Sonosensitive Cavitation Nuclei—A Customisable Platform Technology for Enhanced Therapeutic Delivery
Abstract
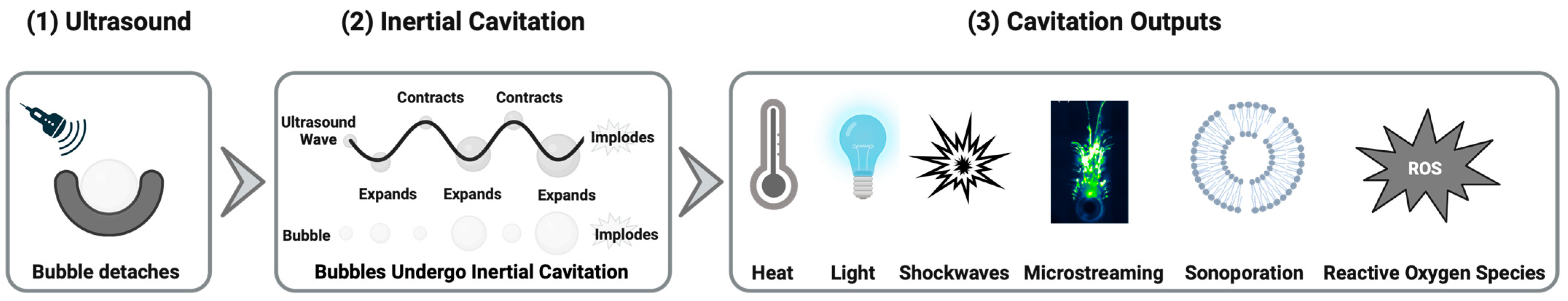
1. Introduction
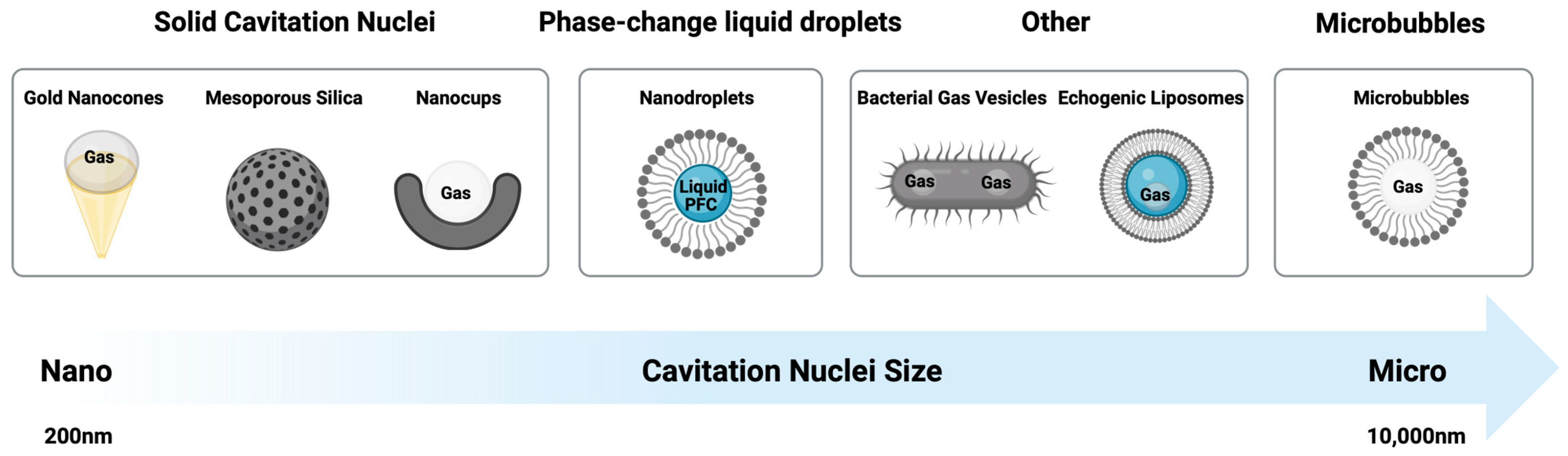
1.1. Gas-Filled Bubbles
1.2. Nanodroplets
1.3. Solid Cavitation Nuclei
1.3.1. Polymeric Nanocups
1.3.2. Gold Nanoparticles
