Mutual Placement of Isocyanide and Phosphine Ligands in Platinum(II) Complexes [PtHal2L1L2] (Hal = Cl, Br, I; L1,L2 = CNCy, PPh3) Leads to Highly-Efficient Photocatalysts for Hydrosilylation of Alkynes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesys and Charactrization
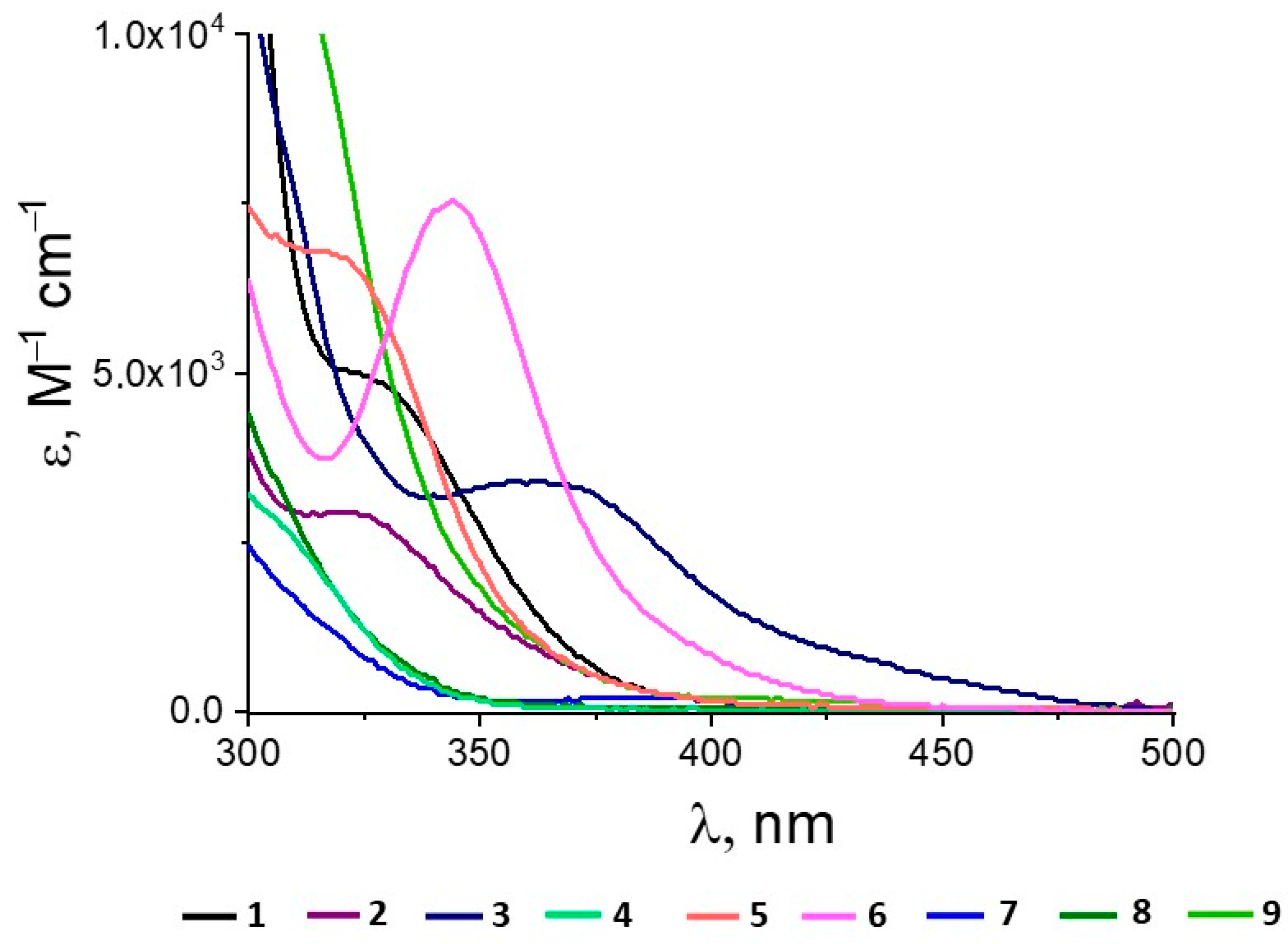
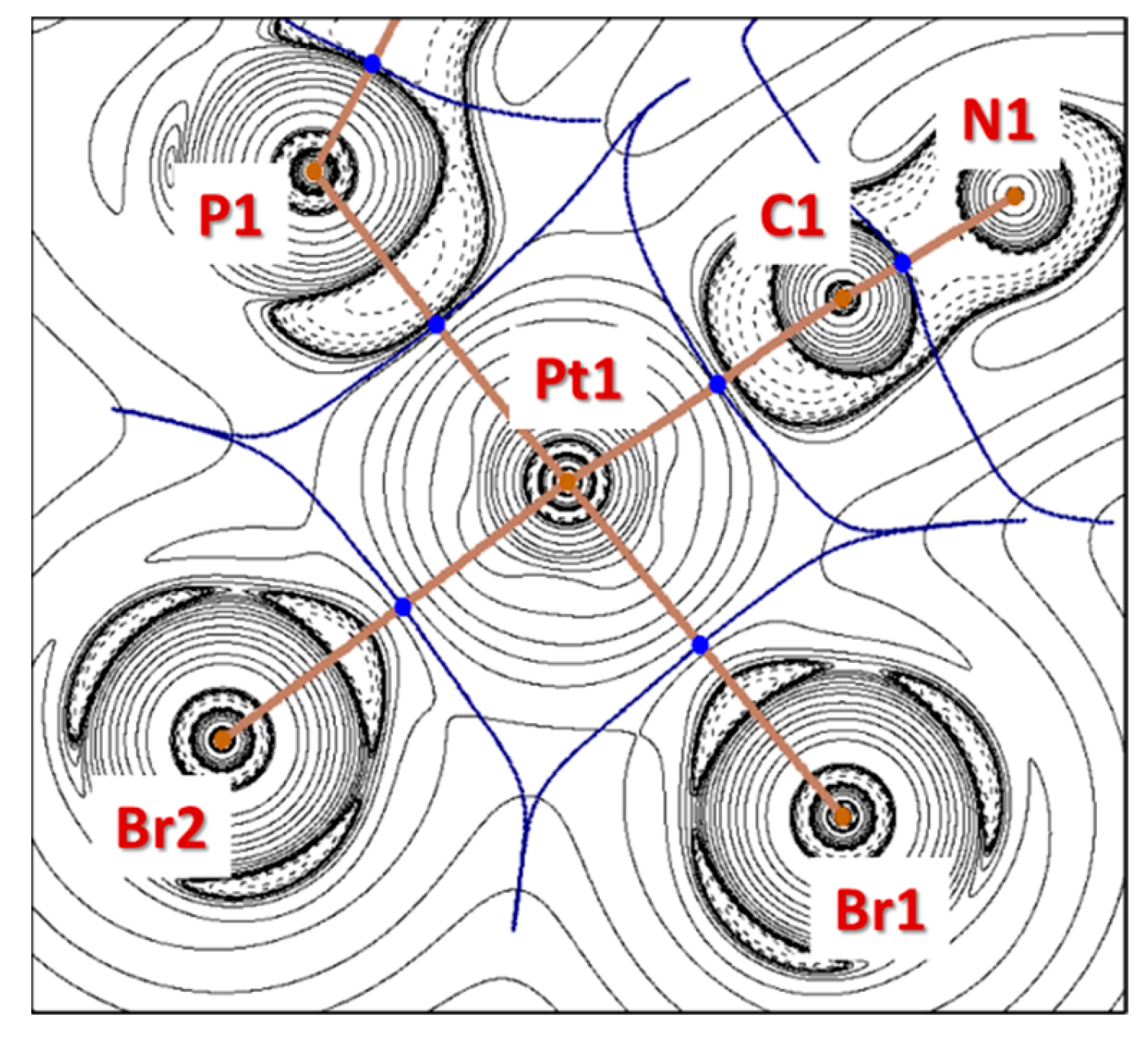
2.2. DFT Analysis of Ligand Properties
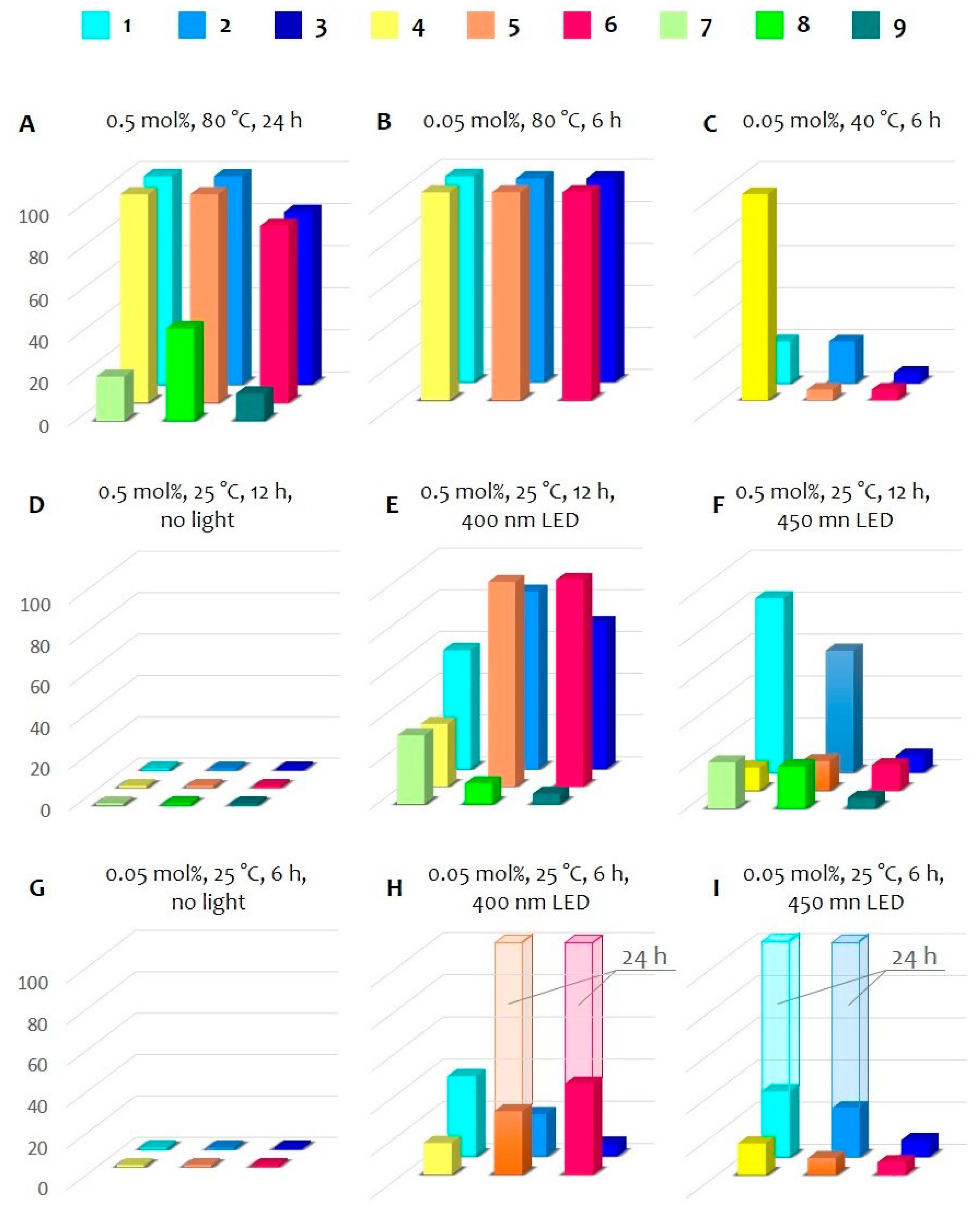
2.3. Catalytic Study
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Synthesis and Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kettler, P.B. Platinum Group Metals in Catalysis: Fabrication of Catalysts and Catalyst Precursors. Org. Process. Res. Dev. 2003, 7, 342–354. [Google Scholar] [CrossRef]
- Kamer, P.C.J.; van Leeuwen, P.W.N.M. Phosphorus(III)Ligands in Homogeneous Catalysis: Design and Synthesis; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Lühr, S.J.; Holz, A.B. The Synthesis of Chiral Phosphorus Ligands for use in Homogeneous Metal Catalysis. ChemCatChem 2011, 3, 1708–1730. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-Mediated and Metal-Catalyzed Reactions of Isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Hu, J.; Shao, G.; Yang, Y.; Wu, Z.Z. Hydration of alkynes at room temperature catalyzed by gold(i) isocyanide compounds. Green Chem. 2015, 17, 532–537. [Google Scholar] [CrossRef]
- Knorn, M.; Rawner, T.; Czerwieniec, R.; Reiser, O. [Copper(phenanthroline)(bisisonitrile)]+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catal. 2015, 5, 5186–5193. [Google Scholar] [CrossRef]
- Liu, M.; Reiser, O. A Copper(I) Isonitrile Complex as a Heterogeneous Catalyst for Azide−Alkyne Cycloaddition in Water. Org. Lett. 2011, 13, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Meina, L.; Zabel, M.; Reiser, O. Efficient Aerobic Wacker Oxidation of Styrenes Using Palladium Bis(isonitrile) Catalysts. Chem. Eur. J. 2010, 16, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Islamova, R.M.; Dobrynin, M.V.; Vlasov, A.V.; Eremina, A.A.; Kinzhalov, M.A.; Kolesnikov, I.E.; Zolotarev, A.A.; Masloborodova, E.A.; Luzyanin, K.V. Iridium(III)-catalysed cross-linking of polysiloxanes leading to the thermally resistant luminescent silicone rubbers. Catal. Sci. Technol. 2017, 7, 5843–5846. [Google Scholar] [CrossRef]
- Gee, J.C.; Fuller, B.A.; Lockett, H.-M.; Sedghi, G.; Robertson, C.M.; Luzyanin, K.V. Visible light accelerated hydrosilylation of alkynes using platinum–[acyclic diaminocarbene] photocatalysts. Chem. Commun. 2018, 54, 9450–9453. [Google Scholar] [CrossRef]
- Vicenzi, D.; Sgarbossa, P.; Biffis, A.; Tubaro, C.; Basato, M.; Michelin, R.A.; Lanza, A.; Nestola, F.; Bogialli, S.; Pastore, P.; et al. Platinum(II) Complexes with Novel Diisocyanide Ligands: Catalysts in Alkyne Hydroarylation. Organometallics 2013, 32, 7153–7162. [Google Scholar] [CrossRef]
- Murakami, M.; Matsuda, T.; Itami, K.; Ashida, S.; Terayama, M. Stereoselective Synthesis of (Z)-1-Silyl-2-stannylethene by Palladium- Catalyzed Silastannation of Ethyne and Its Synthetic Transformations. Synthesis 2004, 2004, 1522–1526. [Google Scholar] [CrossRef]
