Transition-Metal-Free One-Pot Synthesis of Fused Benzofuranamines and Benzo[b]thiophenamines
Abstract
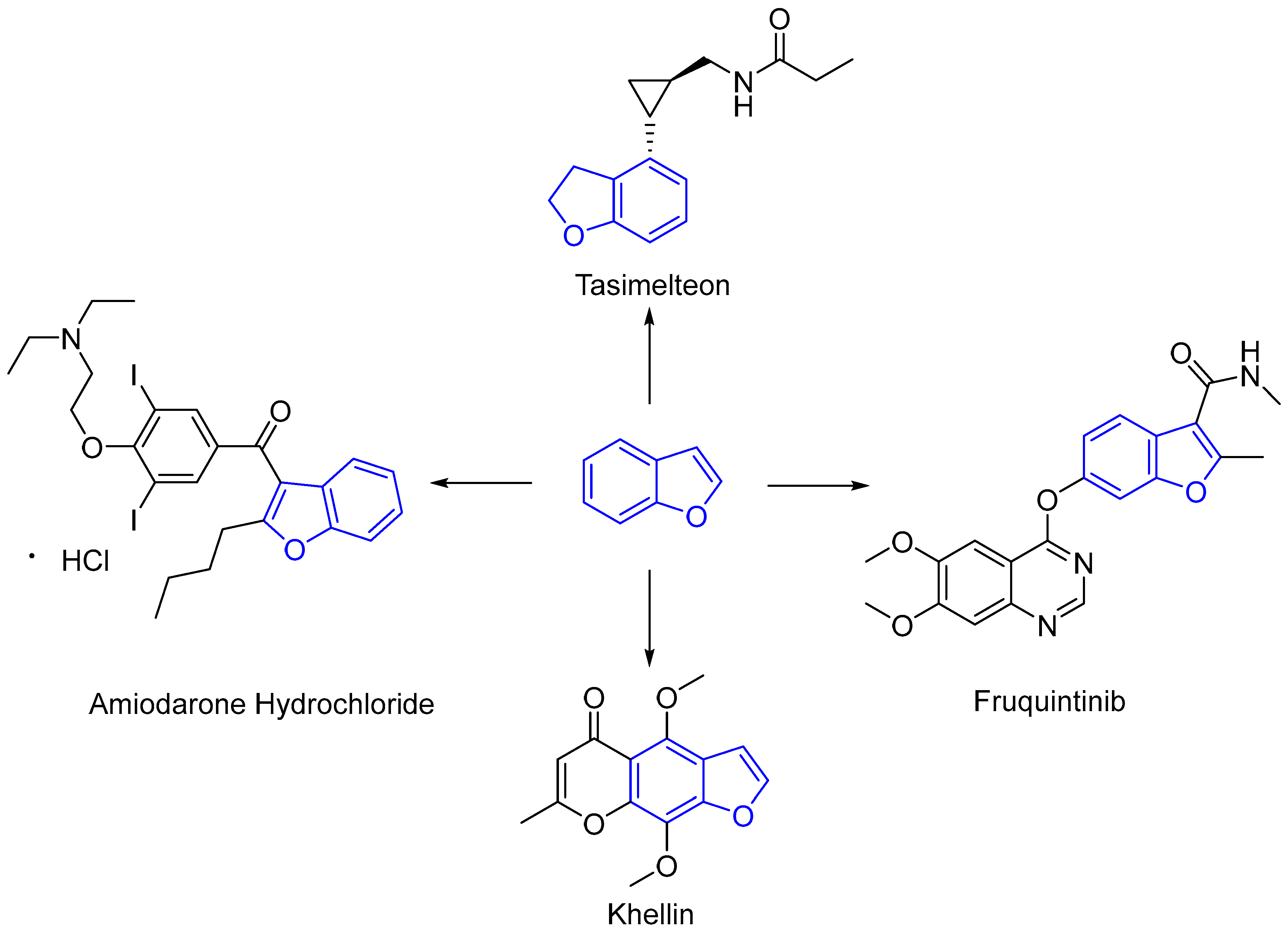
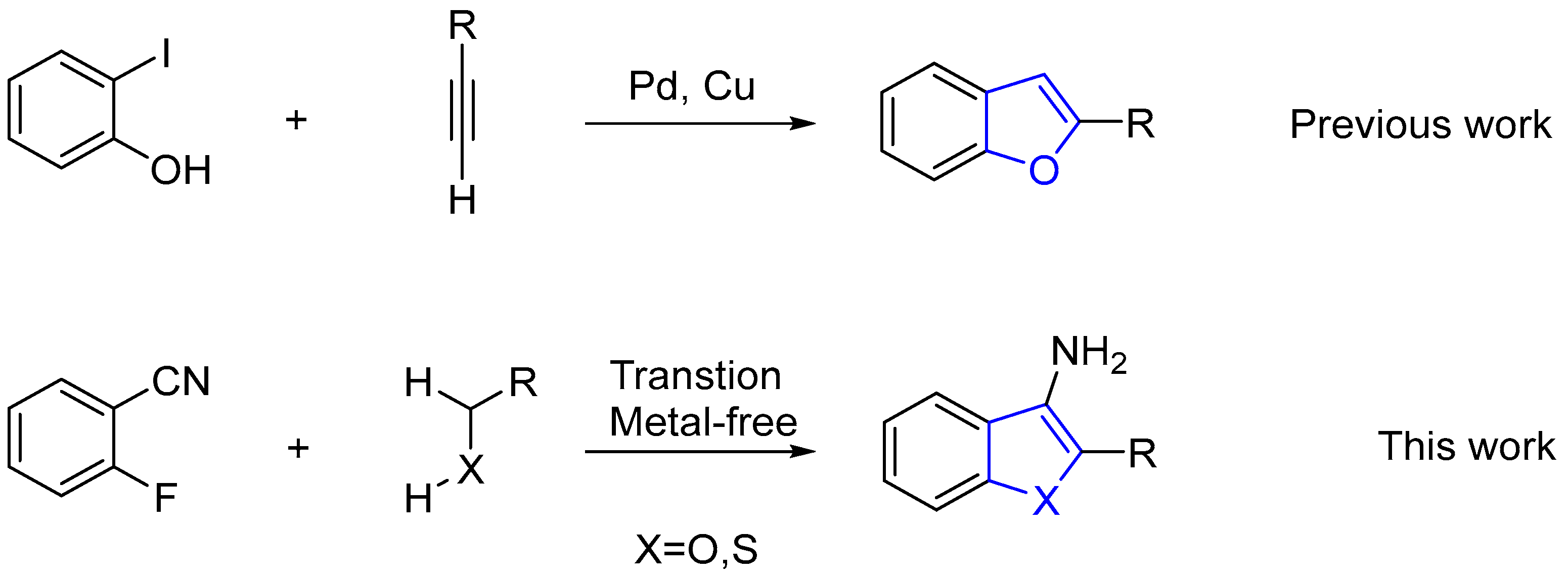
:1. Introduction
2. Results
3. Experimental Section
3.1. General
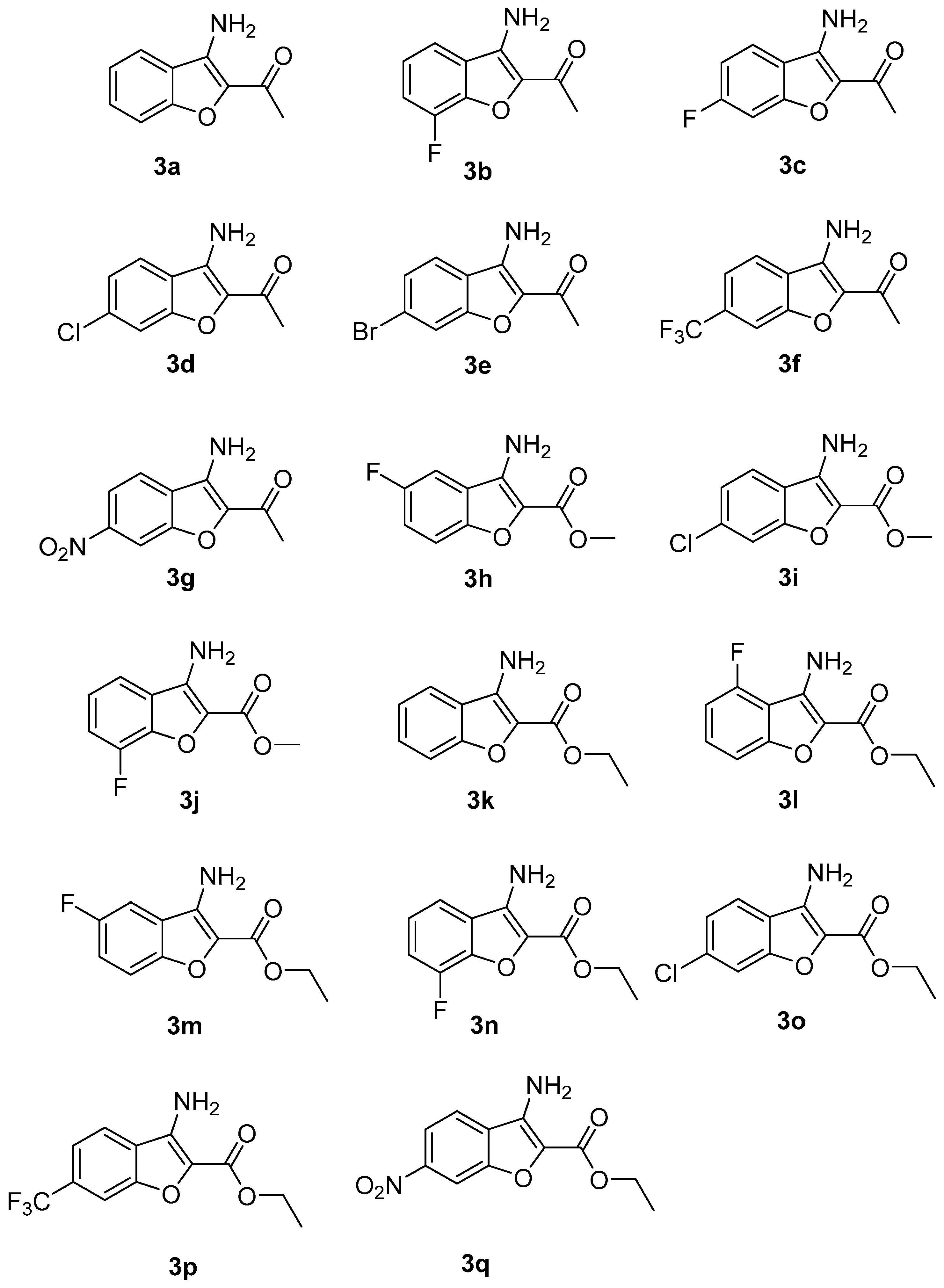
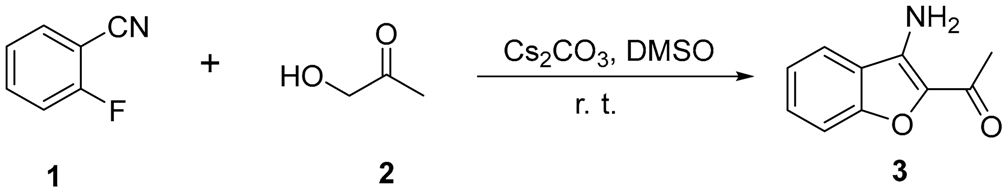
3.2. General Experimental Procedure for 1-(3-Aminobenzofuran-2-yl)ethan-1-one (3a)
