Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications
Abstract
1. Introduction
2. Natural Triterpenoids
3. Antimelanoma Activity of Natural Triterpenoids—Evidence from In Vitro Studies
| Compound | Cell Line | Results | References |
|---|---|---|---|
| Betulin (BT) | SK-MEL-28H | IC50 16.2 μM (48 h) | [1] |
| SK-MEL-2H | IC50 > 45.2 μM (48 h) | ||
| IC50 1.1–4.6 μg/mL (72 h) | [37,38] | ||
| IC50 > 250 μM (48 h) | [1] | ||
| IC50 4.1 μM | [9] | ||
| Me-45H | IC50 24.2–30.5 μM (24–72 h) | [3] | |
| G361H | IC50 12.4 μM (48 h) | [1] | |
| Hs294TH | IC50 44.1 μM | [39] | |
| A-431H | Inhibition of proliferation: ≈ 68% of control (C = no data) | [40] | |
| IC50 6.8 μM (72 h) | [41] | ||
| Inhibition of proliferation: 52–62% of control (C = no data) | [42] | ||
| B164A5A | IC50 4.27 μM (72 h) | [43] | |
| B16OvaA | IC50 3.89 μM (72 h) | ||
| B16-2F2A | IC50 27.4 μM (48 h) | [1] | |
| B16-F10A | IC50 13.8 μM (48 h) | ||
| IC50 14.38 μM (48 h) | |||
| C-32H | IC50 15.61 μM (72 h) | [44] | |
| Betulinic acid (BA) | MEL-1H | ED50 3.3 μg/mL (72 h) | [45] |
| MEL-2H | IC50 1.3 μM | [46] | |
| ED50 1 μg/mL (72 h) | [45] | ||
| ED50 1.2 μg/mL (72 h) | [47] | ||
| G361H | IC50 5.2 μM (48 h) | [1] | |
| SK-MEL-28H | IC50 6.5 μM (48 h) | ||
| IC50 2.21 μM (72 h) | [34] | ||
| Cell viability ~50% (C = 10 μM) (24 h) | [35] | ||
| RPMI-7951H | Cell viability: ~88% (C = 10 μM) (24 h) | ||
| 518A2H | IC50 8.13 μM (96 h) | [48] | |
| Me-45H | IC50 22.7–15.3 μM (24–72 h) | [3] | |
| MEL-1,-3,-4H | IC50 1.1–4.6 μg/mL (72 h) | [37] | |
| MEL-1,-2,-4H | IC50 0.5–4.8 μg/mL (72 h) | ||
| B16-2F2A | IC50 7.9 μM (48 h) | [1] | |
| B16A | IC50 76 μg/mL (48 h) | [49] | |
| B16-F1A | IC50 16.1 μM | [46] | |
| B16-F10A | IC50 16.41 μM (48h | [1] | |
| IC50 70 μM | [50] | ||
| B164A5 metastaticA | Cell viability: 57.89% (C = no data) | [51] | |
| B164A5 non-metastaticA | Cell viability: 61.82% | ||
| WM-266-4H | Inhibition of proliferation: < 20% of control (C > 2 μM) | [52] | |
| A375H | IC50 16.91 µM (24 h) | [53] | |
| IC50 154 μM | [4] | ||
| IC50 15.94 μM (72 h) | [34] | ||
| Cell viability: 75% (C = 50 µM) (24 h) | [54] | ||
| Betulinic acid (BA) | eRGO1A | IC50 22.8 µM/L (24 h) | [55] |
| IC50 20.7 µM/L (48 h) | |||
| IC50 12.7 µM/L (96 h) | |||
| MelDuWiA | IC50 34.6 µM/L (24 h) | ||
| IC50 31.7 µM/L (48 h) | |||
| IC50 23.6 µM/L (96 h) | |||
| FM55PH | IC50 5.62 μM (72 h) | [34] | |
| FM55M2H | IC50 4.08 μM (72 h) | ||
| Lupeol (LU) | WM35H | IC50 32 µM/L (72 h) | [56] |
| 451LuH | IC50 38 µM/L (72 h) | ||
| G361H | Cell growth inhibition: 97.5% (C = 10 µM) (72 h) | [57] | |
| IC50 50 µM (72 h) | [58] | ||
| Mel 928H | IC50 75 µM (48 h) | [59] | |
| Mel 1241H | IC50 72 µM (48 h) | ||
| Mel 1011H | IC50 135 µM (48 h) | ||
| B16F10A | IC50 58.39 µg/mL (24 h) | [32] | |
| A375H | IC50 66.59 µM (24 h) | [60] | |
| RPMI-7951H | IC50 45.54 µM (24 h) | ||
| Oleanolic acid (OA) | A2058H | IC50 60 µM (48 h) | [61] |
| A375H | IC50 75 µM (48 h) | ||
| Cell viability: 74.8% (C = 50 µM) (24 h) | [54] | ||
| WM-266-4H | Inhibition of proliferation: 21% of control (C = 20 μM) (24 h) | [52] | |
| A375SMH | Cell viability: 79.6% (C = 80 µM) | [62] | |
| Cell viability: 41.5% (C = 100 µM) | |||
| A375PH | Cell viability: 82.4% (C = 60 µM) | ||
| Cell viability: 46.4% (C = 100 µM) | |||
| Ursolic acid (UA) | A375H | IC50 75 μM | [30] |
| Cell viability: 85% (C = 50 µM) (24 h) | [54] | ||
| IC50 68.22 μM (48 h) | [63] | ||
| GI50 26 μM | [64] | ||
| IC50 6.95 µg/mL (24 h) | [65] | ||
| IC50 5.20 µg/mL (48 h) | |||
| IC50 32.36 μM (24 h) | [26] | ||
| IC50 19.45 μM (48 h) | |||
| SK-MEL 2H | IC50 58.43 μM (48 h) | [66] | |
| IC50 58.44 μM (48 h) | [63] | ||
| A2058H | IC50 60 μM (48 h) | [61] | |
| B164A5A | IC50 43.59 μM (48 h) | [63] | |
| B16F10A | IC50 31.65 μM (24 h) | [67] | |
| Mel-RMH | IC50 26.25 μM (48 h) | [30] | |
| IC50 40.48 μM (24 h) | |||
| Me4405H | IC50 28.67 μM (24 h) | ||
| IC50 18.15 μM (48 h) | |||
| Ursolic acid (UA) | MM200H | IC50 33.09 μM (48 h) | [30] |
| IC50 61.6 μM (24 h) | |||
| HFFF2H | IC50 46.71 μM (48 h) | ||
| IC50 69.77 μM (24 h) | |||
| HTB-140H | IC50 5.69 µg/mL (24 h) | [65] | |
| IC50 4.13 µg/mL (48 h) | |||
| WM793H | IC50 5.89 µg/mL (24 h) | ||
| IC50 4.08 µg/mL (48 h) | |||
| WM-266-4H | Inhibition of proliferation: 10% of control (C = 10 μM) (24 h) | [52] | |
| Ursolic acid + oleanolic acid (ratio 1:1 and 3.5:1) | WM-266-4H | Inhibition of proliferation: 20% (4 h), 7% (24 h), 6% (48 h) of control (C = 10 μM) | [52] |
| Ursolic acid + oleanolic acid (ratio 1:1) | A375H | IC50 60 µM (48 h) | [61] |
| A2058H |
| Compound | Cell Line | Results | References | |
|---|---|---|---|---|
| 22β-hydroxytingenone | SK-MEL-28H | IC50 4.32 μM (24 h) | [71] | |
| IC50 3.72 μM (48 h) | ||||
| IC50 3.29 μM (72 h) | ||||
| IC50 3.2 μM (72 h) | [72] | |||
| Celastrol (CEL) | B16-F10A | IC50 3.56 μM (48 h) | [69] | |
| Cucurbitacin (Cuc) | A-375.S2H | IC50 15.57 μM (24 h) | [73] | |
| B-16A | IC50 65.31 μM (48 h) | |||
| Cucurbitacin B (Cuc B) | SK-MEL-28H | IC50 0.36 μM (24 h) | [26] | |
| IC50 0.52 μM (24 h) | ||||
| A375H | IC50 1.54 μM (24 h) | |||
| IC50 0.015 μg/mL | [33] | |||
| IC50 1.59 μM (24 h) | [22] | |||
| B16-F10A | IC50 0.32 μM (24/48/72 h) | [74] | ||
| Cucurbitacin D (Cuc D) | SK-MEL-28H | IC50 0.40 μM (24 h) | [26] | |
| A375H | IC50 0.32 μM (24 h) | |||
| Cucurbitacin E (Cuc E) | SK-MEL-28H | IC50 0.70 μM (24 h) | [26] | |
| A375H | IC50 0.54 μM (24 h) | |||
| Benzyl (2α,3β) 2,3-diacetoxy-olean-12-en-28-amide (EM2) | 518A2H | IC50 1.5 μM (72 h) | [31] | |
| NiH 3T3A | IC50 33.8 μM (72 h) | |||
| Erigeronol | B16A | IC50 7.77 μg/mL | [75] | |
| Geoditin A | B16A | IC50 ≈ 10.0 μg/mL (48 h) | [70] | |
| Ilexgenin A | B16-F10A | IC50 27.34 μM (24 h) | [36] | |
| IC50 12.44 μM (48 h) | ||||
| Maslinic acid (MA) | SK-MEL-2H | IC50 14.7 µM | [9] | |
| 518A2H | IC50 13.7 μM (72 h) | [31] | ||
| NiH 3T3A | IC50 38.8 μM (72 h) | |||
| B16-F10A | FBS | NO FBS | [76] | |
| IC50 86.22 μM (24 h) | IC50 3.46 μM (24 h) | |||
| IC50 42.0 μM (24 h) | [77] | |||
| IC50 38.07 μg/mL (72 h) | [78] | |||
| Tyramine-MA conjugate | B16-F10A | IC50 8.06 μg/mL (72 h) | [78] | |
| Xanthoceraside | A-375.S2H | IC50 5.71 μM (24 h) | [79] | |
| Taraxasterol | SK-MEL-28H | Cell viability: ~70% (C = 20 μg/mL) (24 h) | [68] | |