- Murakami, M.; Amii, H.; Takizawa, N.; Ito, Y. Synthesis of acylsilanes via silastannation of alkynes by a palladium-isocyanide catalyst. Organometallics 1993, 12, 4223–4227. [Google Scholar] [CrossRef]
- Villemin, D.A.; Jullien, N. Bar, Isonitriles as efficient ligands in Suzuki–Miyaura reaction. Tetrahedron Lett. 2007, 48, 4191–4193. [Google Scholar] [CrossRef]
- Timofeeva, S.A.; Kinzhalov, M.A.; Valishina, E.A.; Luzyanin, K.V.; Boyarskiy, V.P.; Buslaeva, T.M.; Haukka, M.; Kukushkin, V.Y. Application of palladium complexes bearing acyclic amino(hydrazido)carbene ligands as catalysts for copper-free Sonogashira cross-coupling. J. Catal. 2015, 329, 449–456. [Google Scholar] [CrossRef]
- Büldt, L.A.; Guo, X.; Prescimone, A.; Wenger, A. Molybdenum(0) Isocyanide Analogue of Ru(2,2′-Bipyridine)32+: A Strong Reductant for Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 11247–11250. [Google Scholar] [CrossRef] [PubMed]
- Bigler, R.; Mezzetti, A. Isonitrile Iron(II) Complexes with Chiral N2P2 Macrocycles in the Enantioselective Transfer Hydrogenation of Ketones. Org. Lett. 2014, 16, 6460–6463. [Google Scholar] [CrossRef]
- Cadierno, V.P.; Crochet, J.; Díez, S.E.; García-Garrido, J.G. Efficient Transfer Hydrogenation of Ketones Catalyzed by the Bis(isocyanide)−Ruthenium(II) Complexes trans,cis,cis-[RuX2(CNR)2(dppf)] (X = Cl, Br; dppf = 1,1′-Bis(diphenylphosphino)ferrocene): Isolation of Active Mono- and Dihydride Intermediates. Organometallics 2004, 23, 4836–4845. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Choudhary, S.; Doshi, A.; Liu, F.; Mohan, R.; Ravindra, M.P.; Shah, D.; Yang, X.; Fleming, F.F. Catalytic Isonitrile Insertions and Condensations Initiated by RNC–X Complexation. Adv. Synth. Catal. 2014, 356, 2135–2196. [Google Scholar] [CrossRef]
- Rodriguez, T.M.; Deegbey, M.; Chen, C.-H.; Jakubikova, E.; Dempsey, J.L. Isocyanide Ligands Promote Ligand-to-Metal Charge Transfer Excited States in a Rhenium(II) Complex. Inorg. Chem. 2023, 62, 6576–6585. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Luzyanin, K.V.; Kukushkin, V.Y. Acyclic diaminocarbenes (ADCs) as a promising alternative to N-heterocyclic carbenes (NHCs) in transition metal catalyzed organic transformations. Coord. Chem. Rev. 2012, 256, 2029–2056. [Google Scholar] [CrossRef]
- Slaughter, L.M. Acyclic Aminocarbenes in Catalysis. ACS Catal. 2012, 2, 1802–1816. [Google Scholar] [CrossRef]
- Moncada, A.I.; Manne, S.; Tanski, J.M.; Slaughter, L.M. Modular Chelated Palladium Diaminocarbene Complexes: Synthesis, Characterization, and Optimization of Catalytic Suzuki−Miyaura Cross-Coupling Activity by Ligand Modification. Organometallics 2006, 25, 491–505. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C NMR Spectroscopic Determination of Ligand Donor Strengths Using N-Heterocyclic Carbene Complexes of Palladium(II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Rigamonti, L.C.; Manassero, M.; Rusconi, M.; Manassero, A. Pasini, cis Influence in trans-Pt(PPh3)2 complexes. Dalton Trans. 2009, 7, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Manassero, M.; Manassero, C.A. Pasini, Cooperation between Cis and Trans Influences in cis-PtII(PPh3)2 Complexes: Structural, Spectroscopic, and Computational Studies. Inorg. Chem. 2010, 49, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Haukka, M.; Kukushkin, V.Y. Synthesis and Characterization of cis-(RNC)2PtII Species Useful as Synthons for Generation of Various (Aminocarbene)PtII Complexes. J. Chem. Crystallogr. 2012, 42, 1170–1175. [Google Scholar] [CrossRef]