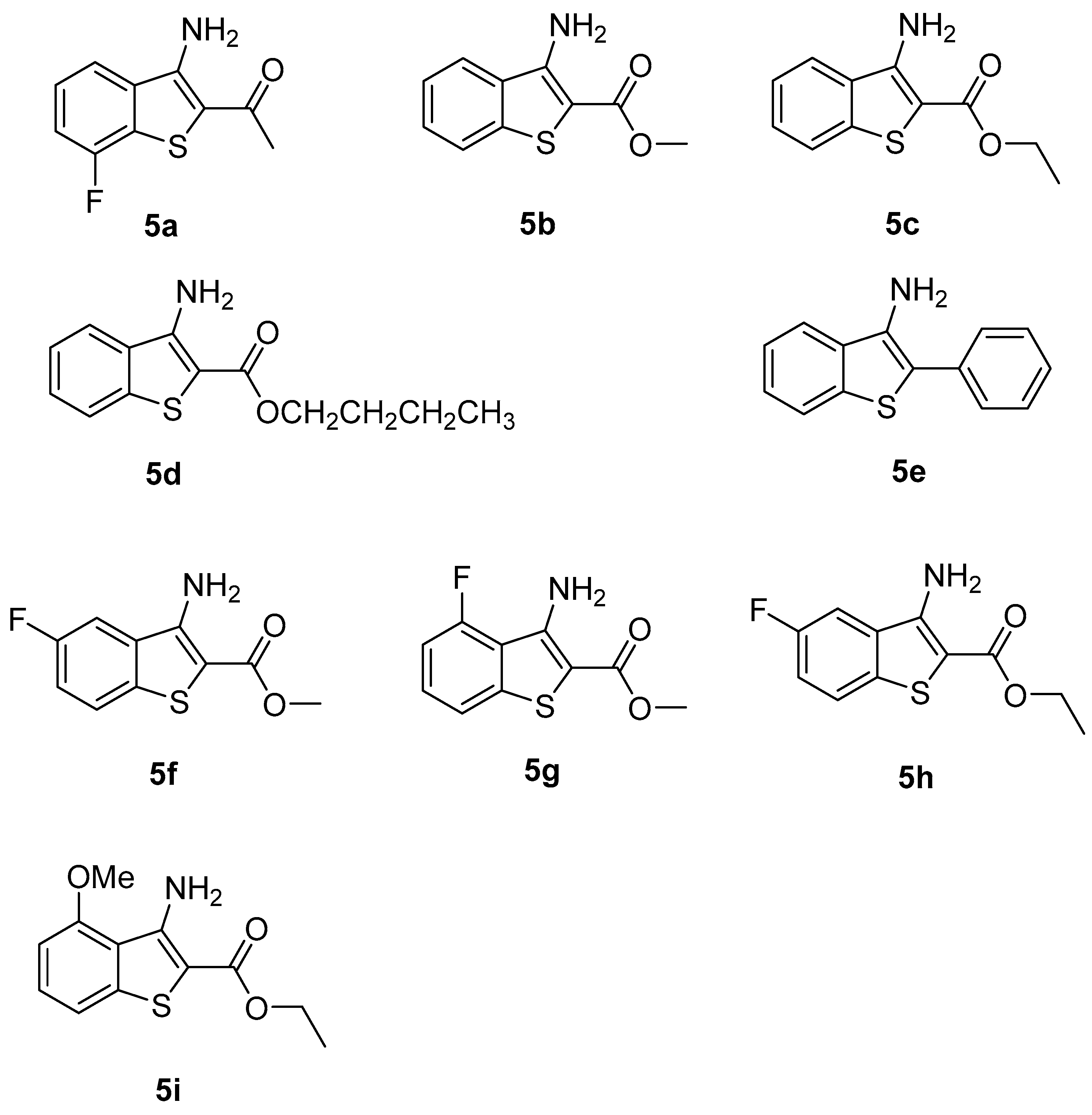
3.3. General Experimental Procedure for 1-(3-Amino-7-fluorobenzo[b]thiophen-2-yl)ethan-1-one (5a)
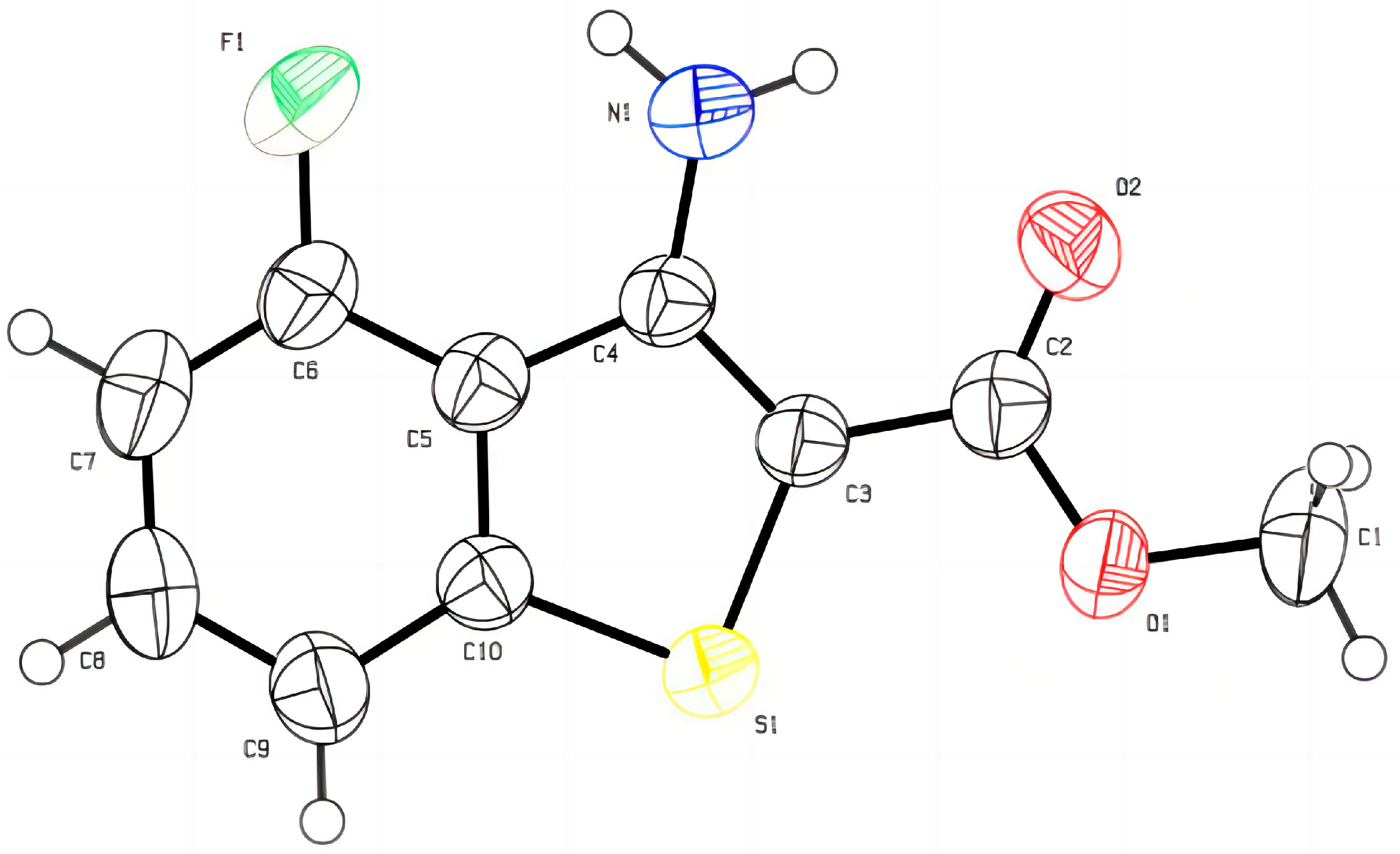
3.4. X-ray Crystal Structure Analysis of Compound 5g [24,25]
3.5. Characterization Data
- 1-(3-Amino-7-fluorobenzofuran-2-yl)ethan-1-one (3b): 155 mg (80% yield), white solid, mp: 188–189 °C; 1H NMR (CDCl3, 300 MHz): δ 7.35 (dd, J = 1.5, 7.5 Hz, 1H), 7.25–7.14 (m, 2H), 5.31 (s, 1H), 2.53 (s, 3H); 13C NMR (CDCl3, 75 MHz): δ 190.0, 148.6 (JC,F = 249 Hz), 141.4 (JC,F = 13 Hz), 138.1, 136.1, 124.8, 122.8 (JC,F = 6 Hz), 115.7, 115.1 (JC,F = 16 Hz), 25.97; FT-HRMS (ESI) calcd for C10H8FNO2 [(M + H)+]: 194.0573; found, 194.0625.