| Cell viability: ~60% (C = 20 μg/mL) (48 h) | ||||
| Cell viability: ~40% (C = 20 μg/mL) (72 h) | ||||
| A375H | Cell viability: ~75% (C = 20 μg/mL) (24 h) | |||
| Cell viability: ~55% (C = 20 μg/mL) (48 h) | ||||
| Cell viability: ~45% (C = 20 μg/mL) (72 h) | ||||
4. Mechanisms of Cytotoxic Activity of Natural Triterpenoids
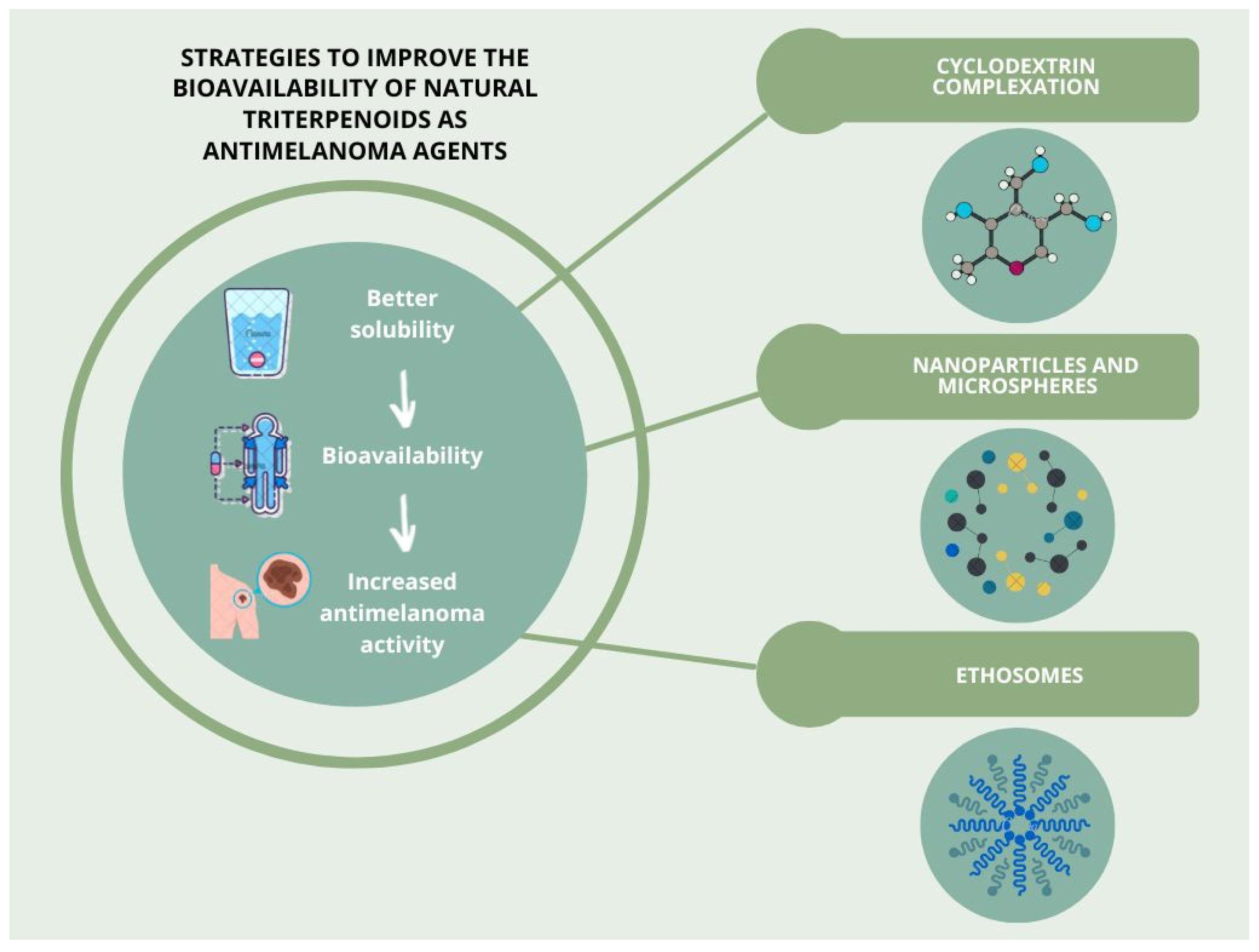
5. Strategies to Improve the Bioavailability of Selected Natural Triterpenoids as Antimelanoma Agents
5.1. Cyclodextrin Complexation
5.1.1. In Vitro Experiments
5.1.2. In Vivo Experiments
5.2. Nanoparticles and Microspheres
5.2.1. In Vitro Experiments
5.2.2. In Vivo Experiments
5.3. Ethosomes
6. Chemical Modifications of Selected Natural Triterpenoids to Improve their Effectiveness as Antimelanoma Agents
6.1. Betulin and Betulinic Acid
6.2. Lupeol
6.3. Oleanolic Acid
6.4. Maslinic Acid
6.5. Celastrol
6.6. Ursolic Acid
7. In Vivo Trials
7.1. Lupeol
7.2. Ursolic Acid
7.3. Ilexgenins
8. Limitations of the Studies Included in the Review
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eade, S. Improved Anti-Melanoma and Anti-Melanogenic Effects of Birch Bark Triterpenes Delivered in Ethosomes. Ph.D. Thesis, Lakehead University, Thunder Bay, ON, Canada, 2018. [Google Scholar]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved Anti-Melanoma Effect of a Transdermal Mitoxantrone Ethosome Gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Drąg, M.; Poręba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer Properties of Ester Derivatives of Betulin in Human Metastatic Melanoma Cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.; Antal, D.; Andrica, F.; Băbuţa, R.; Moacă, A.; Ardelean, F.; Ghiulai, R.; Avram, S.; Danciu, C.; Coricovac, D.; et al. Lupan-Skeleton Pentacyclic Triterpenes with Activity against Skin Cancer: Preclinical Trials Evolution. In Unique Aspects of Anti-cancer Drug Development; Latosińska, J.N., Latosińska, M., Eds.; IntechOpen: London, UK, 2017; pp. 88–114. [Google Scholar]
- Siddiqui, I.A.; Tarapore, R.S.; Chamcheu, J.C.; Mukhtar, H. Bioactive Food Components for Melanoma: An Overview. In Skin Cancer Overview; Xi, Y., Ed.; IntechOpen: London, UK, 2011; pp. 192–214. ISBN 978-953-307-746-8. [Google Scholar]
- Chinembiri, T.; du Plessis, L.; Gerber, M.; Hamman, J.; du Plessis, J. Review of Natural Compounds for Potential Skin Cancer Treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Kroemer, G. Targeting Mitochondrial Apoptosis by Betulinic Acid in Human Cancers. Drug Discov. Today 2009, 14, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Cazal, C.M.; Choosang, K.; Severino, V.G.P.; Soares, M.S.; Sarria, A.L.F.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Pakkong, P.; Almeida, G.M.; et al. Evaluation of Effect of Triterpenes and Limonoids on Cell Growth, Cell Cycle and Apoptosis in Human Tumor Cell Lines. Anticancer Agents Med. Chem. 2010, 10, 769–776. [Google Scholar] [CrossRef]
- Kang, H.R.; Eom, H.J.; Lee, S.R.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. Bioassay-Guided Isolation of Antiproliferative Triterpenoids from Euonymus alatus Twigs. Nat. Prod. Commun. 2015, 10, 1929–1932. [Google Scholar] [CrossRef]
- Peron, G.; Marzaro, G.; Dall‘Acqua, S. Known Triterpenes and Their Derivatives as Scaffolds for the Development of New Therapeutic Agents for Cancer. Curr. Med. Chem. 2018, 25, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, Ursane-, and Quinone Methide Friedelane-Type Triterpenoid Derivatives: Recent Advances in Cancer Treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef]
- Luo, R.; Fang, D.; Chu, P.; Wu, H.; Zhang, Z.; Tang, Z. Multiple Molecular Targets in Breast Cancer Therapy by Betulinic Acid. Biomed. Pharmacother. 2016, 84, 1321–1330. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic Acid in the Treatment of Tumour Diseases: Application and Research Progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, N.; Liu, Y.; Cheng, M.-S. Recent Progress on Betulinic Acid and Its Derivatives as Antitumor Agents: A Mini Review. Chin. J. Nat. Med. 2021, 19, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives. Molecules 2019, 24, 3546. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer Activity of Oleanolic Acid and Its Derivatives: Recent Advances in Evidence, Target Profiling and Mechanisms of Action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Cabaj, J.; Bąk, W.; Wróblewska-Łuczka, P. Anti-Cancer Effect of Betulin and Its Derivatives, with Particular Emphasis on the Treatment of Melanoma. J. Pre-Clin. Clin. Res. 2021, 15, 73–79. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as Cytotoxic Agents: A Review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical Study and Medical Application of Saponins as Anti-Cancer Agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Thakur, M.; Melzig, M.F.; Fuchs, H.; Weng, A. Chemistry and Pharmacology of Saponins: Special Focus on Cytotoxic Properties. Bot. Targets Ther. 2011, 1, 19–29. [Google Scholar] [CrossRef]
- Podolak, I.; Grabowska, K.; Sobolewska, D.; Wróbel-Biedrawa, D.; Makowska-Wąs, J.; Galanty, A. Saponins as Cytotoxic Agents: An Update (2010–2021). Part II—Triterpene Saponins. Phytochem. Rev. 2023, 22, 113–167. [Google Scholar] [CrossRef]
- Patočka, J. Biologically Active Pentacyclic Triterpenes and Their Current Medicine Signification. J. Appl. Biomed. 2003, 1, 7–12. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Halaweish, F.T. Cucurbitacins: Potential Candidates Targeting Mitogen-Activated Protein Kinase Pathway for Treatment of Melanoma. J. Enzym. Inhib. Med. Chem. 2014, 29, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Olayinka, O.A. Search for a Novel Antioxidant, Anti-Inflammatory/Analgesic or Anti-Proliferative Drug: Cucurbitacins Hold the Ace. J. Med. Plants Res. 2010, 4, 2821–2826. [Google Scholar]
- Sun, J.; Blaskovich, M.A.; Jove, R.; Livingston, S.K.; Coppola, D.; Sebti, S.M. Cucurbitacin Q: A Selective STAT3 Activation Inhibitor with Potent Antitumor Activity. Oncogene 2005, 24, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Sliva, D. Ganoderma Lucidum for Cancer Treatment. Integr. Cancer Ther. 2015, 14, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Rabe, S.Z.T.; Balali-Mood, M.; Karimi, G.; Tabasi, N.; Riahi-Zanjani, B. Ursolic Acid Induced Apoptotic Cell Death Following Activation of Caspases in Isolated Human Melanoma Cells. Cell Biol. Int. 2015, 39, 230–236. [Google Scholar] [CrossRef]