- Iggo, J.A.K.V. Luzyanin, Multi-Nuclear Magnetic Resonance Spectroscopy. In Modern NMR Techniques for Synthetic Chemistry; Fisher, J., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 177–224. [Google Scholar]
- Flemming, J.P.; Pilon, M.C.; Borbulevitch, O.Y.; Antipin, M.Y.; Grushin, V.V. The trans influence of F, Cl, Br and I ligands in a series of square-planar Pd(II) complexes. Relative affinities of halide anions for the metal centre in trans-[(Ph3P)2Pd(Ph)X]. Inorganica Chim. Acta 1998, 280, 87–98. [Google Scholar] [CrossRef]
- Reinholdt, A.; Bendix, J. Weakening of Carbide–Platinum Bonds as a Probe for Ligand Donor Strengths. Inorg. Chem. 2017, 56, 12492–12497. [Google Scholar] [CrossRef]
- Dobrynin, M.V.; Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Starova, G.L.; Kukushkin, V.Y.; Islamova, R.M. Cyclometalated Platinum(II) Complexes Simultaneously Catalyze the Cross-Linking of Polysiloxanes and Function as Luminophores. ACS Appl. Polym. Mater. 2021, 3, 857–866. [Google Scholar] [CrossRef]
- Mostafa, S.H.; Khorassani, M.; Maghsoodlou, M.; Nassiri, M.; Zakarianezhad, M.; Fattahi, M. Synthesis of stable phosphorus ylides from 3, 5-dimethylpyrazole and kinetic investigation of the reactions by UV spectrophotometry. Arkivoc 2006, 2006, 168. [Google Scholar] [CrossRef]
- van Wyk, P.-H.; Gerber, W.J.; Koch, K.R. A robust method for speciation, separation and photometric characterization of all [PtCl6−nBrn]2− (n = 0–6) and [PtCl4−nBrn]2− (n = 0–4) complex anions by means of ion-pairing RP-HPLC coupled to ICP-MS/OES, validated by high resolution 195Pt NMR spectroscopy. Anal. Chim. Acta 2011, 704, 154–161. [Google Scholar] [CrossRef]
- Cox, L.E.; Peters, D.G.; Wehry, E.L. Photoaquation of hexachloroplatinate(IV). J. Inorg. Nucl. Chem. 1972, 34, 297–305. [Google Scholar] [CrossRef]
- Phadnis, P.P.; Jain, V.K.; Klein, A.; Weber, M.; Kaim, W. Configurational selectivity in benzyldimethylarsine complexes of palladium(II) and platinum(II): Synthesis, spectroscopy and structures. Inorganica Chim. Acta 2003, 346, 119–128. [Google Scholar] [CrossRef]
- Aktaş, A. A New Palladium Complex Containing the Mixture of Carbene and Phosphine Ligands: Synthesis,Crystal Structure and Spectral FT-IR,NMR and UV-Vis Researches. Chin. J. Struct. Chem. 2019, 38, 1664–1672. [Google Scholar]
- Dolatyari, V.; Shahsavari, H.R.; Habibzadeh, S.; Aghakhanpour, R.B.; Paziresh, S.; Haghighi, M.G.; Halvagar, M.R. Photophysical Properties and Kinetic Studies of 2-Vinylpyridine-Based Cycloplatinated(II) Complexes Containing Various Phosphine Ligands. Molecules 2021, 26, 2034. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Kryukova, M.A.; Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Smirnov, A.S.; Bokach, N.A.; Kukushkin, V.Y. Four-Center Nodes: Supramolecular Synthons Based on Cyclic Halogen Bonding. Chem. Eur. J. 2019, 25, 13671–13675. [Google Scholar] [CrossRef] [PubMed]