- 1-(3-Amino-6-fluorobenzofuran-2-yl)ethan-1-one (3c): 158 mg (82% yield), white solid, mp: 188–189 °C; 1H NMR (CDCl3, 400 MHz): δ 7.56 (t, J = 8.0 Hz, 1H), 6.79–6.71 (m, 2H), 4.70 (s, 2H), 3.83 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 163.1, 159.7 (JC,F = 257 Hz), 157.9 (JC,F = 11 Hz), 129.70, 109.3, 106.7, 98.5 (JC,F = 23 Hz), 89.6 (JC,F = 16 Hz), 26.0; FT-HRMS (ESI) calcd for C10H8FNO2 [(M + H)+]: 194.0573; found, 194.0653
- 1-(3-Amino-6-chlorobenzofuran-2-yl)ethan-1-one (3d): 163 mg (78% yield), white solid, mp: 217–219 °C; 1H NMR (CDCl3, 400 MHz): δ 7.50 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 1.6 Hz, 1H), 7.24 (dd, J = 1.6, 8.4 Hz, 1H), 5.58 (s, 2H), 2.50 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 189.7, 153.9, 137.8, 135.4, 123.3, 120.9, 120.0, 113.1, 110.0, 25.9; FT-HRMS (ESI) calcd for C10H8ClNO2 [(M + H)+]: 211.0214; found, 211.0265.
- 1-(3-Amino-6-bromobenzofuran-2-yl)ethan-1-one (3e): 142 mg (56% yield), white solid, mp: 247–249 °C; 1H NMR (CDCl3, 400 MHz): δ 7.61 (d, J = 1.2 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.37 (dd, J = 1.6, 8.4 Hz, 1H), 5.56 (s, 2H), 2.49 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 189.8, 154.0, 137.8, 135.6, 125.9, 123.1, 121.1, 120.3, 116.1, 26.0; FT-HRMS (ESI) calcd for C10H8BrNO2 [(M + H)+]: 254.9718; found, 254.9816.
- 1-(3-Amino-6-(trifluoromethyl)benzofuran-2-yl)ethan-1-one (3f): 219 mg (90% yield), white solid, mp: 203–204 °C; 1H NMR (CDCl3, 400 MHz): δ 7.70 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.4 Hz, 1H), 5.60 (s, 2H), 2.53 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 190.3, 152.8, 137.1, 136.6, 131.5 (JC,F = 32 Hz), 130.8 (JC,F = 33 Hz) 125.3, 124.1, 122.6, 119.0 (JC,F = 3 Hz), 110.3 (JC,F = 4 Hz), 26.1; FT-HRMS (ESI) calcd for C11H8F3NO2 [(M + H)+]: 244.0541; found, 244.0645.
- 1-(3-Amino-6-nitrobenzofuran-2-yl)ethan-1-one (3g): 178 mg (81% yield), white solid, mp none; 1H NMR (CDCl3, 400 MHz): δ 8.35 (d, J = 1.6 Hz, 1H), 8.16 (dd, J = 2.0, 8.4 Hz, 1H), 7.72 (d, J = 8.8 Hz, 1H), 5.59 (s, 2H), 2.56 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 190.5, 152.3, 148.3, 138.2, 136.5, 126.4, 120.6, 117.5, 109.1, 26.2; FT-HRMS (ESI) calcd for C10H8N2O4 [(M + H)+]: 221.0518; found, 221.0589.
- Methyl 3-amino-5-fluorobenzofuran-2-carboxylate (3h): 161 mg (77% yield), white solid, mp: 180–182 °C; 1H NMR (CDCl3, 400 MHz): δ 7.40–7.34 (m, 1H), 7.23 (d, J = 8.0 Hz, 1H), 6.90–6.86 (m, 1H), 5.21 (s, 2H), 3.96 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 163.4, 161.5, 157.3 (JC,F = 250 Hz), 129.4 (JC,F = 9 Hz), 111.1 (JC,F = 18 Hz), 108.7 (JC,F = 5 Hz), 107.8 (JC,F = 18 Hz), 51.5; FT-HRMS (ESI) calcd for C10H8FNO3 [(M + H)+]: 210.0522; found, 210.0539.
- Methyl 3-amino-6-chlorobenzofuran-2-carboxylate (3i): 173 mg (72% yield), white solid, mp: 210–211 °C; 1H NMR (CDCl3, 400 MHz): δ 7.49–7.45 (m, 2H), 7.24 (dd, J = 1.6, 8.4 Hz, 1H), 4.99 (s, 2H), 3.97 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.6, 153.9, 138.2, 134.9, 125.9, 123.3, 120.4, 120.2, 113.0, 110.0, 103.1, 51.6; FT-HRMS (ESI) calcd for C10H8ClNO3 [(M + H)+]: 227.0163; found, 227.0195.