- Pavel, I.Z.; Danciu, C.; Oprean, C.; Dehelean, C.A.; Muntean, D.; Csuk, R.; Muntean, D.M. In Vitro Evaluation of the Antimicrobial Ability and Cytotoxicity on Two Melanoma Cell Lines of a Benzylamide Derivative of Maslinic Acid. Anal. Cell. Pathol. 2016, 2016, 2787623. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mitra, D.; Ray, S.; Biswas, N.; Banerjee, S.; Majumder, B.; Mustafi, S.M.; Murmu, N. Reversing Effect of Lupeol on Vasculogenic Mimicry in Murine Melanoma Progression. Microvasc. Res. 2019, 121, 52–62. [Google Scholar] [CrossRef]
- Park, H.J.; Jo, D.S.; Choi, D.S.; Bae, J.-E.; Park, N.Y.; Kim, J.-B.; Chang, J.H.; Shin, J.J.; Cho, D.-H. Ursolic Acid Inhibits Pigmentation by Increasing Melanosomal Autophagy in B16F1 Cells. Biochem. Biophys. Res. Commun. 2020, 531, 209–214. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.-C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Zhang, Y.; Ge, L.; Chen, J.; Jia, X.; Gu, R.; Sun, Y.; Sun, W. Ilexgenin A Induces B16-F10 Melanoma Cell G1/S Arrest in Vitro and Reduces Tumor Growth in Vivo. Int. Immunopharmacol. 2015, 24, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Marciniec, K.; Kadela-Tomanek, M.; Trynda, J.; Wietrzyk, J.; Boryczka, S. Biological Activity and In Silico Study of 3-Modified Derivatives of Betulin and Betulinic Aldehyde. Int. J. Mol. Sci. 2019, 20, 1372. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Soica, C.M.; Toma, C.C.; Feflea, S.; Gruia, A.T.; Kasa, P. Antitumoral Activity of Betulin, a Compound Present in Birch Tree, in Formulations with Cyclodextrin. Stud. Univ. Vasile Goldis Arad Ser. Stiint. Vietii 2010, 20, 55–58. [Google Scholar]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an Antitumor Agent Tested in Vitro on A431, HeLa and MCF7, and as an Angiogenic Inhibitor in Vivo in the CAM Assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar] [CrossRef]
- Dehelean, C.; Soica, C.; Peev, C.; Ciurlea, S.; Coneac, G.; Cinta-Pinzaru, S. Pentacyclic Triterpenes Interventions in Skin Pathology/Toxicity and Treatment: In Vitro and in Vivo Correlations. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2008, 65, 370–375. [Google Scholar]
- Danciu, C.; Pinzaru, I.; Coricovac, D.; Andrica, F.; Sizemore, I.; Dehelean, C.; Baderca, F.; Lazureanu, V.; Soica, C.; Mioc, M.; et al. Betulin Silver Nanoparticles Qualify as Efficient Antimelanoma Agents in in Vitro and in Vivo Studies. Eur. J. Pharm. Biopharm. 2019, 134, 1–19. [Google Scholar] [CrossRef]
- Chrobak, E.; Jastrzębska, M.; Bębenek, E.; Kadela-Tomanek, M.; Marciniec, K.; Latocha, M.; Wrzalik, R.; Kusz, J.; Boryczka, S. Molecular Structure, In Vitro Anticancer Study and Molecular Docking of New Phosphate Derivatives of Betulin. Molecules 2021, 26, 737. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kouzi, S.A.; Pezzuto, J.M.; Hamann, M.T. Biotransformation of the Antimelanoma Agent Betulinic Acid by Bacillus Megaterium ATCC 13368. Appl. Environ. Microbiol. 2000, 66, 3850–3855. [Google Scholar] [CrossRef]
- Sarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Dzubak, P.; Hajduch, M. The Potential of Triterpenoids in the Treatment of Melanoma. In Research on Melanoma—A Glimpse into Current Directions and Future Trends; Murph, M., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-293-7. [Google Scholar]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative Terpenoids from Almond Hulls (Prunus dulcis): Identification and Structure-Activity Relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Paschke, R. Lupane Triterpenoids—Betulin and Betulinic Acid Derivatives Induce Apoptosis in Tumor Cells. Investig. New Drugs 2011, 29, 266–272. [Google Scholar] [CrossRef]
- Liu, W.-K.; Ho, J.C.K.; Cheung, F.W.K.; Liu, B.P.L.; Ye, W.-C.; Che, C.-T. Apoptotic Activity of Betulinic Acid Derivatives on Murine Melanoma B16 Cell Line. Eur. J. Pharmacol. 2004, 498, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Ghosh, M.; Dutta, S.K. A Potent Tumoricidal Co-Drug ‘Bet-CA’—An Ester Derivative of Betulinic Acid and Dichloroacetate Selectively and Synergistically Kills Cancer Cells. Sci. Rep. 2015, 5, 7762. [Google Scholar] [CrossRef]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line WM-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef] [PubMed]
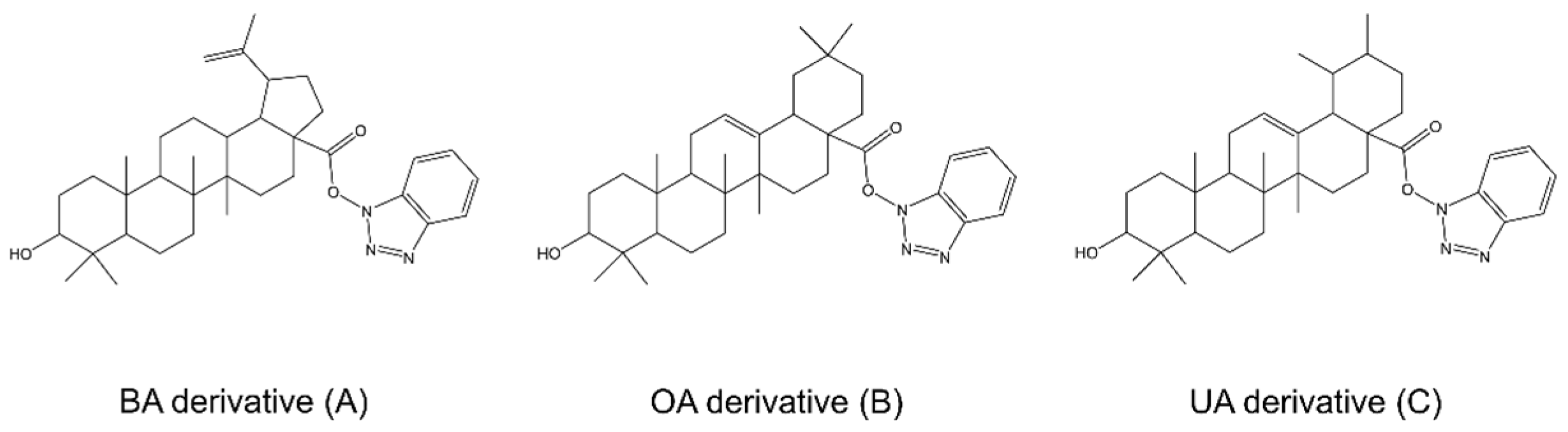
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G.; et al. Novel Triterpenic Acid—Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef]
- Weber, L.A.; Meißner, J.; Delarocque, J.; Kalbitz, J.; Feige, K.; Kietzmann, M.; Michaelis, A.; Paschke, R.; Michael, J.; Pratscher, B.; et al. Betulinic Acid Shows Anticancer Activity against Equine Melanoma Cells and Permeates Isolated Equine Skin in Vitro. BMC Vet. Res. 2020, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Maddodi, N.; Abu Zaid, M.; Khan, N.; bin Hafeez, B.; Asim, M.; Suh, Y.; Yun, J.-M.; Setaluri, V.; Mukhtar, H. Lupeol Inhibits Growth of Highly Aggressive Human Metastatic Melanoma Cells In Vitro and In Vivo by Inducing Apoptosis. Clin. Cancer Res. 2008, 14, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Ogihara, K.; Takahashi, S.; Tsuka, T.; Minami, S.; Okamoto, Y. Effects of Lupeol on Melanoma In Vitro and In Vivo: Fundamental and Clinical Trials. In Basic and Applied Aspects; Kamihira, M., Katakura, Y., Ito, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 339–344. ISBN 9789048138920. [Google Scholar]
- Cmoch, P.; Pakulski, Z.; Swaczynová, J.; Strnad, M. Synthesis of Lupane-Type Saponins Bearing Mannosyl and 3,6-Branched Trimannosyl Residues and Their Evaluation as Anticancer Agents. Carbohydr. Res. 2008, 343, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, R.S.; Siddiqui, I.A.; Saleem, M.; Adhami, V.M.; Spiegelman, V.S.; Mukhtar, H. Specific Targeting of Wnt/-Catenin Signaling in Human Melanoma Cells by a Dietary Triterpene Lupeol. Carcinogenesis 2010, 31, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Bociort, F.; Macasoi, I.G.; Marcovici, I.; Motoc, A.; Grosu, C.; Pinzaru, I.; Petean, C.; Avram, S.; Dehelean, C.A. Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective. Curr. Oncol. 2021, 28, 5054–5066. [Google Scholar] [CrossRef]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.; Munteanu, M. The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef]
