, R. Therapeutic potential of biogenic and optimized silver nanoparticles using Rubia cordifolia L. leaf extract. Sci. Rep. 2022, 12, 8831. [Google Scholar] [CrossRef] [PubMed]
- Tormena, R.P.I.; Rosa, E.V.; Mota, B.d.F.O.; Chaker, J.A.; Fagg, C.W.; Freire, D.O.; Martins, P.M.; da Silva, I.C.R.; Sousa, M.H. Evaluation of the antimicrobial activity of silver nanoparticles obtained by microwave-assisted green synthesis using Handroanthus impetiginosus (Mart. ex DC.) Mattos underbark extract. RSC Adv. 2020, 10, 20676–20681. [Google Scholar] [CrossRef] [PubMed]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Thirumagal, N.; Jeyakumari, A.P. Structural, optical and antibacterial properties of green synthesized silver nanoparticles (AgNPs) using Justicia adhatoda L. leaf extract. J. Clust. Sci. 2020, 31, 487–497. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A.; Fekete, G.; Singh, T. Cytotoxic and radiosensitizing potential of silver nanoparticles against HepG-2 cells prepared by biosynthetic route using Picrasma quassioides leaf extract. J. Drug Deliv. Sci. Technol. 2020, 55, 101479. [Google Scholar] [CrossRef]
- Ghabban, H.; Alnomasy, S.F.; Almohammed, H.; Al Idriss, O.M.; Rabea, S.; Eltahir, Y. Antibacterial, Cytotoxic, and Cellular Mechanisms of Green Synthesized Silver Nanoparticles against Some Cariogenic Bacteria (Streptococcus mutans and Actinomyces viscosus). J. Nanomater. 2022, 2022, 9721736. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Rajendran, R.; Ganesan, N.; Balu, S.K.; Alagar, S.; Thandavamoorthy, P.; Thiruvengadam, D. Green synthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Origanum heracleoticum L. leaf extract. Int. J. Pharm. Pharm. Sci. 2015, 7, 288–293. [Google Scholar]
- Kumar, V.; Singh, S.; Srivastava, B.; Bhadouria, R.; Singh, R. Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti-inflammatory, antidiabetic and antibacterial activities. J. Environ. Chem. Eng. 2019, 7, 103094. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Thiruvengadam, M.; Rajeswari, V.D.; Kalpana, V.N.; Chung, I.-M. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb. Pathog. 2017, 103, 123–128. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Ali Shah, S.A.; Harish, R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Seqqat, R.; Benalcazar, K.; Grijalva, M.; Cumbal, L. In vitro evaluation of silver nanoparticles cytotoxicity on Hepatic cancer (Hep-G2) cell line and their antioxidant activity: Green approach for fabrication and application. J. Photochem. Photobiol. B Biol. 2016, 159, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Ramezani, T.; Mousavi, M.; Asadi-Samani, M. Antioxidant and anti-inflammatory activity of green synthesized silver nanoparticles using Salvia officinalis extract. Ann. Trop. Med. Public Health 2017, 10, 1265–1270. [Google Scholar]