- Methyl 3-amino-7-fluorobenzofuran-2-carboxylate (3j): 164 mg (78% yield), white solid, mp: 180–182 °C; 1H NMR (CDCl3, 400 MHz): δ 7.35–7.32 (m, 1H), 7.22–7.17 (m, 2H), 5.02 (s, 2H), 3.97 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.7, 148.4 (JC,F = 250 Hz), 141.4 (JC,F = 13 Hz), 138.6, 126.2, 125.0 (JC,F = 3 Hz), 123.0 (JC,F = 6 Hz), 115.2 (JC,F = 4 Hz), 114.7 (JC,F = 16 Hz), 51.6; FT-HRMS (ESI) calcd for C10H8FNO3 [(M + H)+]: 210.0522; found, 210.0598.
- Ethyl 3-aminobenzofuran-2-carboxylate (3k): 146 mg (71% yield), white solid, mp: 179–180 °C; 1H NMR (CDCl3, 300 MHz): δ 7.57–7.54 (m, 1H), 7.46–7.44 (m, 2H), 7.27–7.22 (m, 2H), 4.96 (s, 1H), 4.45 (q, J = 6.9 Hz, 2H), 1.44 (t, J = 6.9 Hz, 3H); 13C NMR (CDCl3, 75 MHz): δ 161.67, 154.02, 130.91, 128.85, 128.77, 125.56, 122.30, 121.67, 119.59, 112.66, 65.58, 60.46, 30.59, 29.71, 19.19, 14.66, 13.72; FT-HRMS (ESI) calcd for C11H11NO3 [(M + H)+]: 206.0772; found, 206.0785.
- Ethyl 3-amino-4-fluorobenzofuran-2-carboxylate (3l): 162 mg (73% yield), white solid, mp: 192–193 °C; 1H NMR (CDCl3, 400 MHz): δ 7.39–7.34 (m, 1H), 7.24 (d, J = 8.4 Hz, 1H), 6.88 (dd, J = 8.0, 9.6 Hz, 1H), 5.19 (s, 2H), 4.44 (q, J = 7.2 Hz, 2H), 1.44 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.4, 157.3 (JC,F = 250 Hz), 155.2, 129.3 (JC,F = 7 Hz), 111.1 (JC,F = 20 Hz), 108.8 (JC,F = 5 Hz), 107.7, (JC,F = 18 Hz), 60.5, 14.6; FT-HRMS (ESI) calcd for C11H10FNO3 [(M + H)+]: 224.0678; found, 224.0693.
- Ethyl 3-amino-5-fluorobenzofuran-2-carboxylate (3m): 179 mg (80% yield), white solid, mp: 192–193 °C; 1H NMR (CDCl3, 400 MHz): δ 7.44 (d, J = 8.0 Hz, 1H), 7.37–7.32 (m, 1H), 6.94 (dd, J = 8.0, 9.6 Hz, 1H), 6.31 (s, 2H), 4.34 (q, J = 7.2 Hz, 2H), 1.38 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.2, 159.6 (JC,F = 250 Hz), 147.6, 142.1, 128.8 (JC,F = 9 Hz), 120.3 (JC,F = 14 Hz), 119.2 (JC,F = 4 Hz), 109.3 (JC,F = 20 Hz), 97.6, 60.4, 14.49; FT-HRMS (ESI) calcd for C11H10FNO3 [(M + H)+]: 224.0678; found, 224.0695.
- Ethyl 3-amino-7-fluorobenzofuran-2-carboxylate (3n): 181 mg (81% yield), white solid, mp: 192–193 °C; 1H NMR (CDCl3, 400 MHz): δ 7.34–7.32 (m, 1H), 7.22–7.17 (m, 2H), 5.00 (s, 2H), 4.45 (q, J = 7.2 Hz, 2H), 1.44 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.4, 148.4 (JC,F = 250 Hz), 141.4 (JC,F = 13 Hz), 138.4, 126.5, 125.1 (JC,F = 3 Hz), 122.8 (JC,F = 6 Hz), 115.1 (JC,F = 4 Hz), 114.5 (JC,F = 15 Hz), 60.6, 14.5; FT-HRMS (ESI) calcd for C11H10FNO3 [(M + H)+]: 224.0678; found, 224.0689.
- Ethyl 3-amino-6-chlorobenzofuran-2-carboxylate (3o): 177 mg (74% yield), white solid, mp: 221–222 °C; 1H NMR (CDCl3, 400 MHz): δ 7.47 (d, J = 8.8 Hz, 2H), 7.24 (dd, J = 2.0, 8.4 Hz, 1H), 4.97 (s, 2H), 4.44 (q, J = 7.2 Hz, 2H), 1.44 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.4, 153.9, 134.7, 123.3, 120.3, 113.0, 60.6, 14.6; FT-HRMS (ESI) calcd for C11H10ClNO3 [(M + H)+]: 241.0320; found.
- Ethyl 3-amino-6-(trifluoromethyl)benzofuran-2-carboxylate (3p): 246 mg (90% yield), white solid, mp: 207–208 °C; 1H NMR (CDCl3, 400 MHz): δ 7.73 (s, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 5.02 (s, 2H), 4.46 (q, J = 7.2 Hz, 2H), 1.45 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 161.3, 152.8, 137.5, 131.1, 130.8, 130.3 (JC,F = 30 Hz), 128.0, 127.3, 125.3, 124.4, 122.6, 120.4, 119.1 (JC,F = 3 Hz), 110.2 (JC,F = 4 Hz), 60.8, 14.6; FT-HRMS (ESI) calcd for C12H10F3NO3 [(M + H)+]: 274.0646; found, 274.0686.
- Ethyl 3-amino-6-nitrobenzofuran-2-carboxylate (3q): 218 mg (87% yield), white solid, mp none; 1H NMR (CDCl3, 400 MHz): δ 7.68 (d, J = 1.2 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.20 (dd, J = 1.6, 8.4 Hz, 1H), 5.88 (s, 2H), 4.35 (q, J = 7.2 Hz, 2H), 1.38 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.2, 162.6, 147.7, 140.9, 134.4, 129.8, 124.7, 122.9, 122.0, 99.8, 60.6, 14.5; FT-HRMS (ESI) calcd for C11H10N2O5 [(M + H)+]: 251.0623; found, 251.0635.
- Methyl 3-aminobenzo[b]thiophene-2-carboxylate (5b): 157 mg (76% yield), pale yellow solid, mp: 224–225 °C; 1H NMR (CDCl3, 300 MHz): δ 7.73 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.49–7.44 (m, 1H), 7.39–7.34 (m, 1H), 5.80 (s, 2H), 3.89 (s, 3H); 13C NMR (CDCl3, 75 MHz): δ 165.9, 148.5, 140.0, 131.3, 128.2, 123.9, 123.4, 121.2, 99.0, 51.5; FT-HRMS (ESI) calcd for C10H9NO2S [(M + H)+]: 208.0388; found, 208.0398.