- Kashina, M.V.; Kinzhalov, M.A.; Smirnov, A.S.; Ivanov, D.M.; Novikov, A.S.; Kukushkin, V.Y. Dihalomethanes as Bent Bifunctional XB/XB-Donating Building Blocks for Construction of Metal-involving Halogen Bonded Hexagons. Chem. Asian J. 2019, 14, 3915–3920. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Luzyanin, K.V.; Boyarskaya, I.A.; Starova, G.L.; Boyarskiy, V.P. Synthetic and structural investigation of [PdBr2(CNR)2] (R = Cy, Xyl). J. Mol. Struct. 2014, 1068, 222–227. [Google Scholar] [CrossRef]
- Singleton, E.; Oosthuizen, H.E. Metal Isocyanide Complexes; Elsevier: Amsterdam, The Netherlands, 1983; pp. 209–310. [Google Scholar]
- Appleton, T.G.; Clark, H.C.; Manzer, L.E. The trans-influence: Its measurement and significance. Coord. Chem. Rev. 1973, 10, 335–422. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent⋅⋅⋅Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Farrugia, L.J.; Evans, C.; Tegel, M. Chemical Bonds without “Chemical Bonding”? A Combined Experimental and Theoretical Charge Density Study on an Iron Trimethylenemethane Complex. J. Phys. Chem. A 2006, 110, 7952–7961. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Bridgeman, A.J.; Cavigliasso, G.; Ireland, L.R.; Rothery, J. The Mayer bond order as a tool in inorganic chemistry. Dalton Trans. 2001, 14, 2095–2108. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Kashina, M.V.; Luzyanin, K.V.; Katlenok, E.A.; Novikov, A.S.; Kinzhalov, M.A. Experimental and computational tuning of metalla-N-heterocyclic carbenes at palladium(ii) and platinum(ii) centers. Dalton Trans. 2022, 51, 6718–6734. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Huynh, H.V. A unified ligand electronic parameter based on 13C NMR spectroscopy of N-heterocyclic carbene complexes. Dalton Trans. 2016, 46, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.D.; Olumba, M.E.; Nguyen, Y.H.; Teets, T.S. The diverse functions of isocyanides in phosphorescent metal complexes. Dalton Trans. 2021, 50, 17851–17863. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Patro, A.G.; Vadavi, R.S.; Nembenna, S. Coordination Chemistry of Main Group Metals with Organic Isocyanides. Eur. J. Inorg. Chem. 2022, 2022, e202200469. [Google Scholar] [CrossRef]
- Eseola, A.O.; Görls, H.; Orighomisan Woods, J.A.; Plass, W. Single monodentate N-donor ligands versus multi-ligand analogues in Pd(II)-catalysed C–C coupling at reduced temperatures. Polyhedron 2020, 182, 114507. [Google Scholar] [CrossRef]
- Hu, J.J.; Li, F.; Hor, T.S.A. Novel Pt(II) Mono- and Biscarbene Complexes: Synthesis, Structural Characterization and Application in Hydrosilylation Catalysis. Organometallics 2009, 28, 1212–1220. [Google Scholar] [CrossRef]
- Pertschi, R.; Hatey, D.; Pale, P.; de Frémont, P.; Blanc, A. Synthesis, Characterization, and Catalytic Activity of Chiral NHC Platinum(II) Pyridine Dihalide Complexes. Organometallics 2020, 39, 804–812. [Google Scholar] [CrossRef]
- Brissy, D.; Skander, M.; Retailleau, P.; Frison, G.; Marinetti, A. Platinum(II) Complexes Featuring Chiral Diphosphines and N-Heterocyclic Carbene Ligands: Synthesis and Evaluation as Cycloisomerization Catalysts. Organometallics 2009, 28, 140–151. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Strieth-Kalthoff, F.; James, M.J.; Teders, M.; Pitzer, L.; Glorius, F. Energy transfer catalysis mediated by visible light: Principles, applications, directions. Chem. Soc. Rev. 2018, 47, 7190–7202. [Google Scholar] [CrossRef] [PubMed]