- Tyagi, P.K.; Tyagi, S.; Gola, D.; Arya, A.; Ayatollahi, S.A.; Alshehri, M.M.; Sharifi-Rad, J. Ascorbic acid and polyphenols mediated green synthesis of silver nanoparticles from Tagetes erecta L. aqueous leaf extract and studied their antioxidant properties. J. Nanomater. 2021, 2021, 6515419. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Bunghez, I.R.; Somoghi, R.; Fierascu, I.; Ion, R.M. Characterization of silver nanoparticles obtained by using Rosmarinus officinalis extract and their antioxidant activity. Rev. Roum. Chim. 2014, 59, 213–218. [Google Scholar]
- Rajakannu, S.; Shankar, S.; Perumal, S.; Subramanian, S.; Dhakshinamoorthy, G.P. Biosynthesis of silver nanoparticles using Garcinia mangostana fruit extract and their antibacterial, antioxidant activity. Int. J. Curr. Microbiol. Appl. Sci 2015, 4, 944–952. [Google Scholar]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif. Cells Nanomed. Biotechnol 2017, 45, 1234–1240. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef]
- Younas, M.; Rasool, M.H.; Khurshid, M.; Khan, A.; Nawaz, M.Z.; Ahmad, I.; Lakhan, M.N. Moringa oleifera leaf extract mediated green synthesis of silver nanoparticles and their antibacterial effect against selected gram-negative strains. Biochem. Syst. Ecol. 2023, 107, 104605. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Qamer, S.; Romli, M.H.; Che-Hamzah, F.; Misni, N.; Joseph, N.M.S.; Al-Haj, N.A.; Amin-Nordin, S. Systematic review on biosynthesis of silver nanoparticles and antibacterial activities: Application and theoretical perspectives. Molecules 2021, 26, 5057. [Google Scholar] [CrossRef]
- Some, S.; Sen, I.K.; Mandal, A.; Aslan, T.; Ustun, Y.; Yilmaz, E.Ş.; Katı, A.; Demirbas, A.; Mandal, A.K.; Ocsoy, I. Biosynthesis of silver nanoparticles and their versatile antimicrobial properties. Mater. Res. Express 2018, 6, 12001. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L. V Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Farshori, N.N.; Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Saquib, Q.; Siddiqui, M.A.; Wahab, R.; Al-Khedhairy, A.A. Green synthesis of silver nanoparticles using Phoenix dactylifera seed extract and its anticancer effect against human lung adenocarcinoma cells. J. Drug Deliv. Sci. Technol. 2022, 70, 103260. [Google Scholar] [CrossRef]
- Saber, M.M.; Mirtajani, S.B.; Karimzadeh, K. Green synthesis of silver nanoparticles using Trapa natans extract and their anticancer activity against A431 human skin cancer cells. J. Drug Deliv. Sci. Technol. 2018, 47, 375–379. [Google Scholar] [CrossRef]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Gurumallesh Prabu, H. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-Mediated Green Synthesis of Silver Nanoparticles Exhibiting Antioxidant and Anticancer Activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S. Green synthesis and anticancer activity of silver nanoparticles prepared using fruit extract of Azadirachta indica. J. Radiat. Res. Appl. Sci. 2022, 15, 335–345. [Google Scholar] [CrossRef]