- Ethyl 3-aminobenzo[b]thiophene-2-carboxylate (5c): 164 mg (74% yield), pale yellow solid, mp: 235–237 °C; 1H NMR (CDCl3, 300 MHz): δ 7.72 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 8.1 Hz, 1H), 7.49–7.43 (m, 1H), 7.39–7.34 (m, 1H), 5.61 (s, 1H), 4.36 (q, J = 7.2 Hz, 2H), 1.39 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 75 MHz): δ 165.6, 148.3, 140.0, 131.5, 128.1, 123.8, 123.4, 121.2, 99.5, 60.4, 14.5; FT-HRMS (ESI) calcd for C11H11NO2S [(M + H)+]: 222.0544; found, 222.0609.
- Butyl 3-aminobenzo[b]thiophene-2-carboxylate (5d): 192 mg (77% yield), pale yellow solid, mp: 258–259 °C; 1H NMR (CDCl3, 300 MHz): δ 7.73–7.66 (m, 2H), 7.48–7.42 (m, 1H), 7.38–7.33 (m, 1H), 5.26 (s, 2H), 4.30 (t, J = 6.6 Hz, 2H), 1.80–––1.69 (m, 2H), 1.54–1.41 (m, 2H), 0.98 (t, J = 7.5 Hz, 3H); 13C NMR (CDCl3, 75 MHz): δ 165.6, 147.9, 140.0, 131.5, 128.2, 123.9, 123.4, 121.2, 100.0, 64.3, 30.9, 19.3, 13.8; FT-HRMS (ESI) calcd for C13H15NO2S [(M + H)+]: 250.0857; found, 250.0903.
- 2-Phenylbenzo[b]thiophen-3-amine (5e): 191 mg (85% yield), pale yellow solid, mp: 253–255 °C; 1H NMR (CDCl3, 300 MHz): δ 7.78 (dd, J = 1.5, 6.9 Hz, 1H), 7.64–7.57 (m, 3H), 7.49–7.44 (m, 2H), 7.42–7.29 (m, 3H), 4.07 (s, 2H); 13C NMR (CDCl3, 75 MHz): δ 137.6, 134.5, 134.3, 133.6, 129.2, 128.4, 127.0, 124.8, 123.9, 122.7, 119.9, 115.6; FT-HRMS (ESI) calcd for C14H11NS [(M + H)+]: 226.0646; found, 226.0649.
- Methyl 3-amino-5-fluorobenzo[b]thiophene-2-carboxylate (5f): 169 mg (75% yield), pale yellow solid, mp: 237–238 °C; 1H NMR (CDCl3, 400 MHz): δ 7.67 (dd, J = 4.8, 8.8 Hz, 1H), 7.30 (dd, J = 2.4, 8.8 Hz, 1H), 7.25–7.21 (m, 1H), 5.83 (s, 2H), 3.90 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.6, 160.4 (JC,F = 242 Hz), 147.8, 136.4, 135.2, 132.2 (JC,F = 8 Hz), 130.7, 124.8 (JC,F = 8 Hz), 117.2 (JC,F = 25 Hz), 109.9, 106.9 (JC,F = 23 Hz), 101.2, 51.7; FT-HRMS (ESI) calcd for C10H8FNO2S [(M + H)+]: 226.0293; found, 226.0356.
- Methyl 3-amino-4-fluorobenzo[b]thiophene-2-carboxylate (5g): 171 mg (76% yield), pale yellow solid, mp: 237–238 °C; 1H NMR (CDCl3, 400 MHz): δ 7.44 (d, J = 8.0 Hz, 1H), 7.37–7.32 (m, 1H), 7.17 (t, J = 8.8 Hz, 1H), 5.92 (s, 2H), 3.90 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.6, 157.9 (JC,F = 246 Hz), 148.3, 134.5 (JC,F = 10 Hz), 127.1 (JC,F = 20 Hz), 125.4 (JC,F = 7 Hz), 117.1, 117.0, 113.2 (JC,F = 18 Hz), 99.9, 53.4, 51.7; FT-HRMS (ESI) calcd for C10H8FNO2S [(M + H)+]: 226.0293; found, 226.0348.
- Ethyl 3-amino-5-fluorobenzo[b]thiophene-2-carboxylate (5h): 179 mg (75% yield), pale yellow solid, mp: 249–250 °C; 1H NMR (CDCl3, 400 MHz): δ 7.67 (dd, J = 4.8, 8.8 Hz, 1H), 7.30 (dd, J = 2.0, 8.8 Hz, 1H), 7.25–7.21 (m, 1H), 5.80 (s, 2H), 4.36 (q, J = 7.2 Hz, 2H), 1.40 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.3, 160.4 (JC,F = 242 Hz), 147.6, 135.2, 132.3 (JC,F = 8 Hz), 124.8 (JC,F = 9 Hz), 117.1 (JC,F = 25 Hz), 106.9 (JC,F = 23 Hz), 60.6, 14.5; FT-HRMS (ESI) calcd for C11H10FNO2S [(M + H)+]: 240.0450; found, 240.0468.