- Woo, J.-S.; Yoo, E.-S.; Kim, S.-H.; Lee, J.-H.; Han, S.-H.; Jung, S.-H.; Jung, G.-H.; Jung, J.-Y. Anticancer Effects of Oleanolic Acid on Human Melanoma Cells. Chem. Biol. Interact. 2021, 347, 109619. [Google Scholar] [CrossRef]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C.; et al. Improvement of Ursolic and Oleanolic Acids’ Antitumor Activity by Complexation with Hydrophilic Cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef]
- AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Zagrodzki, P.; Grabowska, K.; Malarz, J.; Podolak, I. Sorbus intermedia (EHRH.) PERS. Fruits as a Novel Source of Biologically Active Triterpenoids—Comparative Studies of Ursolic Acid Derivatives with Cytotoxic Potential. Biomed. Pharmacother. 2022, 154, 113592. [Google Scholar] [CrossRef]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of Ursolic and Oleanolic on SK-MEL-2 Melanoma Cells: In Vitro and in Vivo Assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef]
- Xiang, L.; Chi, T.; Tang, Q.; Yang, X.; Ou, M.; Chen, X.; Yu, X.; Chen, J.; Ho, R.J.Y.; Shao, J.; et al. A Pentacyclic Triterpene Natural Product, Ursolic Acid and Its Prodrug US597 Inhibit Targets within Cell Adhesion Pathway and Prevent Cancer Metastasis. Oncotarget 2015, 6, 9295–9312. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Q.; Wang, F.; Li, Y.; Zhang, G.; Tao, S. Taraxasterol Attenuates Melanoma Progression via Inactivation of Reactive Oxygen Species-Mediated PI3K/Akt Signaling Pathway. Hum. Exp. Toxicol. 2022, 41, 09603271211069034. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.; Zhang, P.; Yang, H.; Cong, X.; An, L.; Xiao, C. Celastrol Self-Stabilized Nanoparticles for Effective Treatment of Melanoma. Int. J. Nanomed. 2020, 15, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.W.K.; Guo, J.; Ling, Y.-H.; Che, C.-T.; Liu, W.-K. Anti-Melanogenic Property of Geoditin A in Murine B16 Melanoma Cells. Mar. Drugs 2012, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Aranha, E.S.P.; da Silva, E.L.; Mesquita, F.P.; Bentes de Sousa, L.; da Silva, F.M.; Rocha, W.C.; Lima, E.S.; Koolen, H.H.F.; de Moraes, M.E.A.; Montenegro, R.C.; et al. 22β-Hydroxytingenone Reduces Proliferation and Invasion of Human Melanoma Cells. Toxicol. Vitr. 2020, 66, 104879. [Google Scholar] [CrossRef] [PubMed]
- Aranha, E.S.P.; de Sousa Portilho, A.J.; Bentes de Sousa, L.; da Silva, E.L.; Mesquita, F.P.; Rocha, W.C.; Araújo da Silva, F.M.; Lima, E.S.; Alves, A.P.N.N.; Koolen, H.H.F.; et al. 22β-Hydroxytingenone Induces Apoptosis and Suppresses Invasiveness of Melanoma Cells by Inhibiting MMP-9 Activity and MAPK Signaling. J. Ethnopharmacol. 2021, 267, 113605. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, X.; Yu, H.; Tang, Z.; Tang, X.; Cai, C.; Xu, J.; Xu, H. The Anti-Melanoma Efficiency of the Intratumoral Injection of Cucurbitacin-Loaded Sustained-Release Carriers: A PLGA Particle System. J. Pharm. Sci. 2013, 102, 2550–2563. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, D.; Xu, L.; Ji, Y.; Zha, Q.; Cai, J.; He, X. Cucurbitacin B Induces Rapid Depletion of the G-Actin Pool through Reactive Oxygen Species-Dependent Actin Aggregation in Melanoma Cells. Acta Biochim. Biophys. Sin. 2011, 43, 556–567. [Google Scholar] [CrossRef]
- Yan, M.M.; Li, T.Y.; Zhao, D.Q.; Shao, S.; Bi, S.N. A New Derivative of Triterpene with Anti-Melanoma B16 Activity from Conyza canadensis. Chin. Chem. Lett. 2010, 21, 834–837. [Google Scholar] [CrossRef]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid. Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.A.; Lupiáñez, J.; Rufino-Palomares, E.E. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules 2020, 25, 4020. [Google Scholar] [CrossRef]
- Fuentes-Rios, D.; Cepero, A.; García-Castro, M.; Contreras-Cáceres, R.; López-Romero, J.M.; Luque, C.; Cabeza, L.; Melguizo, C.; Prados, J. Synthesis, Solubility and Antitumor Activity of Maslinic Acid Derivatives. Eur. J. Med. Chem. Rep. 2022, 4, 100032. [Google Scholar] [CrossRef]
- Jiao, Q.; Zou, L.; Liu, P.; Xu, Q.; Zhang, Y.; Yu, Y.; Zou, L.; Chi, T.; Ji, X. Xanthoceraside Induces Apoptosis in Melanoma Cells Through the Activation of Caspases and the Suppression of the IGF-1R/Raf/MEK/ERK Signaling Pathway. J. Med. Food 2014, 17, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chodurek, E.; Orchel, A.; Dzierzewicz, Z.; Boryczka, S. Antiproliferative Activity of Novel Acetylenic Derivatives of Betulin against G-361 Human Melanoma Cells. Acta Pol. Pharm. Drug Res. 2015, 72, 699–703. [Google Scholar]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic Acid as a Potent and Complex Antitumor Phytochemical: A Minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and Betulinic Acid: Triterpenoids Derivatives with a Powerful Biological Potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Hossain, A.; Radwan, F.F.Y.; Doonan, B.P.; God, J.M.; Zhang, L.; Bell, P.D.; Haque, A. A Possible Cross-Talk between Autophagy and Apoptosis in Generating an Immune Response in Melanoma. Apoptosis 2012, 17, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Kaps, A.; Chodurek, E.; Orchel, A.; Jaworska-Kik, M.; Bębenek, E.; Boryczka, S.; Kasperczyk, J. Influence of 28-O-Propynoylbetulin on Proliferation and Apoptosis of Melanotic and Amelanotic Human Melanoma Cells. Postepy Hig. Med. Dosw. 2016, 70, 1404–1408. [Google Scholar]
- Kikuchi, T.; Uchiyama, E.; Ukiya, M.; Tabata, K.; Kimura, Y.; Suzuki, T.; Akihisa, T. Cytotoxic and Apoptosis-Inducing Activities of Triterpene Acids from Poria cocos. J. Nat. Prod. 2011, 74, 137–144. [Google Scholar] [CrossRef]
- Kuttan, G.; Pratheeshkumar, P.; Manu, K.A.; Kuttan, R. Inhibition of Tumor Progression by Naturally Occurring Terpenoids. Pharm. Biol. 2011, 49, 995–1007. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic Acid, a Natural Compound with Potent Anticancer Effects. Anti-Cancer Drugs 2010, 21, 215–227. [Google Scholar] [CrossRef]