- Gomathi, A.C.; Rajarathinam, S.R.X.; Sadiq, A.M.; Rajeshkumar, S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Narasimha, V.R.; Latha, T.S.; Pallu, R.; Panati, K.; Narala, V.R. Anticancer activities of biogenic silver nanoparticles targeting apoptosis and inflammatory pathways in colon cancer cells. J. Clust. Sci. 2022, 33, 2215–2231. [Google Scholar] [CrossRef]
- El Raey, M.A.; El-Hagrassi, A.M.; Osman, A.F.; Darwish, K.M.; Emam, M. Acalypha wilkesiana flowers: Phenolic profiling, cytotoxic activity of their biosynthesized silver nanoparticles and molecular docking study for its constituents as Topoisomerase-I inhibitors. Biocatal. Agric. Biotechnol. 2019, 20, 101243. [Google Scholar] [CrossRef]
- Rajawat, S.; Malik, M.M. Anticancer activity of green silver nanoparticles against He-La cervical cancer cell lines. Mater. Today Proc. 2019, 18, 841–847. [Google Scholar] [CrossRef]
- Yadav, A.; Mendhulkar, V.D. Antiproliferative activity of Camellia sinensis mediated silver nanoparticles on three different human cancer cell lines. J. Cancer Res. Ther. 2018, 14, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Datkhile, K.D.; Durgawale, P.P.; Patil, M.N. Biogenic silver nanoparticles are equally Cytotoxic as Chemically Synthesized silver nanoparticles. Biomed. Pharmacol. J. 2017, 10, 337–344. [Google Scholar] [CrossRef]
- Lydia, E.; John, S.; Mohammed, R.; Sivapriya, T. Investigation on the Phytochemicals present in the Fruit peel of Carica papaya and evaluation of its Antioxidant and Antimicrobial property. Res. J. Pharmacogn. Phytochem. 2016, 8, 217. [Google Scholar] [CrossRef]
- De Caro, C.; Claudia, H. UV/VIS Spectrophotometry—Fundamentals and Applications; Mettler-Toledo Publication: Columbus, OH, USA, 2015. [Google Scholar]
- Khanal, L.N.; Sharma, K.R.; Paudyal, H.; Parajuli, K.; Dahal, B.; Ganga, G.C.; Pokharel, Y.R.; Kalauni, S.K. Green Synthesis of Silver Nanoparticles from Root Extracts of Rubus ellipticus Sm. and Comparison of Antioxidant and Antibacterial Activity. J. Nanomater. 2022, 2022, 1832587. [Google Scholar] [CrossRef]
- Velusamy, P.; Das, J.; Pachaiappan, R.; Vaseeharan, B.; Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind. Crops Prod. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R. Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Pungle, R.; Nile, S.H.; Kharat, A.S. Green synthesis and characterization of Solanum xanthocarpum capped silver nanoparticles and its antimicrobial effect on multidrug-resistant bacterial (MDR) isolates. Chem. Biol. Drug Des. 2023, 101, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J.; Massey, F.J., Jr. Introduction to Statistical Analysis; McGraw-Hill: New York, NY, USA, 1951. [Google Scholar]

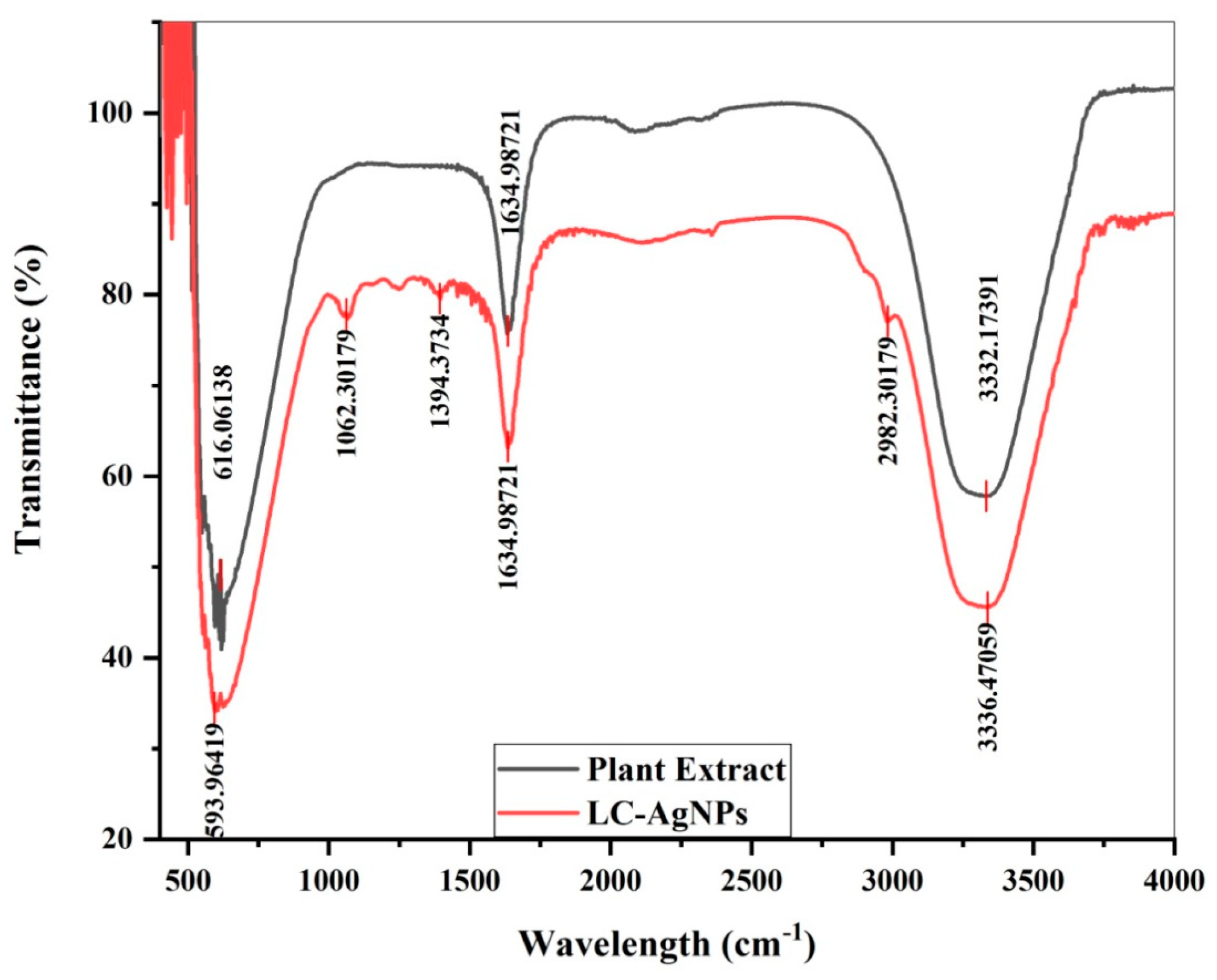



| Sample | Wavelength (cm−1) | Bond Stretching | Functional Groups |
|---|---|---|---|
| Plant extract | 616.06 | C–Cl stretching | Alkyl group |
| 1634.98 | C=O stretching | Ketones | |
| 3332.17 | OH stretching | Phenolic compound | |
| LC-AgNPs | 593.96 | C–C–C–N bonding | Nitrites |
| 1062.30 | C–H stretching | Cellulose | |
| 1394.37 | –C–H– Bending | Polyphenols | |