- Ethyl 3-amino-4-methoxybenzo[b]thiophene-2-carboxylate (5i): 78 mg (31% yield), pale yellow solid, mp: 282–283 °C; 1H NMR (CDCl3, 400 MHz): δ 7.37–7.28 (m, 1H), 7.24 (d, J = 4.0 Hz, 1H), 6.75 (s, 2H), 6.71–6.64 (m, 1H), 4.32 (q, J = 8.0 Hz, 2H), 2.62 (s, 3H), 1.37 (t, J = 8.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 165.5, 157.7, 148.8, 142.0, 129.0, 120.9, 115.8, 104.0, 60.0, 55.6, 14.5; FT-HRMS (ESI) calcd for C12H13NO3S [(M + H)+]: 252.0650; found, 252.0687.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.A. An overview of benzo [b] thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- de Brito, D.H.A.; Almeida-Neto, F.W.Q.; Ribeiro, L.R. Synthesis, structural and spectroscopic analysis, and antiproliferative activity of chalcone derivate (E)-1-(4-aminophenyl)-3-(benzo [b] thiophen-2-yl) prop-2-en-1-one in Trypanosoma cruzi. J. Mol. Struct. 2022, 1253, 132197. [Google Scholar] [CrossRef]
- Algso, M.A.S.; Kivrak, A. New strategy for the synthesis of 3-ethynyl-2-(thiophen-2-yl) benzo [b] thiophene derivatives. Chem. Pap. 2019, 73, 977–985. [Google Scholar] [CrossRef]
- Dhillon, S.; Clarke, M. Tasimelteon: First global approval. Drugs 2014, 74, 505–511. [Google Scholar] [CrossRef]
- Mhoumadi, A.; Elkhashab, M.; Prillieux, S.; Dumas, J.; Collas, F.; Louvain, N.; Fraisse, B.; Espeau, P. Characterization of the heat behavior of amiodarone hydrochloride. Thermochim. Acta 2022, 708, 179121. [Google Scholar] [CrossRef]
- McGovern, B.; Garan, H.; Kelly, E.; Ruskin, J.N. Adverse reactions during treatment with amiodarone hydrochloride. Br. Med. J. (Clin. Res. Ed.) 1983, 287, 175–180. [Google Scholar] [CrossRef]
- Shirley, M. Fruquintinib: First global approval. Drugs 2018, 78, 1757–1761. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.H. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef]
- Valkova, S.; Trashlieva, M.; Christova, P. Treatment of vitiligo with local khellin and UVA: Comparison with systemic PUVA. Clin. Exp. Dermatol. 2004, 29, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel Benzo[b]thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Zhou, S.; Wang, L.; Zhang, Y.; Wang, J. Pd-catalyzed cyclization and carbene migratory insertion: New approach to 3-vinylindoles and 3-vinylbenzofurans. Org. Lett. 2013, 15, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, C.; Cui, M.; Du, M.; Li, W.; Jia, Z.; Zhao, Q. Synthesis of difluoroalkylated benzofuran, benzothiophene, and indole derivatives via palladium-catalyzed cascade difluoroalkylation and arylation of 1, 6-enynes. Org. Lett. 2020, 22, 1149–1154. [Google Scholar] [CrossRef]
- Liao, J.; Fan, L.; Guo, W.; Zhang, Z.; Li, J.; Zhu, C.; Ren, Y.; Wu, W.; Jiang, H. Palladium-Catalyzed Fluoroalkylative Cyclization of Olefins. Org. Lett. 2017, 19, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Kuram, M.R.; Bhanuchandra, M.; Sahoo, A.K. Direct access to benzo [b] furans through Palladium-catalyzed oxidative annulation of phenols and unactivated internal alkynes. Angew. Chem. Int. Ed. 2013, 52, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Rong, N.; Li, P.; Shen, G.; Li, Q.; Xin, N.; Cui, C.; Cui, J.; Yang, B.; Li, D.; et al. AIBN-promoted synthesis of bibenzo [b][1, 4] thiazines by the condensation of 2, 2′-dithiodianiline with methyl aryl ketones. Org. Lett. 2018, 20, 3332–3336. [Google Scholar] [CrossRef]
- Wang, D.; Lu, Q.; Li, Z.; Fang, C.; Liu, R.; Yang, B.; Shen, G. “One-Pot” CuCl2-Mediated Condensation/C–S Bond Coupling Reactions to Synthesize Dibenzothiazepines by Bi-Functional-Reagent N, N′-Dimethylethane-1, 2-Diamine. Molecules 2022, 27, 7392. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Li, Z.; Chen, C.; Li, J.; Zhang, Y.; Shen, G.; Yang, B.; Hu, X.; Huang, X. Metal-and oxidant-free electrochemically promoted oxidative coupling of amines. RSC Adv. 2022, 12, 118–122. [Google Scholar] [CrossRef]
- Khan, I.; Zaib, S.; Ibrar, A. New frontiers in the transition-metal-free synthesis of heterocycles from alkynoates: An overview and current status. Org. Chem. Front. 2020, 7, 3734–3791. [Google Scholar] [CrossRef]
- Suleymanov, A.A.; Scopelliti, R.; Fadaei Tirani, F.; Severin, K. One-Pot Synthesis of Trisubstituted Triazenes from Grignard Reagents and Organic Azides. Org. Lett. 2018, 20, 3323–3326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, C.; Yang, B.; Cao, X.; Ma, C. A one-pot transition-metal-free tandem process to 1, 4-benzodiazepine scaffolds. Synthesis 2013, 45, 111–117. [Google Scholar]
- Crystal Data for 5g: See the Supporting Information. CCDC 2285416 (5g) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crysallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 31 July 2023).