- Yakushev, A.A.; Abel, A.S.; Averin, A.D.; Beletskaya, I.P.; Cheprakov, A.V.; Ziankou, I.S.; Bonneviot, L.; Bessmertnykh-Lemeune, A. Visible-light photocatalysis promoted by solid- and liquid-phase immobilized transition metal complexes in organic synthesis. Coord. Chem. Rev. 2022, 458, 214331. [Google Scholar] [CrossRef]
- Parasram, M.; Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: Beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef]
- Chuentragool, P.; Kurandina, D.; Gevorgyan, V. Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem. Int. Ed. 2019, 58, 11586–11598. [Google Scholar] [CrossRef]
- Steinke, S.J.; Gupta, S.; Piechota, E.J.; Moore, C.E.; Kodanko, J.J.; Turro, C. Photocytotoxicity and photoinduced phosphine ligand exchange in a Ru(ii) polypyridyl complex. Chem. Sci. 2022, 13, 1933–1945. [Google Scholar] [CrossRef]
- Katkova, S.A.; Kinzhalov, M.A.; Tolstoy, P.M.; Novikov, A.S.; Boyarskiy, V.P.; Ananyan, A.Y.; Gushchin, P.V.; Haukka, M.; Zolotarev, A.A.; Ivanov, A.Y.; et al. Diversity of Isomerization Patterns and Protolytic Forms in Aminocarbene PdII and PtII Complexes Formed upon Addition of N,N′-Diphenylguanidine to Metal-Activated Isocyanides. Organometallics 2017, 36, 4145–4159. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS-2008/1-Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Baldes, A. Segmented contracted basis sets for one- and two-component Dirac–Fock effective core potentials. J. Chem. Phys. 2010, 133, 174102. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Vreven, Gaussian 09, Revision B.01.; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Jens, A.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Mayer, I.P.S. Overlap populations, bond orders and valences for ‘fuzzy’ atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Sueki, S.; Kuninobu, Y. Rhodium-catalysed synthesis of multi-substituted silylindenes from aryl alkynes and hydrosilanes via C–H bond activation. Chem. Commun. 2015, 51, 7685–7688. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Hounjet, L.J.; Caputo, C.B.; Dobrovetsky, R.; Stephan, D.W. Olefin Isomerization and Hydrosilylation Catalysis by Lewis Acidic Organofluorophosphonium Salts. J. Am. Chem. Soc. 2013, 135, 18308–18310. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Katkova, S.A.; Suslonov, V.V. Synthesis of Platinum(II) Phoshine Isocyanide Complexes and Study of Their Stability in Isomerization and Ligand Disproportionation Reactions. Russ. J. Gen. Chem. 2018, 88, 1180–1187. [Google Scholar] [CrossRef]
- Manojlovicmuir, L.; Muir, K.W.; Solomun, T. Cis-influence and trans-influence of ligands in platinum(ii) complexes—crystal and molecular-structure of cis-PtCl2(PET3)(CO). J. Organomet. Chem. 1977, 142, 265–280. [Google Scholar] [CrossRef]
- Briggs, J.R.; Crocker, C.; Shaw, B.L. Complexes of the type trans-PtCl2(PR3)(CNR’) and the carbene complex trans-PtCl2(PR3) C(NHC6H4ME-4)2. Inorganica Chim. Acta-Artic. 1981, 51, 15–18. [Google Scholar] [CrossRef]