- Oprean, C.; Ivan, A.; Bojin, F.; Cristea, M.; Soica, C.; Drăghia, L.; Caunii, A.; Paunescu, V.; Tatu, C. Selective in Vitro Anti-Melanoma Activity of Ursolic and Oleanolic Acids. Toxicol. Mech. Methods 2017, 28, 148–156. [Google Scholar] [CrossRef]
- Orchel, A.; Kulczycka, A.; Chodurek, E.; Bębenek, E.; Borkowska, P.; Boryczka, S.; Kowalski, J.; Dzierżewicz, Z. Influence of Betulin and 28-O-Propynoylbetulin on Proliferation and Apoptosis of Human Melanoma Cells (G-361). Adv. Hyg. Exp. Med. 2014, 68, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Mukhtar, H. Botanicals for the Prevention and Treatment of Cutaneous Melanoma. Pigment. Cell Melanoma Res. 2011, 24, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-S.; Lin, L.-W.; Wu, C.-R. Lupeol and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature. Advances in Experimental Medicine and Biology; Gupta, S., Prasad, S., Aggarwal, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 929, pp. 145–175. ISBN 9783319413426. [Google Scholar]
- Blom van Staden, A.; Lall, N. Medicinal Plants as Alternative Treatments for Progressive Macular Hypomelanosis. In Medicinal Plants for Holistic Health and Well-Being; Lall, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–182. ISBN 9780128124758. [Google Scholar]
- Wal, A.; Wal, P.; Sharma, G.; Rai, A. Biological Activities of Lupeol. Syst. Rev. Pharm. 2011, 2, 96–103. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Jaswal, V.; Sharma, A.; Kashyap, D.; Tuli, H.S.; Garg, V.K.; Das, S.K.; Srinivas, R. Celastrol as a Pentacyclic Triterpenoid with Chemopreventive Properties. Pharm. Pat. Anal. 2018, 7, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Men, X.; Lei, P. Review on Anti-Tumor Effect of Triterpene Acid Compounds. J. Cancer Res. Ther. 2014, 10, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kanjoormana, M.; Kuttan, G. Antiangiogenic Activity of Ursolic Acid. Integr. Cancer Ther. 2010, 9, 224–235. [Google Scholar] [CrossRef]
- Hata, K.; Hori, K.; Murata, J.; Takahashi, S. Remodeling of Actin Cytoskeleton in Lupeol-Induced B16 2F2 Cell Differentiation. J. Biochem. 2005, 138, 467–472. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted Inhibition of Tumor Proliferation, Survival, and Metastasis by Pentacyclic Triterpenoids: Potential Role in Prevention and Therapy of Cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T.; Matsumoto, M.; Sotozono, Y.; Fukami, S.; Nugroho, A.E.; Hirasawa, Y.; Hamid, H.A.; Morita, H. Cycloartane Triterpenoid (23R, 24E)-23-Acetoxymangiferonic Acid Inhibited Proliferation and Migration in B16-F10 Melanoma via MITF Downregulation Caused by Inhibition of Both β-Catenin and c-Raf–MEK1–ERK Signaling Axis. J. Nat. Med. 2019, 73, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Soica, C.; Antal, D.; Alexa, E.; Pavel, I.Z.; Ghiulai, R.; Ardelean, F.; Babuta, R.M.; Popescu, A.; Dehelean, C.A. Natural Compounds in the Chemoprevention of Malignant Melanoma. Anti-Cancer Agents Med. Chem. 2018, 18, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Mukaiyama, T.; Tsujimura, N.; Sato, Y.; Kosaka, Y.; Sakamoto, K.; Hori, K. Differentiation-Inducing Activity of Lupane Triterpenes on a Mouse Melanoma Cell Line. Cytotechnology 2006, 52, 151–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, D.; Wakimoto, N.; Xing, H.; Lu, D.; Huynh, T.; Wang, X.; Black, K.L.; Koeffler, H.P. Cucurbitacin B Markedly Inhibits Growth and Rapidly Affects the Cytoskeleton in Glioblastoma Multiforme. Int. J. Cancer 2008, 123, 1364–1375. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Zhang, H.; Sun, C.; Yang, X.; Deng, Y.; Ji, W. Combined Antitumor Activity of Cucurbitacin B and Docetaxel in Laryngeal Cancer. Eur. J. Pharmacol. 2008, 587, 78–84. [Google Scholar] [CrossRef]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-Bark Triterpenoids and Their Derivatives in Anticancer Research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Soica, C.; Trandafirescu, C.; Danciu, C.; Muntean, D.; Dehelean, C.; Simu, G. New Improved Drug Delivery Technologies for Pentacyclic Triterpenes: A Review. Protein Pept. Lett. 2014, 21, 1137–1145. [Google Scholar] [CrossRef]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of Terpenes in Cyclodextrins: Preparation, Characterization and Pharmacological Approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-Coated Silver Nanoparticles—A Comprehensive Toxicological Profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Ghiulai, R.; Mioc, A.; Racoviceanu, R.; Mioc, M.; Milan, A.; Prodea, A.; Semenescu, A.; Dehelean, C.; Barbu Tudoran, L.; Avram, Ș.; et al. The Anti-Melanoma Effect of Betulinic Acid Functionalized Gold Nanoparticles: A Mechanistic In Vitro Approach. Pharmaceuticals 2022, 15, 1362. [Google Scholar] [CrossRef]
- Mioc, M.; Mioc, A.; Racoviceanu, R.; Ghiulai, R.; Prodea, A.; Milan, A.; Barbu Tudoran, L.; Oprean, C.; Ivan, V.; Șoica, C. The Antimelanoma Biological Assessment of Triterpenic Acid Functionalized Gold Nanoparticles. Molecules 2023, 28, 421. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Cai, C.; Xu, J.; Yu, H.; Xu, H.; Xing, T. The Anti-Melanoma Efficiency of the Intratumoral Injection of Cucurbitacin-Loaded Sustained Release Carriers: In Situ-Forming Implants. AAPS PharmSciTech 2015, 16, 973–985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bębenek, E.; Chrobak, E.; Rzepka, Z.; Wrześniok, D. New Betulin Derivatives with Nitrogen Heterocyclic Moiety—Synthesis and Anticancer Activity In Vitro. Biomolecules 2022, 12, 1540. [Google Scholar] [CrossRef]
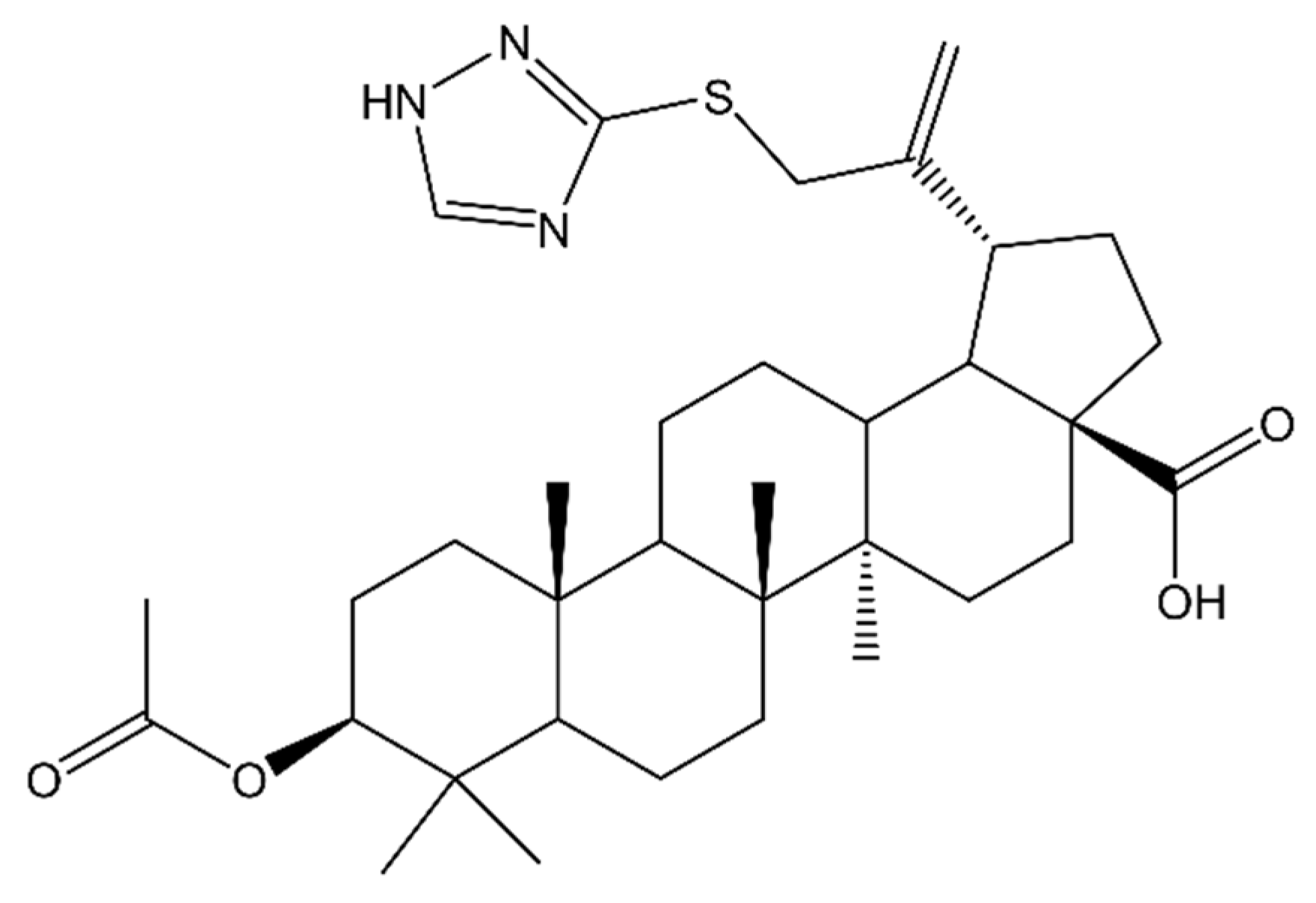
- Nistor, G.; Mioc, M.; Mioc, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Ghiulai, R.; Semenescu, A.; Dehelean, C.; et al. The C30-Modulation of Betulinic Acid Using 1,2,4-Triazole: A Promising Strategy for Increasing Its Antimelanoma Cytotoxic Potential. Molecules 2022, 27, 7807. [Google Scholar] [CrossRef] [PubMed]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.-P.; Csuk, R. Betulinic Acid Derived Amides Are Highly Cytotoxic, Apoptotic and Selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Chen, L. Synthesis and Cytotoxic Activity of Nitric Oxide-Releasing Isosteviol Derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 2202–2205. [Google Scholar] [CrossRef]