- Sheldrick, G.M. SHELX-97. Program for the Refinement of Crystal Structure; University of Götingen: Götingen, Germany, 1997. [Google Scholar]
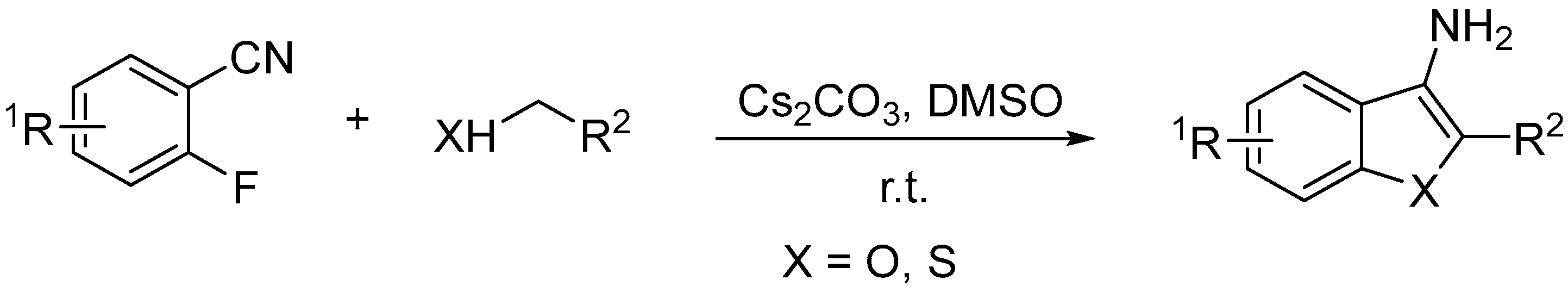

 | |||||
| Entry | Base | Solvent | T (°C) | Time (h) | Yield (%) b |
| 1 | K2CO3 | DMSO | r.t. | 4 | n.d. |
| 2 | K2CO3 | DMSO | 60 | 6 | 6 |
| 3 | K3PO4 | DMSO | r.t. | 4 | n.d. |
| 4 | K3PO4 | DMSO | 60 | 6 | 18 |
| 5 | Cs2CO3 | DMSO | r.t. | 4 | 76 |
| 6 | KOH | DMSO | r.t. | 4 | 36 |
| 7 | t-BuOK | DMSO | r.t. | 1 | 56 |
| 8 | Et3N | DMSO | r.t. | 5 | n.d. |
| 9 | Cs2CO3 | THF | r.t. | 4 | n.d. |
| 10 | Cs2CO3 | CH3CN | r.t. | 4 | n.d. |
| 11 | Cs2CO3 | DMF | r.t. | 4 | n.d. |
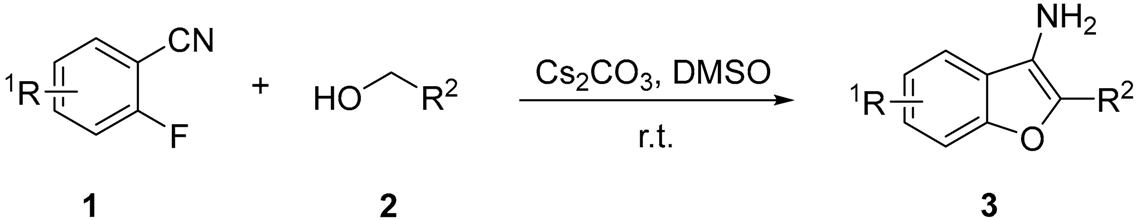 | |||||
| Entry | R1 | R2 | Time (h) | Product | Yield (%) b |
| 1 | H | -COCH3 | 6 | 3a | 76 |
| 2 | 3-F | -COCH3 | 6 | 3b | 80 |
| 3 | 4-F | -COCH3 | 5 | 3c | 82 |
| 4 | 4-Cl | -COCH3 | 6 | 3d | 78 |
| 5 | 4-Br | -COCH3 | 6 | 3e | 56 |
| 6 | 4-CF3 | -COCH3 | 6 | 3f | 90 |
| 7 | 4-NO2 | -COCH3 | 6 | 3g | 81 |
| 8 | 5-F | -COOCH3 | 6 | 3h | 77 |
| 9 | 4-Cl | -COOCH3 | 6 | 3i | 72 |
| 10 | 3-F | -COOCH3 | 6 | 3j | 78 |
| 11 | H | -COOCH2CH3 | 6 | 3k | 71 |
| 12 | 6-F | -COOCH2CH3 | 6 | 3l | 73 |
| 13 | 5-F | -COOCH2CH3 | 6 | 3m | 80 |
| 14 | 3-F | -COOCH2CH3 | 6 | 3n | 81 |
| 15 | 4-Cl | -COOCH2CH3 | 6 | 3o | 74 |
| 16 | 4-CF3 | -COOCH2CH3 | 6 | 3p | 90 |
| 17 | 4-NO2 | -COOCH2CH3 | 6 | 3q | 87 |
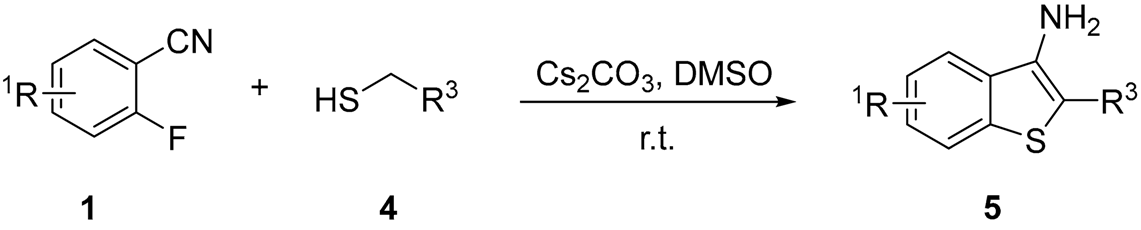 | |||||
| Entry | R1 | R3 | Time (h) | Product | Yield (%) b |
| 1 | 3-F | -COCH3 | 6 | 5a | 78 |
| 2 | H | -COOCH3 | 6 | 5b | 76 |
| 3 | H | -COOCH2CH3 | 5 | 5c | 74 |
| 4 | H | -COOCH2CH2CH2CH3 | 6 | 5d | 77 |
| 5 | H | -ph | 6 | 5e | 85 |
| 6 | 5-F | -COCH3 | 6 | 5f | 75 |
| 7 | 3-F | -COCH3 | 6 | 5g | 76 |
| 8 | 5-F | -COOCH3 | 6 | 5h | 75 |
| 9 | 4-OMe | -COOCH2CH3 | 6 | 5i | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Lv, L.; Yang, B.; Gu, Z.; Li, C.; Lv, X.; Ding, C.; Huang, X.; Yuan, D. Transition-Metal-Free One-Pot Synthesis of Fused Benzofuranamines and Benzo[b]thiophenamines. Molecules 2023, 28, 7738. https://doi.org/10.3390/molecules28237738
Liu R, Lv L, Yang B, Gu Z, Li C, Lv X, Ding C, Huang X, Yuan D. Transition-Metal-Free One-Pot Synthesis of Fused Benzofuranamines and Benzo[b]thiophenamines. Molecules. 2023; 28(23):7738. https://doi.org/10.3390/molecules28237738
Chicago/Turabian StyleLiu, Ran, Lili Lv, Bingchuan Yang, Ziyi Gu, Chenglong Li, Xueyan Lv, Chengcheng Ding, Xianqiang Huang, and Dong Yuan. 2023. "Transition-Metal-Free One-Pot Synthesis of Fused Benzofuranamines and Benzo[b]thiophenamines" Molecules 28, no. 23: 7738. https://doi.org/10.3390/molecules28237738
APA StyleLiu, R., Lv, L., Yang, B., Gu, Z., Li, C., Lv, X., Ding, C., Huang, X., & Yuan, D. (2023). Transition-Metal-Free One-Pot Synthesis of Fused Benzofuranamines and Benzo[b]thiophenamines. Molecules, 28(23), 7738. https://doi.org/10.3390/molecules28237738