- Zhang, B.; Kurpiewska, K.; Dömling, A. Highly Stereoselective Ugi/Pictet–Spengler Sequence. J. Org. Chem. 2022, 87, 7085–7096. [Google Scholar] [CrossRef]
- Katkova, S.A.; Luzyanin, K.V.; Novikov, A.S.; Kinzhalov, M.A. Modulation of luminescence properties for [cyclometalated]-PtII(isocyanide) complexes upon co-crystallisation with halosubstituted perfluorinated arenes. New J. Chem. 2021, 45, 2948–2952. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Mayr, A.; Cheung, K.-K. Synthesis and formation of metal complexes of 4-alkynyl and 4-cyano-2,6-diisopropylphenylisocyanides. Inorganica Chim. Acta 1999, 284, 205–214. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Cheranyova, A.M.; Ivanov, D.M.; Kukushkin, V.Y.; Bokach, N.A. Polymorph-Dependent Phosphorescence of Cyclometalated Platinum(II) Complexes and Its Relation to Non-covalent Interactions. ACS Omega 2022, 7, 34454–34462. [Google Scholar] [CrossRef]
- Stephany, R.W.; de Bie, M.J.A.; Drenth, W. A 13C-NMR and IR study of isocyanides and some of their complexes. Org. Magn. Reson. 1974, 6, 45–47. [Google Scholar] [CrossRef]
- Knorr, M.; Jourdain, I.; Crini, G.; Frank, K.; Sachdev, H.; Strohmann, C. Synthesis, Reactivity and Molecular Structures of Bis(diphenylphosphanyl)methane-Bridged Heterobimetallic Iron−Platinum Isocyanide Complexes: Breaking and Formation of Metal−Metal Bonds. Eur. J. Inorg. Chem. 2002, 2002, 2419–2426. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, E.-H.; Lee, S.W. Reactivity of Bis(silyl) Platinum(II) Complexes toward Isocyanides: Preparation and Structure of cis-[Pt(SiHPh2)2(CNR)(PR‘3)] (R = t-Bu, cyclohexyl, i-Pr; R‘ = Me, Et). Organometallics 2003, 22, 3316–3319. [Google Scholar] [CrossRef]
- Jameson, C.J. Multinuclear NMR; Mason, J., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 89–131. [Google Scholar]
- Pidcock, A.; Richards, R.E.; Venanzi, L.M. 195Pt-31P nuclear spin coupling constants and the nature of the trans-effect in platinum complexes. J. Chem. Soc. A 1966, 1707–1710. [Google Scholar] [CrossRef]
- Sluch, I.M.; Miranda, A.J.; Slaughter, L.M. Channeled Polymorphs of cis-M(CNPh)2Cl2 (M = Pt, Pd) with Extended Metallophilic Interactions. Cryst. Growth Des. 2009, 9, 1267–1270. [Google Scholar] [CrossRef]
- Kashina, M.V.; Ivanov, D.M.; Kinzhalov, M.A. The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals 2021, 11, 799. [Google Scholar] [CrossRef]
- Harvey, P.D.; Truong, K.D.; Aye, K.T.; Drouin, M.; Bandrauk, A.D. Resonance-Enhanced Intraligand and Metal-Metal Raman Modes in Weakly Metal-Metal-Interacting Platinum(II) Complexes and Long-Range Relationship between Metal-Metal Separations and Force Constants. Inorg. Chem. 1994, 33, 2347–2354. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hagiwara, T.; Yamazaki, H. Axially dissymmetric 2,2′-diisocyano-1,1′-binaphthyl and its platinum complexes. Inorganica Chim. Acta 1986, 115, L35–L37. [Google Scholar] [CrossRef]
- Barnett, B.R.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Cooperative Transition Metal/Lewis Acid Bond-Activation Reactions by a Bidentate (Boryl)iminomethane Complex: A Significant Metal–Borane Interaction Promoted by a Small Bite-Angle LZ Chelate. J. Am. Chem. Soc. 2014, 136, 10262–10265. [Google Scholar] [CrossRef]