- Du, Z.; Li, G.; Zhou, X.; Zhang, J. Synthesis of MeON-Glycoside Derivatives of Oleanolic Acid by Neoglycosylation and Evaluation of Their Cytotoxicity against Selected Cancer Cell Lines. Molecules 2021, 26, 772. [Google Scholar] [CrossRef]
- Macașoi, I.; Pavel, I.Z.; Moacă, A.E.; Avram, Ș.; David, V.L.; Coricovac, D.; Mioc, A.; Spandidos, D.A.; Tsatsakis, A.; Șoica, C.; et al. Mechanistic Investigations of Antitumor Activity of a Rhodamine B-oleanolic Acid Derivative Bioconjugate. Oncol. Rep. 2020, 44, 1169–1183. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, W.; Chen, W.; Song, X.; Han, C.; Wang, Y.; Chen, G. Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii. Molecules 2013, 18, 14496–14504. [Google Scholar] [CrossRef] [PubMed]
- Farabi, K.; Harneti, D.; Darwati; Mayanti, T.; Nurlelasari; Maharani, R.; Sari, A.P.; Herlina, T.; Hidayat, A.T.; Supratman, U.; et al. Dammarane-Type Triterpenoid from the Stem Bark of Aglaia elliptica (Meliaceae) and Its Cytotoxic Activities. Molecules 2022, 27, 6757. [Google Scholar] [CrossRef]
- Nazir, L.A.; Shahid, N.H.; Amit, K.; Umar, S.A.; Rajni, S.; Bharate, S.; Sangwan, P.L.; Tasduq, S.A. Synthesis and Anti-Melanoma Effect of 3-O-Prenyl Glycyrrhetinic Acid against B16F10 Cells via Induction of Endoplasmic Reticulum Stress-Mediated Autophagy through ERK/AKT Signaling Pathway. Front. Oncol. 2022, 12, 890299. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Chai, H.-B.; Park, S.-Y.; Kim, D.S.H.L. Preparation of Amino Acid Conjugates of Betulinic Acid with Activity against Human Melanoma. Bioorg. Med. Chem. Lett. 1999, 9, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as New Promising Anticancer Drugs. Anti-Cancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Khan, M.F.; Sangwan, R.; Khan, M.A.; Kumar, A.; Verma, R.; Ahamad, T.; Jain, S. Design, Synthesis and In-Vitro Antitumor Activity of Lupeol Derivatives via Modification at C-3 and C-30 Positions. ChemistrySelect 2019, 4, 1800–1805. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The Oleanolic Acid Derivative, 3-O-Succinyl-28-O-Benzyl Oleanolate, Induces Apoptosis in B16–F10 Melanoma Cells via the Mitochondrial Apoptotic Pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Heller, L.; Schwarz, S.; Perl, V.; Köwitsch, A.; Siewert, B.; Csuk, R. Incorporation of a Michael Acceptor Enhances the Antitumor Activity of Triterpenoic Acids. Eur. J. Med. Chem. 2015, 101, 391–399. [Google Scholar] [CrossRef]
- Huang, D.; Ding, Y.; Li, Y.; Zhang, W.; Fang, W.; Chen, X. Anti-Tumor Activity of a 3-Oxo Derivative of Oleanolic Acid. Cancer Lett. 2006, 233, 289–296. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic Acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Parra, A.; Rivas, F.; Martin-Fonseca, S.; Garcia-Granados, A.; Martinez, A. Maslinic Acid Derivatives Induce Significant Apoptosis in B16f10 Murine Melanoma Cells. Eur. J. Med. Chem. 2011, 46, 5991–6001. [Google Scholar] [CrossRef]
- Yu, L.; Xie, X.; Cao, X.; Chen, J.; Chen, G.; Chen, Y.; Li, G.; Qin, J.; PENG, F.; Peng, C. The Anticancer Potential of Maslinic Acid and Its Derivatives: A Review. Drug Des. Dev. Ther. 2021, 15, 3863–3879. [Google Scholar] [CrossRef]
- Siewert, B.; Pianowski, E.; Csuk, R. Esters and Amides of Maslinic Acid Trigger Apoptosis in Human Tumor Cells and Alter Their Mode of Action with Respect to the Substitution Pattern at C-28. Eur. J. Med. Chem. 2013, 70, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Bhoumik, A.; Dahl, R.; Vasile, S.; Krajewski, S.; Cosford, N.D.P.; Ronai, Z.A. Preclinical Studies of Celastrol and Acetyl Isogambogic Acid in Melanoma. Clin. Cancer Res. 2007, 13, 6769–6778. [Google Scholar] [CrossRef]
- Bai, K.-K.; Yu, Z.; Chen, F.-L.; Li, F.; Li, W.-Y.; Guo, Y.-H. Synthesis and Evaluation of Ursolic Acid Derivatives as Potent Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2012, 22, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Z.; Xiang, L.; Li, Y.; Ou, M.; Yang, X.; Shao, J.; Lu, Y.; Lin, L.; Chen, J.; et al. Synergism of Ursolic Acid Derivative US597 with 2-Deoxy-D-Glucose to Preferentially Induce Tumor Cell Death by Dual-Targeting of Apoptosis and Glycolysis. Sci. Rep. 2015, 4, 5006. [Google Scholar] [CrossRef]
- Nitta, M.; Azuma, K.; Hata, K.; Takahashi, S.; Ogiwara, K.; Tsuka, T.; Imagawa, T.; Yokoe, I.; Osaki, T.; Minami, S.; et al. Systemic and Local Injections of Lupeol Inhibit Tumor Growth in a Melanoma-Bearing Mouse Model. Biomed. Rep. 2013, 1, 641–645. [Google Scholar] [CrossRef]

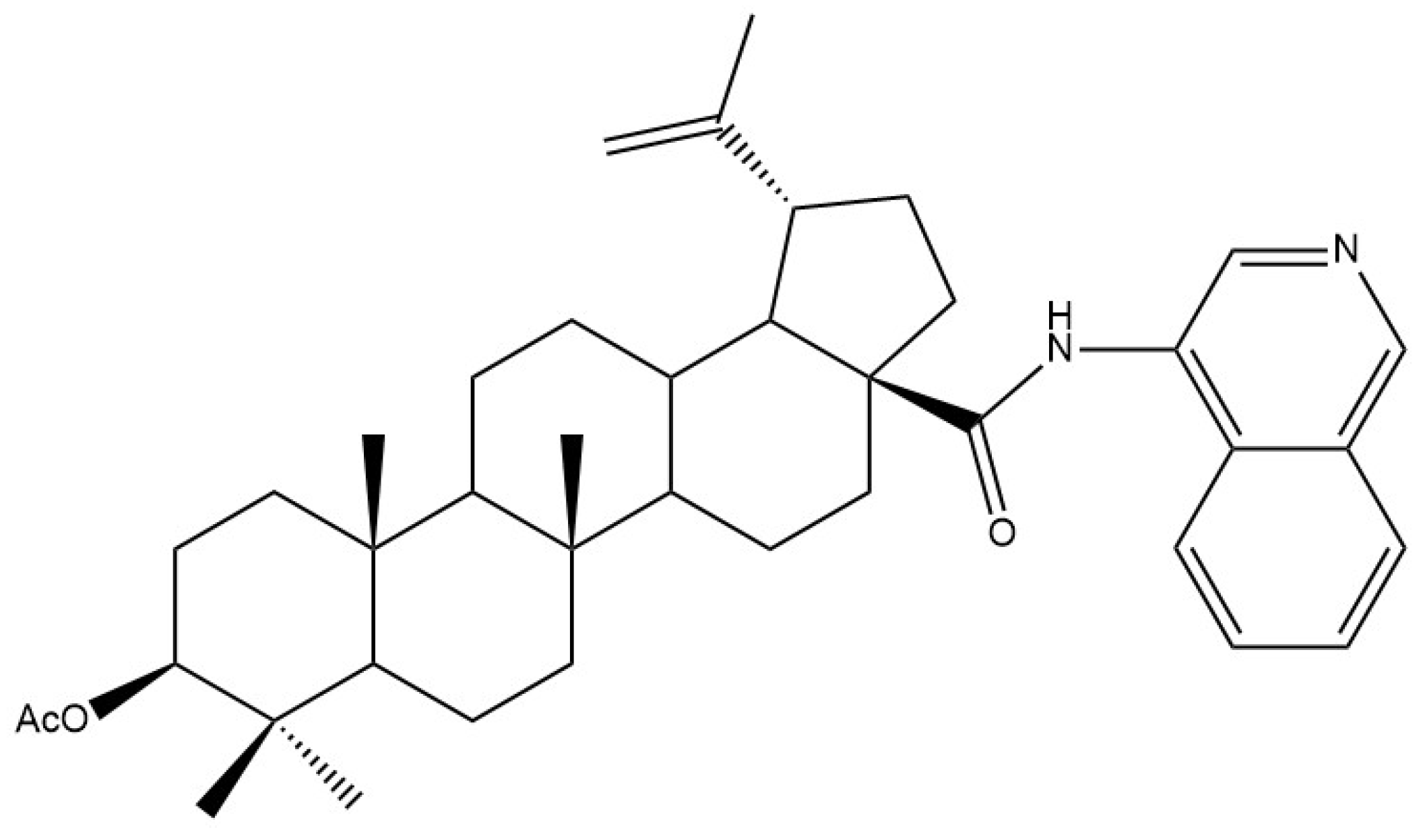
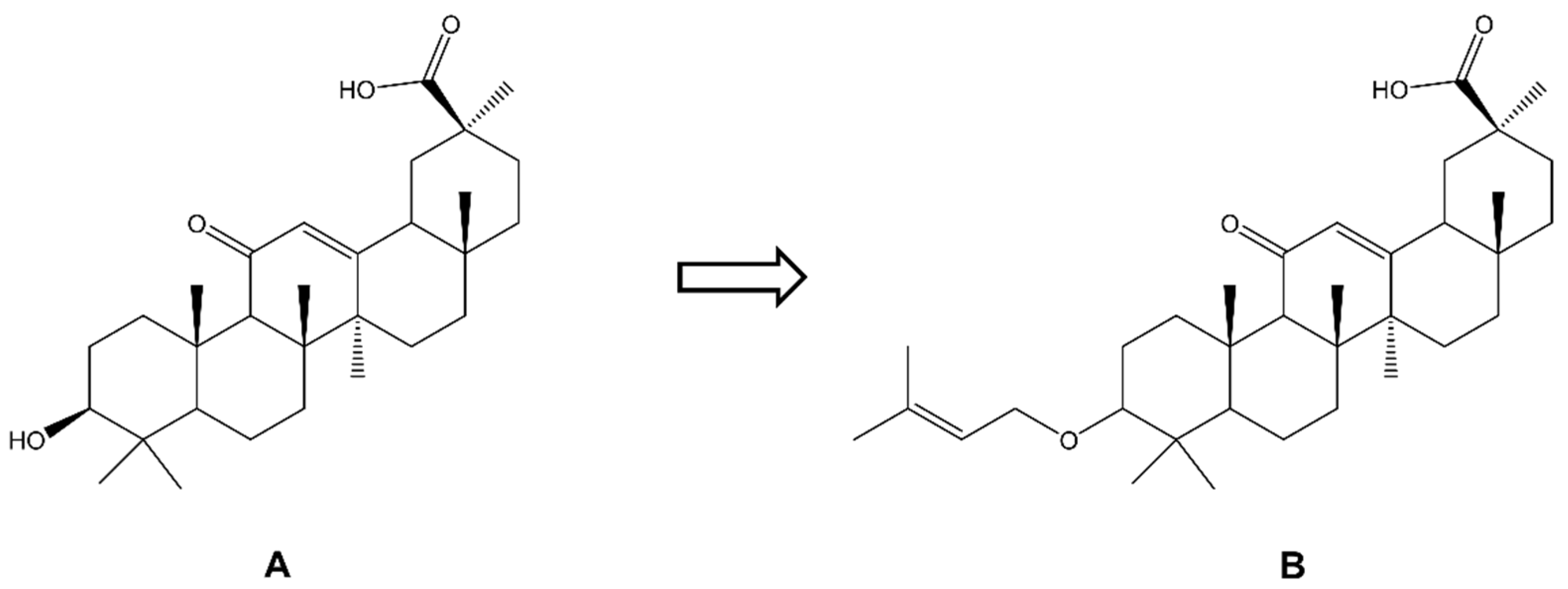
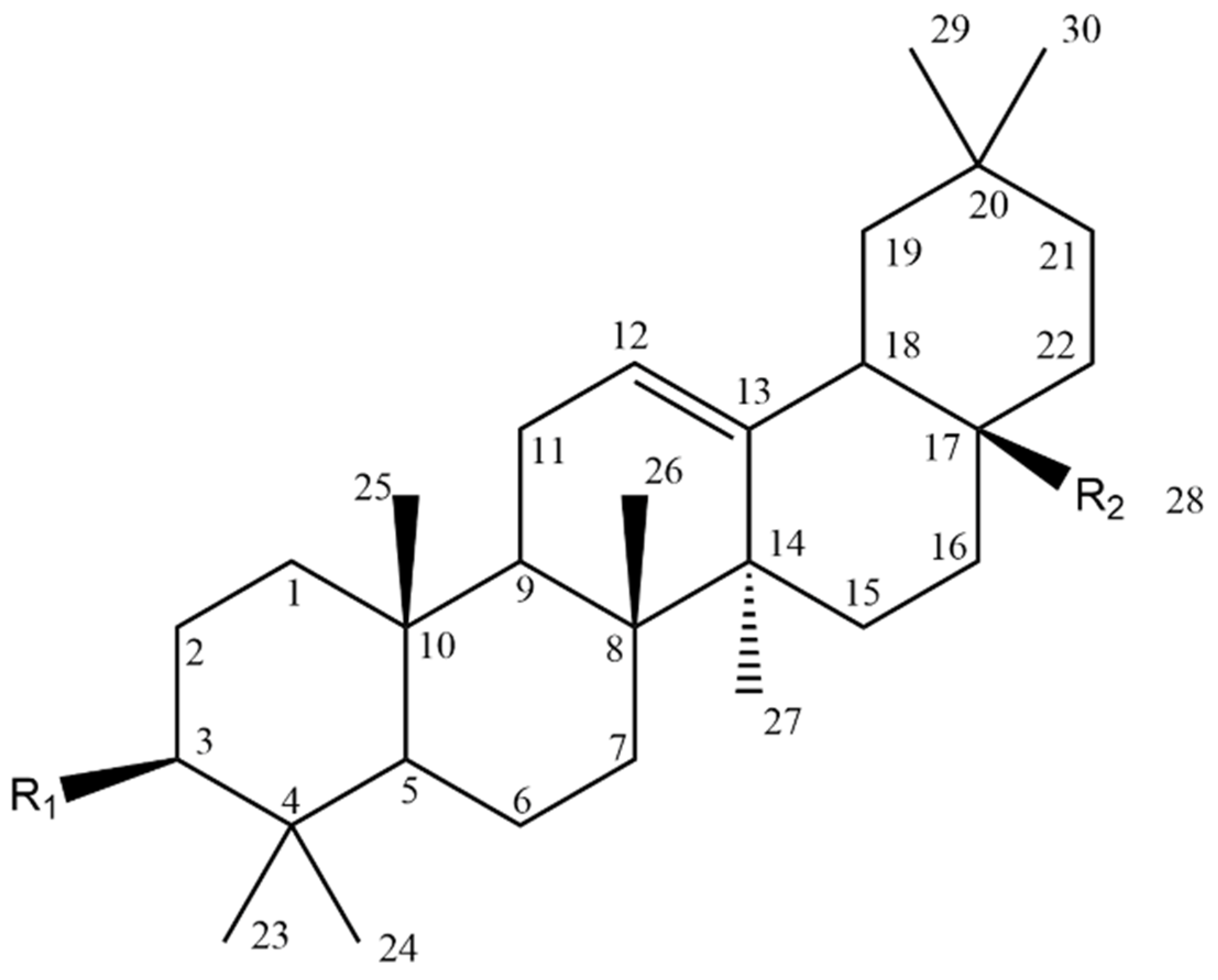


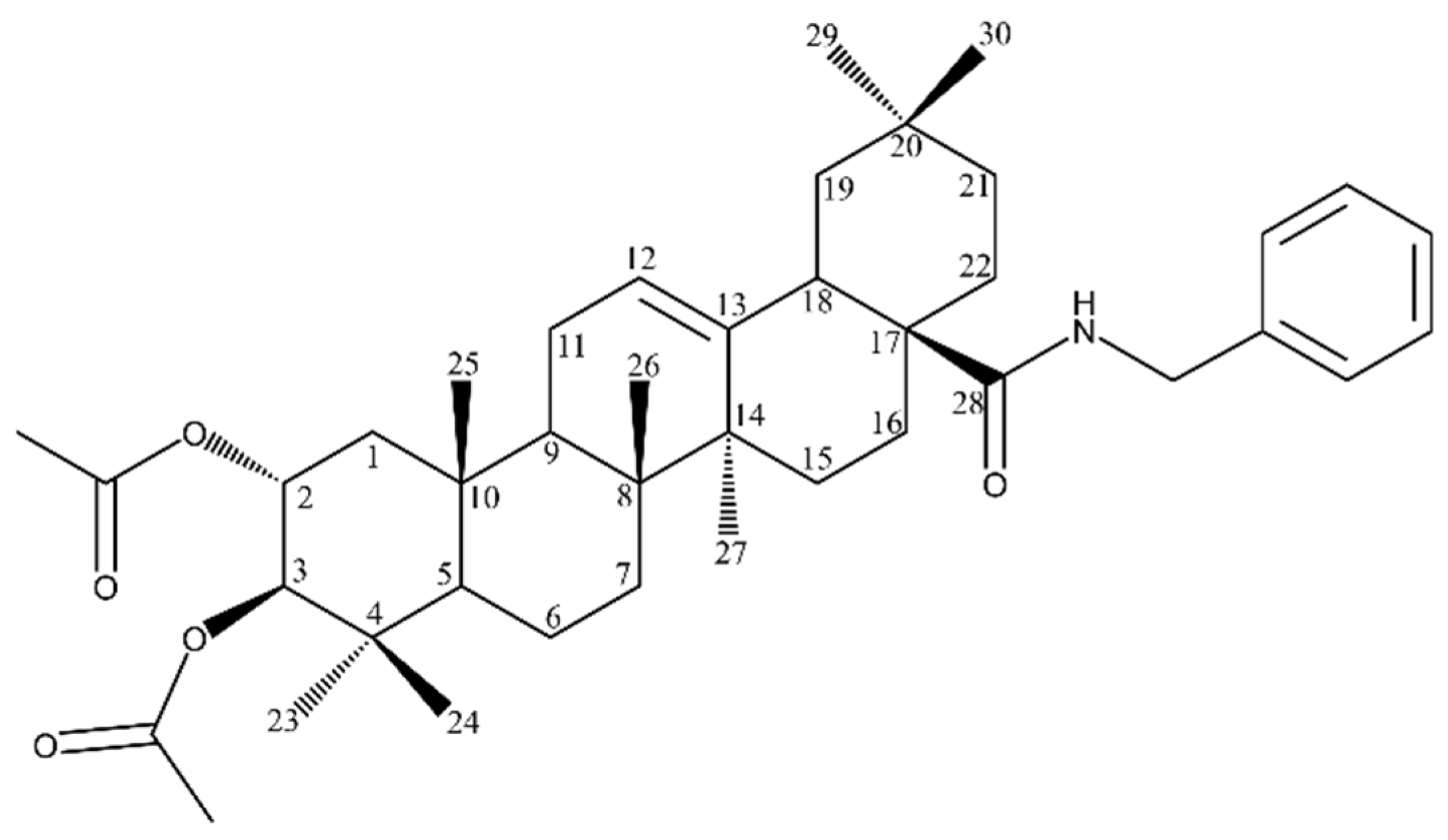


| Biological Effect and Cellular Mechanism | Compound | References |
|---|---|---|
| Apoptosis intrinsic pathway: | ||
| cytochrome c release; mitochondria membrane depolymerization; caspase 3 and 9 activation; PARP-1 cleavage; MAPK cascade activation; Bcl-2, survivin downregulation; p53 upregulation; NF-κB inhibition; TNFα stimulation | Betulin, lupeol, betulinic acid, erythrodiol, oleanolic acid, ursolic acid, Cucurbitacins, masilinic acid, asiatic acid, poricoic acids, 25-hydroxy-3-epidehydrotumulosic acid, dehydroeburiconic acid, glycyrrhizic acid, boswellic acid, celastrol, ganoderic acid, 3-O-acetylursolic acid, taraxasterol | [3,4,7,25,30,32,45,46,51,56,64,66,68,73,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] |
| Autophagy: | ||
| increase in Beclin-1 protein; activation of LC3 protein; depletion of autophagy-related gene 5 | Ganoderic acid, ursolic acid | [33,80] |
| Inhibition of angiogenesis: | ||
| inhibition of capillary formation and endthelial cells proliferation; upregulation of mTOR | Lupeol, ursolic acid, celastrol | [32,95,97] |
| Cell cycle arrest: | ||
| cyclin D1, D2 downregulation; p21 upregulation; CDK2 inhibition | Odoratol, betulinic acid, betulin, lupeol, ursolic acid, oleanolic acid, maslinic acid, celastrol, ilexgenin A | [4,8,30,36,46,56,64,66,83,95] |
| Cytoskeleton disruption: | ||
| cytoskeletal remodeling; attenuated stress fibre assembly; decrease in phospho-cofilin level; actin cytoskeleton disassembling by inhibition of Rho signaling | Lupeol | [57,98] |
| Inhibition of metastasis/migration: | ||