| 1634.98 | C=O stretching | Ketones | |
| 2982.30 | C–H stretching | Alkanes | |
| 3336.47 | C=C stretching | Alkenes |
| Samples | Concentrations (µg/mL) | Wavelength | |
|---|---|---|---|
| 515 nm | |||
| Scavenging Activity (%) | IC50 Value (µg/mL) | ||
| Plant extract | 50 | 16.70 ± 1.13 | 209.44 ± 0.24 |
| 100 | 25.08 ± 1.30 | ||
| 150 | 33.72 ± 1.22 | ||
| 200 | 45.94 ± 1.17 | ||
| 250 | 55.95 ± 1.51 | ||
| 300 | 68.00 ± 1.39 | ||
| LC-AgNPs | 50 | 41.62 ± 1.30 | 85.01 ± 0.17 |
| 100 | 50.25 ± 1.26 | ||
| 150 | 58.11 ± 1.04 | ||
| 200 | 69.94 ± 1.38 | ||
| 250 | 76.12 ± 1.22 | ||
| 300 | 84.24 ± 1.43 | ||
| Ascorbic acid (standard) | 50 | 55.61 ± 1.35 | 47.63 ± 0.21 |
| 100 | 64.37 ± 1.26 | ||
| 150 | 73.83 ± 1.12 | ||
| 200 | 81.26 ± 1.60 | ||
| 250 | 89.50 ± 1.38 | ||
| 300 | 91.84 ± 1.47 | ||
| Bacterial Strains | Various Conc. (mM) | |||||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | ||||
| AgNO3 | LC-AgNPs | AgNO3 | LC-AgNPs | AgNO3 | LC-AgNPs | |
| E. coli | 9 ± 0.3 | 11 ± 0.5 | 8 ± 0.3 | 13 ± 0.4 | 10 ± 0.5 | 10 ± 0.4 |
| S. aureus | 12 ± 0.4 | 14 ± 0.4 | 14 ± 0.6 | 15 ± 0.3 | 14 ± 0.3 | 14 ± 0.3 |
| B. subtilis | 9 ± 0.4 | 8 ± 0.6 | 10 ± 0.4 | 10 ± 0.3 | 7 ± 0.3 | 9 ± 0.5 |
| P. aeruginosa | 9 ± 0.4 | 15 ± 0.7 | 9 ± 0.9 | 17 ± 0.7 | 8 ± 0.5 | 11 ± 0.5 |
| Exposure Time (min) | Various Samples vs. Dilutions (%) | Estimated No. of Cells/Cycle for Variable Cell Lines (%) | ||
|---|---|---|---|---|
| Plant Extract | Free AgNPs | LC-AgNPs | ||
| 30 | Control | 88 | 88 | 88 |
| 30 | 10 | 52 | 63 | 46 |
| 15 | 45 | 56 | 38 | |
| 60 | 5 | 60 | 71 | 57 |
| 10 | 64 | 76 | 61 | |
| 90 | 5 | 76 | 82 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, A.; Ali, S.; Aftab, M.N.; Shami, A.; Al-Saeed, F.A.; Mustafa, B.; Paray, B.A. The Characterization and Study of Antibacterial, Free Radical Scavenging, and Anticancer Potential of Livistona chinensis-Mediated Silver Nanoparticles. Molecules 2023, 28, 7773. https://doi.org/10.3390/molecules28237773
Saleem A, Ali S, Aftab MN, Shami A, Al-Saeed FA, Mustafa B, Paray BA. The Characterization and Study of Antibacterial, Free Radical Scavenging, and Anticancer Potential of Livistona chinensis-Mediated Silver Nanoparticles. Molecules. 2023; 28(23):7773. https://doi.org/10.3390/molecules28237773
Chicago/Turabian StyleSaleem, Aroona, Sikander Ali, Muhammad Nauman Aftab, Ashwag Shami, Fatimah A. Al-Saeed, Bilal Mustafa, and Bilal Ahamad Paray. 2023. "The Characterization and Study of Antibacterial, Free Radical Scavenging, and Anticancer Potential of Livistona chinensis-Mediated Silver Nanoparticles" Molecules 28, no. 23: 7773. https://doi.org/10.3390/molecules28237773
APA StyleSaleem, A., Ali, S., Aftab, M. N., Shami, A., Al-Saeed, F. A., Mustafa, B., & Paray, B. A. (2023). The Characterization and Study of Antibacterial, Free Radical Scavenging, and Anticancer Potential of Livistona chinensis-Mediated Silver Nanoparticles. Molecules, 28(23), 7773. https://doi.org/10.3390/molecules28237773