- Sluch, I.M.; Miranda, A.J.; Elbjeirami, O.; Omary, M.A.; Slaughter, L.M. Interplay of Metallophilic Interactions, π–π Stacking, and Ligand Substituent Effects in the Structures and Luminescence Properties of Neutral PtII and PdII Aryl Isocyanide Complexes. Inorg. Chem. 2012, 51, 10728–10746. [Google Scholar] [CrossRef]
- Hubbert, C.; Breunig, M.; Carroll, K.J.; Rominger, F.; Hashmi, A.S.K. Simple Synthesis of New Mixed Isocyanide-NHC-Platinum(II) Complexes and Their Catalytic Activity. Aust. J. Chem. 2014, 67, 469–474. [Google Scholar] [CrossRef]
- Bulatova, M.; Ivanov, D.M.; Rautiainen, J.M.; Kinzhalov, M.A.; Truong, K.-N.; Lahtinen, M.; Haukka, M. Studies of Nature of Uncommon Bifurcated I–I···(I–M) Metal-Involving Noncovalent Interaction in Palladium(II) and Platinum(II) Isocyanide Cocrystals. Inorg. Chem. 2021, 60, 13200–13211. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Wang, S.; Cheung, K.-K.; Hong, M. Synthesis, structure, and liquid crystal properties of a series of platinum(II) complexes containing chiral 4-(4-alkoxyphenylethynyl) phenylisocyanide ligands. J. Organomet. Chem. 2003, 684, 287–299. [Google Scholar] [CrossRef]
- Wang, S.; Mayr, A.; Cheung, K.-k. Mesogenic palladium and platinum-diiodide complexes of4-isocyanobenzylidene-4-alkoxyphenylimines. J. Mater. Chem. 1998, 8, 1561–1565. [Google Scholar] [CrossRef]
- Kaharu, T.T.; Tanaka, M.; Sawada, S.T. Liquid-crystalline palladium–and platinum–isonitrile complexes: Synthesis, mesomorphic properties and molecular structure. J. Mater. Chem. 1994, 4, 859–865. [Google Scholar] [CrossRef]


| [PtX2L1L2] | L1 = L2 = PPh3 | L1 = PPh3, L2 = CNCy | L1 = L2 = CNCy |
|---|---|---|---|
| X = Cl | cis-[PtCl2(PPh3)2] (1) | cis-[PtCl2(PPh3)(CNCy)] (4) | cis-[PtCl2(CNCy)2] (7) |
| X = Br | cis-[PtBr2(PPh3)2] (2) | cis-[PtBr2(PPh3)(CNCy)] (5) | cis-[PtBr2(CNCy)2] (8) |
| X = I | trans-[PtI2(PPh3)2] (3) | cis-[PtI2(PPh3)(CNCy)] (6) | trans-[PtI2(CNCy)2] (9) |
| Bond | MBO | WI | ρ | G(r) | V(r) | H(r) | ∇2ρ(r)) | |V(r)|/G(r) |
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| Pt1–P1 | 0.7300 | 1.066 | 0.116 | 0.071 | −0.127 | −0.056 | 0.059 | 1.79 |
| Pt1–P2 | 0.6888 | 1.026 | 0.111 | 0.066 | −0.118 | −0.052 | 0.061 | 1.79 |
| Pt1–Cl1 | 0.6800 | 1.093 | 0.087 | 0.071 | −0.100 | −0.029 | 0.173 | 1.41 |
| Pt1–Cl2 | 0.7279 | 1.185 | 0.092 | 0.075 | −0.106 | −0.032 | 0.176 | 1.41 |
| 2 | ||||||||
| Pt1–P1 | 0.7118 | 1.031 | 0.112 | 0.068 | −0.121 | −0.053 | 0.064 | 1.78 |
| Pt1–P2 | 0.6971 | 0.997 | 0.107 | 0.064 | −0.112 | −0.048 | 0.066 | 1.75 |
| Pt1–Br1 | 0.6591 | 1.147 | 0.078 | 0.052 | −0.076 | −0.023 | 0.118 | 1.46 |
| Pt1–Br2 | 0.6610 | 1.227 | 0.082 | 0.055 | −0.080 | −0.026 | 0.118 | 1.45 |
| 3 | ||||||||
| Pt1–P1 | 0.6871 | 0.998 | 0.108 | 0.065 | −0.114 | −0.048 | 0.069 | 1.75 |
| Pt1–P2 | 0.6547 | 0.962 | 0.102 | 0.062 | −0.105 | −0.044 | 0.072 | 1.69 |
| Pt1–I1 | 0.6680 | 1.131 | 0.066 | 0.033 | −0.051 | −0.018 | 0.062 | 1.55 |
| Pt1–I2 | 0.6603 | 1.211 | 0.070 | 0.035 | −0.055 | −0.020 | 0.061 | 1.57 |
| 4 | ||||||||
| Pt1–P1 | 0.8866 | 1.028 | 0.116 | 0.068 | −0.124 | −0.056 | 0.049 | 1.82 |
| Pt1–C1 | 1.0264 | 1.428 | 0.171 | 0.185 | −0.289 | −0.105 | 0.335 | 1.56 |
| Pt1–Cl1 | 0.6986 | 1.151 | 0.089 | 0.071 | −0.101 | −0.030 | 0.169 | 1.42 |