| MITF downregulation via β-catenin and c-Raf-MEK1-ERK signaling pathways; decrease in production of VEGF, MMP-2, MMP-9 and NO modulating tumor adhesion and invasion steps by inhibition of focal adhesion signaling pathway including alterations in ICAM-1, VCAM-1, E-selectin, P-selectin, integrin α6β1, FAK, Src, paxillin and PTEN; inhibition of haptotaxis | Oleanolic acid, (23R, 24E)-acetoxymangiferonic acid, ursolic acid, lupeol, celastrol, betulinic acid, 22β-hydroxytingenone, 3-O-acetylursolic acid, taraxasterol | [64,67,68,71,95,97,98,99,100,101] |
| Inhibition of melanin production: | ||
| downregulation of melanogenic proteins, aggravated with adenylate cyclase inhibitor SQ22536; increase in expression of MITF, a transcriptional factor of tyrosinase, Rab27a and myosin-Va; suppression of melanin accumulation; inhibition of melanogenesis by blockade of the mitogenic and differentiating signals from MAPK and Ras-MAPK kinase cascades; inhibition of α-melanocyte-stimulating hormone (MSH) | Geodotin A, lupeol, (23R, 24E)-acetoxymangiferonic acid, botulin, ursolic acid | [33,57,70,93,98,100] |
| Anti-inflammatory | Lupeol, betulin, betulinic acid, erythrodiol, oleanolic acid, ursolic acid, celastrol | [101,102] |
| Antioxidant | ||
| increase in the activity of superoxide dismutase, glutathione S-transferase and glutathione peroxidase | Lupeol, betulin, betulinic acid, erythrodiol, oleanolic acid, ursolic acid, maslinic acid, celastrol | [76,77,95] |
| Compound | Cell Line | Results | References |
|---|---|---|---|
| BT complexed with cyclodextrin | A-431H | Inhibition of proliferation ≈ 76% of control (C = no data) | [40] |
| BT silver nanoparticles | B164A5A | IC50 0.9301 μM | [43] |
| B16OvaA | IC50 20.26 μM | ||
| BT gold nanoparticles | RPMI-7951H | Cell viability: 75.1% (C = 25 μM) (24 h) | [111] |
| Cell viability: 63.4% (C = 50 μM) (24 h) | |||
| BT-ethosome formulation | B16-F10A | IC50 2.43 μM (48 h) | [1] |
| PEGylated formulation of AgNP-B | B164A5A | IC50 2.47 μM | [43] |
| B16OvaA | IC50 5.74 μM | ||
| BA with cyclodextrin GCDG complex | B164A5 metastaticA | Cell viability: 42.33% (C = no data) | [51] |
| B164A5 non-metastaticA | Cells viability: 50.3% | ||
| BA-ethosomes formulation | B16F10A | IC50 3.07 μM (48 h) | [1] |
| BA-HOBt loaded nanoparticles | A375H | Cell viability: 77.2% (C = 25 μM) (24 h) | [112] |
| Cell viability: 69% (C = 50 μM) (24 h) | |||
| OA-HOBt loaded nanoparticles | A375H | Cell viability: 81.2% (C = 25 μM) (24 h) | [112] |
| Cell viability: 59.3% (C = 50 μM) (24 h) | |||
| UA-HOBt loaded nanoparticles | A375H | Cell viability: 86.8% (C = 25 μM) (24 h) | [112] |
| Cell viability: 74.8% (C = 50 μM) (24 h) | |||
| CEL-NPs | B16-F10A | IC50 2.81 μM (48 h) | [69] |
| Cuc-loaded L-MPs | B-16A | IC50 464.37 μg/mL (48 h) | [73] |
| Cuc-loaded NPs | B-16A | IC50 82.94 μg/mL (48 h) | [73] |
| Cuc-loaded S-MPs | B-16A | IC50 283.41 μg/mL (48 h) | [73] |
| Compound | Cell line | Results | References |
|---|---|---|---|
| Betulin derivatives: | |||
| 28-O-propynoylbetulin | G361H | Cell growth: 10–32% of control (C = 10–20 μg/mL) | [80] |
| 28-O-(3-butynyloxycarbonyl)betulin | G361H | Cell growth: 20–44% of control (C = 10–20 μg/mL) | |
| 28-O-propargyloxycarbonylbetulin | G361H | Cell growth: 10–41% of control (C = 10–20 μg/mL) | |
| 28-O-propynoylbetulin | C32H | Cell growth: 28.8% (C = 3 μg/mL); 3.6% of control (C = 10 μg/mL) | [84] |
| A2058H | Cell growth: 45.9% (C = 3 μg/mL); 10.3% of control (C = 10 μg/mL) | ||
| 3-(2-propenoyl)betulin | Hs294TH | IC50 69 μM | [39] |
| 3-(3-butynyloxycarbonyl)betulin | Hs294TH | IC50 164 μM | |
| 3-propargyloxycarbonylbetulin | Hs294TH | IC50 82.7 μM | |
| 2-butynoyl derivative | Hs294TH | IC50 10.6 μM | |
| Betulinic aldehyde derivative | Hs294TH | IC50 74.4 μM | |
| Betulin-l-Ala-NH2 | Me-45H | IC50 86.6–10.1 μM (24–72 h) | [3] |
| Betulin-l-Dab-NH2 | Me-45H | IC50 66.5–9.3 μM (24–72 h) | |
| Betulin-l-Dap-NH2 | Me-45H | IC50 107.7–75.5 μM (24–72 h) | |
| Betulin-l-Lys-NH2 | Me-45H | IC50 55.3–2.5 μM (24–72 h) | |
| Betulin-l-Orn-NH2 | Me-45H | IC50 70.7–2.5 μM (24–72 h) | |
| Betulone | SK-MEL-2H | IC50 3.3 μM | [9] |
| 28-Hydroxy-(lup-20(29)-ene)-3-yl-2-(1H-indol-3-yl) acetate containing at the C-28 position a free hydroxyl group | A375H | Cell viability: ~70–65% (C = 83 and 167 µM) | [114] |
| C32H | Cell viability: ~70–65% (C = 83 and 167 µM) | ||
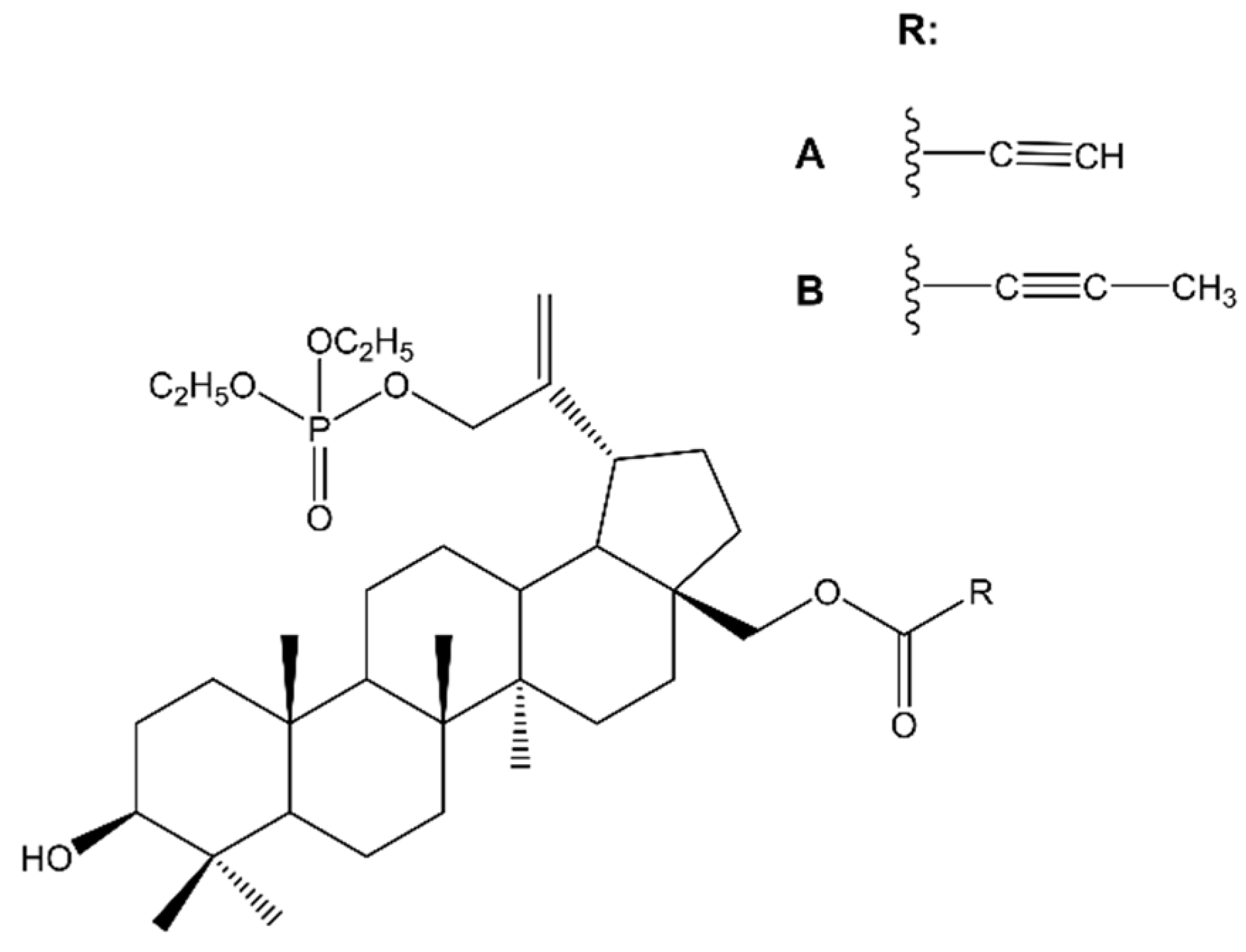
| 30-Diethoxyphosphoryloxy-28-propynoylbetulin | C32H | IC50 2.15 μM (72 h) | [44] |
| 28-(2-Butynoyl)-30-diethoxyphosphoryloxybetulin | C32H | IC50 0.76 μM (72 h) | |
| Betulinic acid derivatives: | |||
| bet-CA (a DCA molecule has been appended on C-3 hydroxyl group of BA) | B16-F10A | IC50 9.89 μM | [50] |
| BA + DCA | B16-F10A | IC50 27.6 μM | |
| BA-TZ | RPMI-7951H | Cell viability: 54.7% (C = 10 μM) | [115] |
| Cell viability: 24.5% (C = 50 μM) | |||
| 1H-Benzotriazole-1-yl (3β) 3-hydroxy-20(29)-lupaene-28-oate | A375H | Cell viability: 81.25% (C = 25 μM) (24 h) | [54] |
| Cell viability: 69.8% (C = 50 μM) (24 h) | |||
| 4-isoquinolinyl amide of 3-O-acetyl-betulinic acid | A375H | EC50 ¼ 1.48 μM (72 h) | [116] |
| Lupeol derivatives: | |||
| 3β,28,30-lup-20(29)-ene triol | SK-MEL-2H | IC50 14.5 µM | [9] |
| lupenone | SK-MEL-2H | IC50 9.2 µM | |
| 28,30-dihydroxy-3-oxolup-20(29)-ene | SK-MEL-2H | IC50 10.8 µM | |
| Oleanolic acid derivatives: | |||
| CDDO-Me (2-cyano-3,12-dioxo- oleana-1,9(11)-dien-28-acid methyl ester) | B16F10A | IC50 5.85 µM | [117] |
| hederagenin | SK-MEL-2H | IC50 13.8 µM | [9] |
| 3-oxo-11α-methoxyolean-12-ene | SK-MEL-2H | IC50 > 30 µM (48 h) | [61] |
| 3β-hydroxy-1-oxo-olean-12-en-28-oic acid | SK-MEL-2H | IC50 11.2 µM (48 h) | |
| glut-5-en-3β-ol | SK-MEL-2H | IC50 > 30 µM (48 h) | |
| (3S)-O-(N-Methoxy-N-a-d-arabinosylglycyl) oleanolic acid | A375H | IC50 9.6 µM (72 h) | [118] |
| (3S)-O-(N-Methoxy-N-β-l-xylosylglycyl) oleanolic acid | A375H | IC50 9.7 µM (72 h) | |
| (3S)-O-(N-Methoxy-N-β-l-lyxosylglycyl) oleanolic acid | A375H | IC50 8.4 µM (72 h) | |
| (3S)-O-(N-Methoxy-N-d-ribosylglycyl) oleanolic acid | A375H | IC50 6 µM (72 h) | |
| 9-[2-[[4-(3β-Acetyloxy-olean-12-en-28-oyl)-1-piperazinyl] carbonyl] phenyl]-3,6-bis(diethylamino]-xanthylium chloride (RhodOA) | 375H | Cell viability: ~75% (C= 80 nM) (72 h) | [119] |
| Cell viability: ~65% (C = 100 nM) (72 h) | |||