| Pt1–Cl2 | 0.6711 | 1.146 | 0.091 | 0.076 | −0.107 | −0.031 | 0.181 | 1.41 |
| 5 | ||||||||
| Pt1–P1 | 0.8682 | 1.004 | 0.113 | 0.066 | −0.120 | −0.053 | 0.053 | 1.81 |
| Pt1–C1 | 0.9955 | 1.394 | 0.168 | 0.179 | −0.279 | −0.100 | 0.327 | 1.56 |
| Pt1–Br1 | 0.6763 | 1.187 | 0.080 | 0.052 | −0.077 | −0.024 | 0.114 | 1.48 |
| Pt1–Br2 | 0.6832 | 1.202 | 0.081 | 0.056 | −0.082 | −0.026 | 0.121 | 1.46 |
| 6 | ||||||||
| Pt1–P1 | 0.8141 | 0.983 | 0.110 | 0.064 | −0.115 | −0.051 | 0.057 | 1.80 |
| Pt1–C1 | 0.9355 | 1.347 | 0.161 | 0.171 | −0.264 | −0.093 | 0.322 | 1.54 |
| Pt1–I1 | 0.6811 | 1.192 | 0.069 | 0.035 | −0.056 | −0.020 | 0.061 | 1.60 |
| Pt1–I2 | 0.6686 | 1.166 | 0.067 | 0.034 | −0.053 | −0.019 | 0.061 | 1.56 |
| 7 | ||||||||
| Pt1–C1 | 0.9703 | 1.343 | 0.164 | 0.172 | −0.269 | −0.097 | 0.314 | 1.56 |
| Pt1–C2 | 0.9572 | 1.335 | 0.162 | 0.171 | −0.266 | −0.095 | 0.318 | 1.56 |
| Pt1–Cl1 | 0.6820 | 1.163 | 0.091 | 0.074 | −0.106 | −0.032 | 0.171 | 1.43 |
| Pt1–Cl2 | 0.7101 | 1.180 | 0.093 | 0.075 | −0.108 | −0.033 | 0.171 | 1.44 |
| 8 | ||||||||
| Pt1–C1 | 0.9411 | 1.310 | 0.160 | 0.167 | −0.259 | −0.092 | 0.314 | 1.55 |
| Pt1–C2 | 0.9369 | 1.310 | 0.159 | 0.167 | −0.259 | −0.091 | 0.316 | 1.55 |
| Pt1–Br1 | 0.6984 | 1.220 | 0.083 | 0.055 | −0.082 | −0.027 | 0.113 | 1.49 |
| Pt1–Br2 | 0.7097 | 1.220 | 0.083 | 0.055 | −0.081 | −0.027 | 0.113 | 1.47 |
| 9 | ||||||||
| Pt1–C1 | 0.9001 | 1.282 | 0.155 | 0.163 | −0.250 | −0.087 | 0.315 | 1.53 |
| Pt1–C2 | 0.9063 | 1.285 | 0.156 | 0.164 | −0.251 | −0.087 | 0.316 | 1.53 |
| Pt1–I1 | 0.7198 | 1.215 | 0.071 | 0.035 | −0.056 | −0.021 | 0.054 | 1.60 |
| Pt1–I2 | 0.7150 | 1.202 | 0.070 | 0.035 | −0.056 | −0.021 | 0.055 | 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashina, M.V.; Karcheuski, A.A.; Kinzhalov, M.A.; Luzyanin, K.V.; Katkova, S.A. Mutual Placement of Isocyanide and Phosphine Ligands in Platinum(II) Complexes [PtHal2L1L2] (Hal = Cl, Br, I; L1,L2 = CNCy, PPh3) Leads to Highly-Efficient Photocatalysts for Hydrosilylation of Alkynes. Molecules 2023, 28, 7764. https://doi.org/10.3390/molecules28237764
Kashina MV, Karcheuski AA, Kinzhalov MA, Luzyanin KV, Katkova SA. Mutual Placement of Isocyanide and Phosphine Ligands in Platinum(II) Complexes [PtHal2L1L2] (Hal = Cl, Br, I; L1,L2 = CNCy, PPh3) Leads to Highly-Efficient Photocatalysts for Hydrosilylation of Alkynes. Molecules. 2023; 28(23):7764. https://doi.org/10.3390/molecules28237764
Chicago/Turabian StyleKashina, Maria V., Andrei A. Karcheuski, Mikhail A. Kinzhalov, Konstantin V. Luzyanin, and Svetlana A. Katkova. 2023. "Mutual Placement of Isocyanide and Phosphine Ligands in Platinum(II) Complexes [PtHal2L1L2] (Hal = Cl, Br, I; L1,L2 = CNCy, PPh3) Leads to Highly-Efficient Photocatalysts for Hydrosilylation of Alkynes" Molecules 28, no. 23: 7764. https://doi.org/10.3390/molecules28237764
APA StyleKashina, M. V., Karcheuski, A. A., Kinzhalov, M. A., Luzyanin, K. V., & Katkova, S. A. (2023). Mutual Placement of Isocyanide and Phosphine Ligands in Platinum(II) Complexes [PtHal2L1L2] (Hal = Cl, Br, I; L1,L2 = CNCy, PPh3) Leads to Highly-Efficient Photocatalysts for Hydrosilylation of Alkynes. Molecules, 28(23), 7764. https://doi.org/10.3390/molecules28237764