| 1H-Benzotriazole-1-yl (3β) 3-hydroxyolean-12-en-28-oate | 375H | Cell viability: 87.4% (C = 25 μM) (24 h) | [54] |
| Cell viability 62.5% (C = 50 μM) (24 h) | |||
| Ursolic acid derivatives: | |||
| 3α, 6α, 30-trihydroxy-ursan-28-oic acid | B16F10A | IC50 72.72 μM (48 h) | [120] |
| UA: HPgammaCD (ratio 1:2) | A375H | IC50 31.38 μM (48 h) | [63] |
| SK-MEL 2H | IC50 9.26 μM (48 h) | ||
| B164A5A | IC50 16.08 μM (48 h) | ||
| UA:HPbCD (ratio 1:2) | A375H | IC50 51.73 μM (48 h) | |
| B164A5A | IC50 40.84 μM (48 h) | ||
| 3β-acetoxy-urs-12-en-28-oic acid hexamethylenediamine (US597) | B16F10A | IC50 8.57 μM (24 h) | [67] |
| Ursolic acid derivatives: | |||
| 3-O-β-acetoxy-ursolic acid | HTB-140H | IC50 19.85 µg/mL (24 h) | [65] |
| IC50 8.75 µg/mL (48 h) | |||
| A375H | GI50 32 μM | [64] | |
| IC50 30.08 µg/mL (24 h) | [65] | ||
| IC50 25.92 µg/mL (48 h) | |||
| WM793H | IC50 30.99 µg/mL (24 h) | ||
| IC50 14.18 µg/mL (48 h) | |||
| Ursolic aldehyde | HTB-140H | IC50 > 100 µg/mL (24 h) | |
| IC50 19.28 µg/mL (48 h) | |||
| A375H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| WM793H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| 3-O-β-acetoxy-19α-hydroxy-ursolic acid | HTB-140H | IC50 > 100 µg/mL (24 h) | |
| IC50 > 100 µg/mL (48 h) | |||
| A375H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| WM793H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| Uvaol | HTB-140H | IC50 > 100 µg/mL (24 h) | |
| IC50 93.62 µg/mL (48 h) | |||
| A375H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| WM793H | IC50 > 100 µg/mL (24 h) | ||
| IC50 > 100 µg/mL (48 h) | |||
| 1H-Benzotriazole-1-yl (3β) 3-hydroxyurs-12-en-28-oate | A375H | Cell viability: 77% (C = 50 µM) (24 h) | [54] |
| Dammarane derivatives: | |||
| 3β-oleate-20S-hydroxydammar-24-en | B16F10A | IC50 181.34 μg/mL (24 h) | [121] |
| 20S,24S-epoxy-3β-oleate-25-hydroxydammarane | B16F10A | IC50 98.4 μg/mL (24 h) | |
| 20S-hydroxydammar-24-en-3-on | B16F10A | IC50 22.95 μg/mL (24 h) | |
| 3β,20S-dihydroxydammar-24-en | B16F10A | IC50 49.57 μg/mL (24 h) | |
| 20S,24S-epoxy-3β,25-dihydroxydammarane | B16F10A | IC50 95.27 μg/mL (24 h) | |
| Glycyrrhetinic acid derivatives: | |||
| NPC-402 | B16F10A | IC50 16 μM (24 h) | [122] |
| A375H | IC50 27 μM (24 h) | ||
| SK-MEL-28H | IC50 33.5 μM (24 h) |
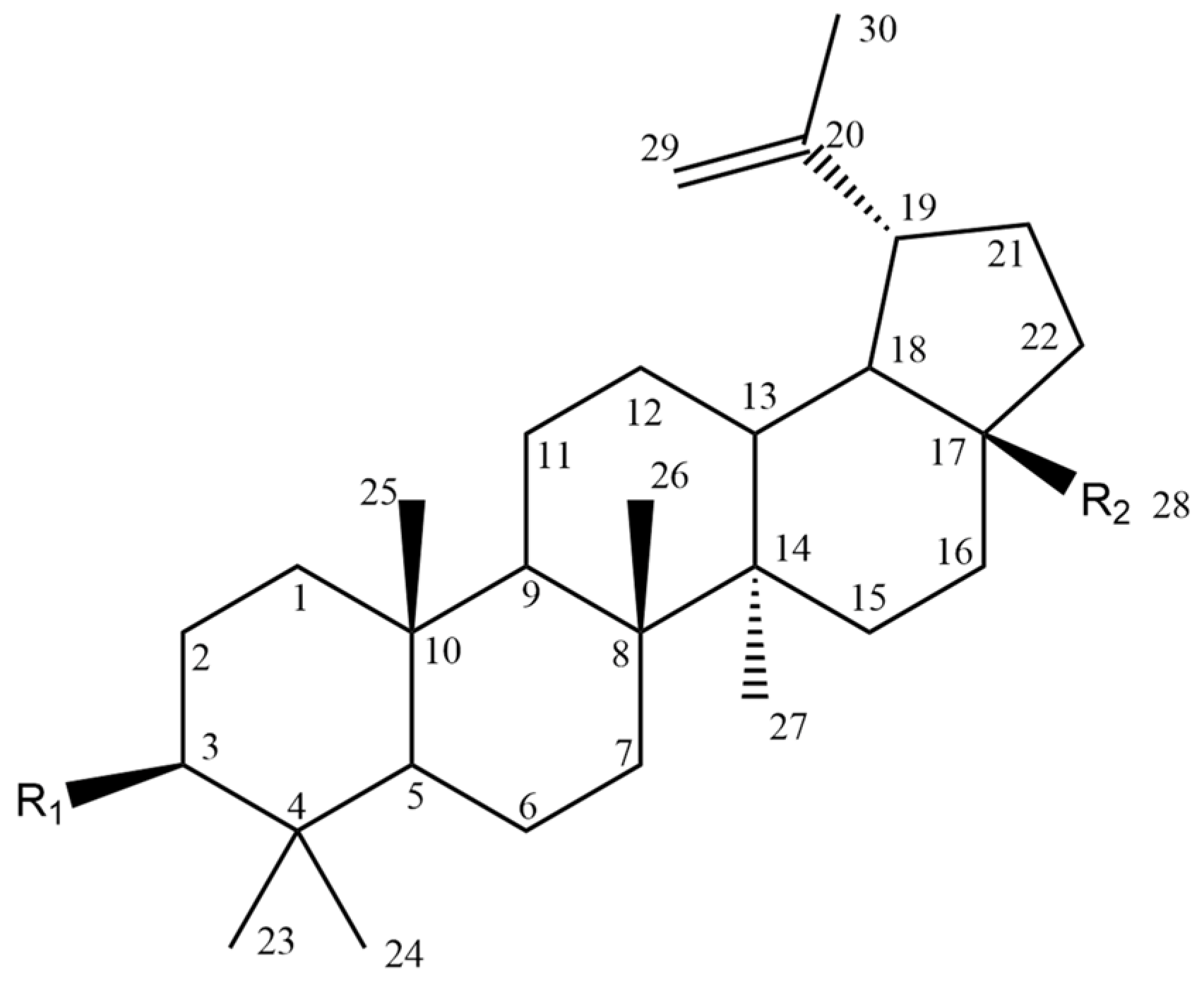
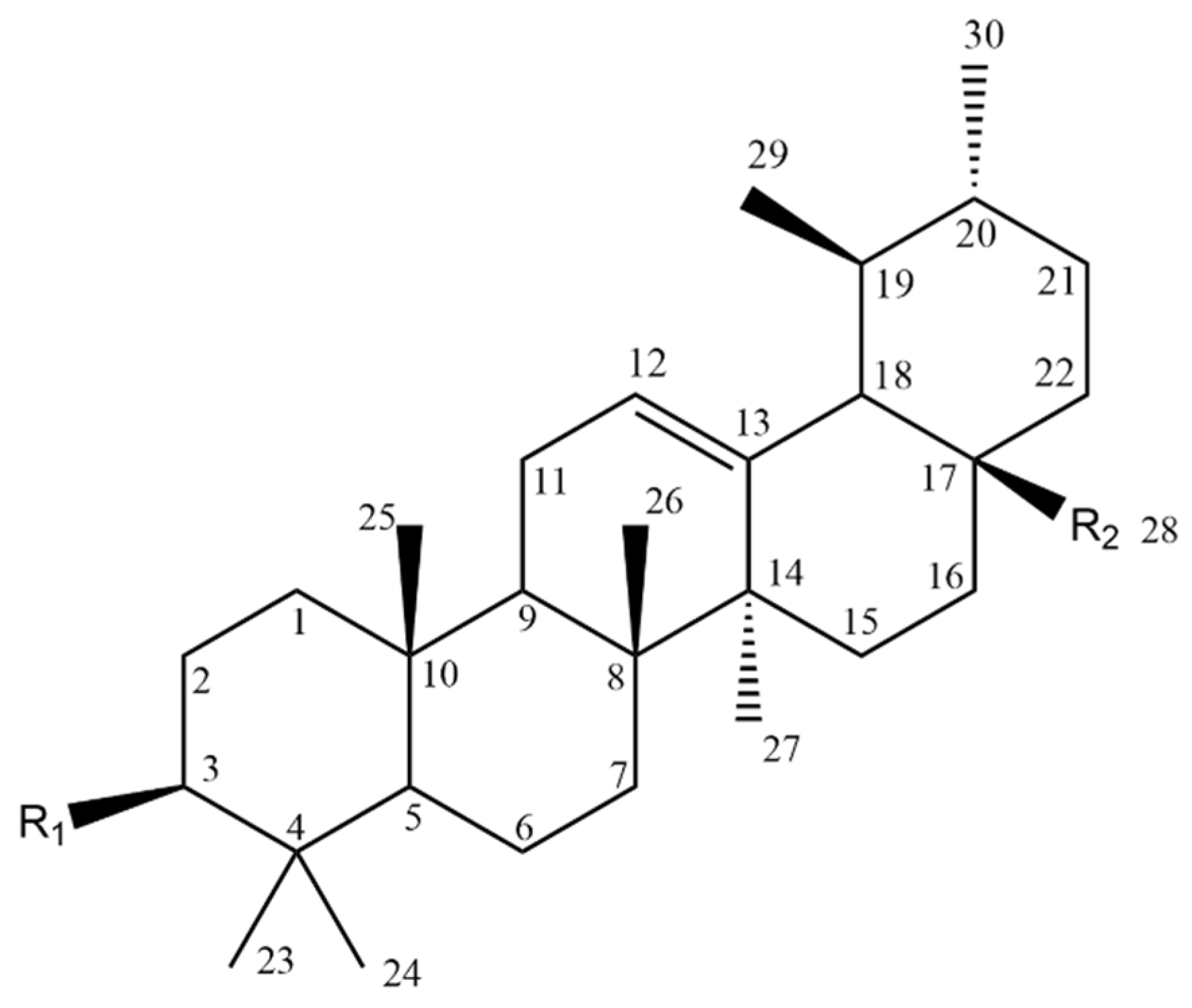
| Compound | Figure 2 Substituents | |
|---|---|---|
| R1 | R2 | |
| 1 |  |  |
| 2 |  |  |
| 3 |  | 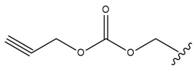 |
| 4 |  | 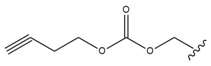 |
| 5 |  | 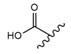 |
| 6 |  |  |
| 7 |  | 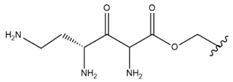 |
| 8 |  |  |
| 9 |  | 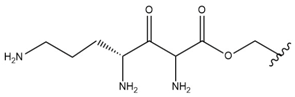 |
| 10 |  | 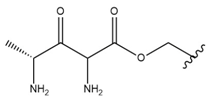 |
| 11 | 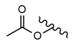 |  |
| 12 | 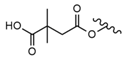 |  |
| 13 |  |  |
| 14 | 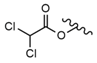 |  |
| 15 |  | 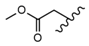 |
| 16 |  | 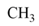 |
| 17 | 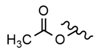 | 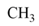 |
| 18 | 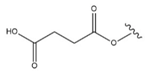 | 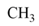 |
| 19 | 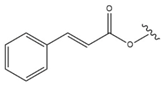 | 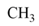 |
| 20 |  | 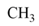 |
| 21 |  | 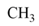 |
| 22 |  | 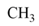 |
| 23 |  | 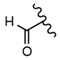 |
| 24 |  |  |
| 25 | 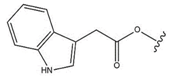 |  |
| Compound | Figure 8 Substituents | |
|---|---|---|
| R1 | R2 | |
| 26 |  |  |
| 27 | 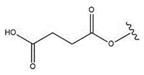 | 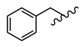 |
| 28 |  |  |
| 29 | 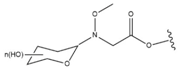 |  |
| 30 |  | 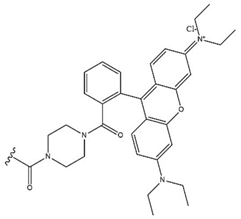 |
| Compound | Figure 10 Substituents | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| 31 |  |  |  |
| 32 |  |  | 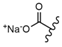 |
| 33 |  |  | 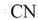 |
| 34 |  |  | 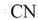 |
| 35 |  |  | 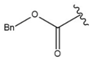 |
| 36 |  |  |  |
| Compound | Figure 12 Substituents |
|---|---|
| R1 | |
| 37 |  |
| 38 |  |
| 39 | 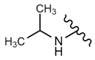 |
| 40 |  |
| 41 |  |
| 42 | 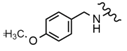 |
| 43 | 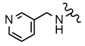 |
| 44 | 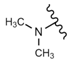 |
| 45 | 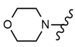 |
| 46 | 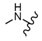 |
| 47 | 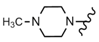 |
| 48 |  |
| 49 | 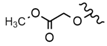 |
| 50 | 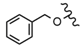 |
| 51 | 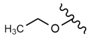 |
| 52 | 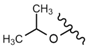 |
| Compound | Figure 13 Substituents | |
|---|---|---|
| R1 | R2 | |
| 53 |  |  |
| 54 | 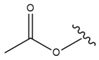 |  |
| 55 |  | 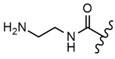 |
| 56 |  |  |
| 57 |  | 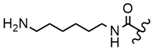 |
| 58 |  | 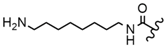 |
| 59 |  | 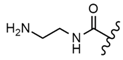 |
| 60 |  | 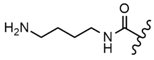 |
| 61 |  | 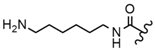 |
| 62 |  | 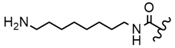 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudzińska, M.; Stachnik, B.; Galanty, A.; Sołtys, A.; Podolak, I. Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules 2023, 28, 7763. https://doi.org/10.3390/molecules28237763
Grudzińska M, Stachnik B, Galanty A, Sołtys A, Podolak I. Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules. 2023; 28(23):7763. https://doi.org/10.3390/molecules28237763
Chicago/Turabian StyleGrudzińska, Marta, Bogna Stachnik, Agnieszka Galanty, Agnieszka Sołtys, and Irma Podolak. 2023. "Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications" Molecules 28, no. 23: 7763. https://doi.org/10.3390/molecules28237763
APA StyleGrudzińska, M., Stachnik, B., Galanty, A., Sołtys, A., & Podolak, I. (2023). Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules, 28(23), 7763. https://doi.org/10.3390/molecules28237763