1.3.3. Mesoporous Silica
1.4. Alternative Cavitation Nuclei
1.5. Summary
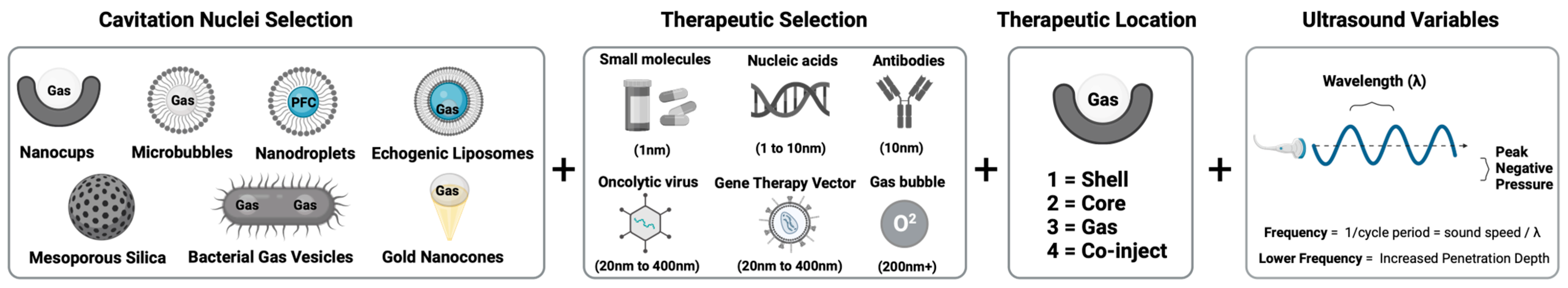
2. Composition Optimization of Cavitation Nuclei for Enhanced Delivery of Therapeutics
2.1. Co-Injection
2.2. Shell
2.3. Core
2.4. Gas
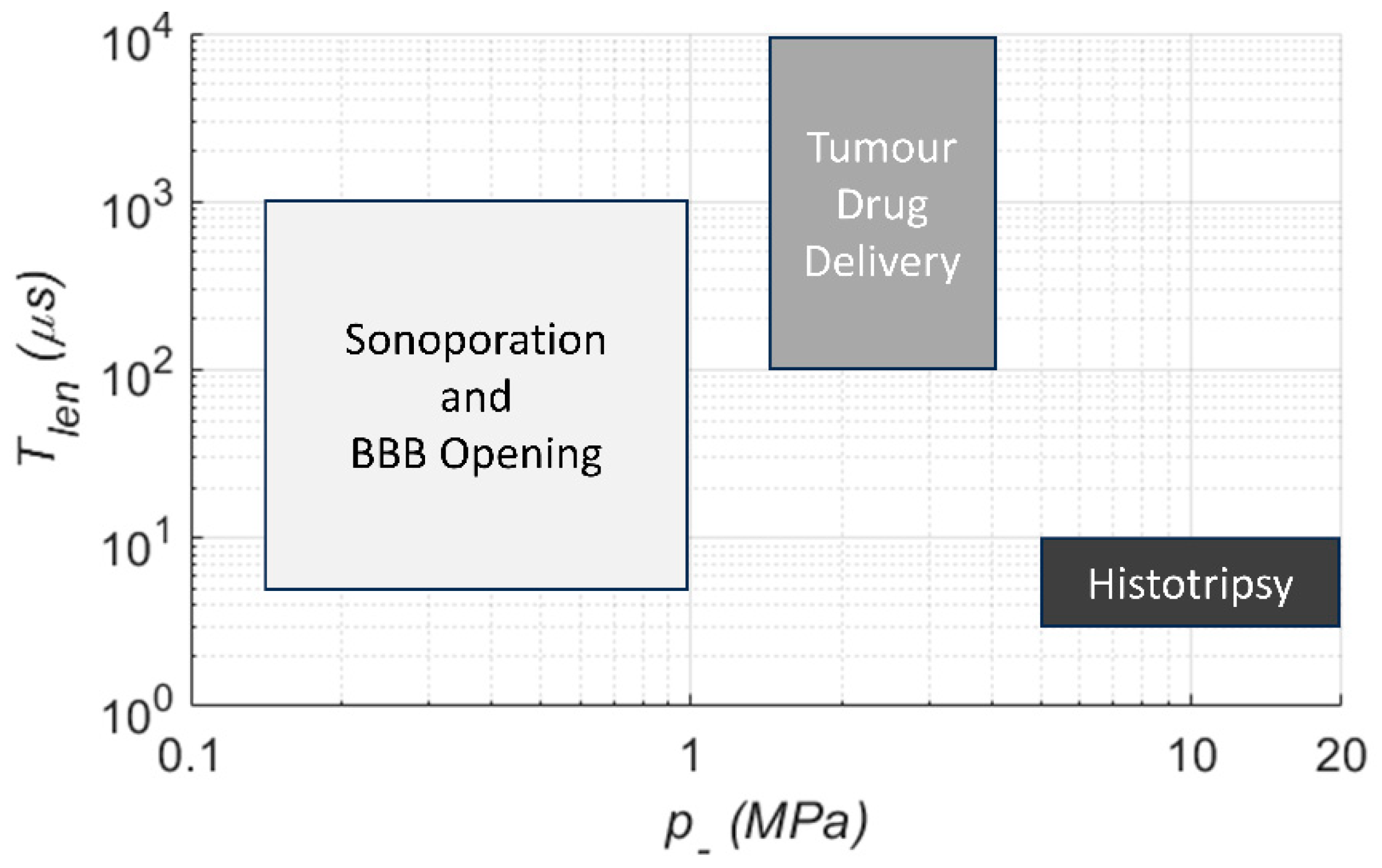
3. Key Ultrasound Considerations for Drug Delivery
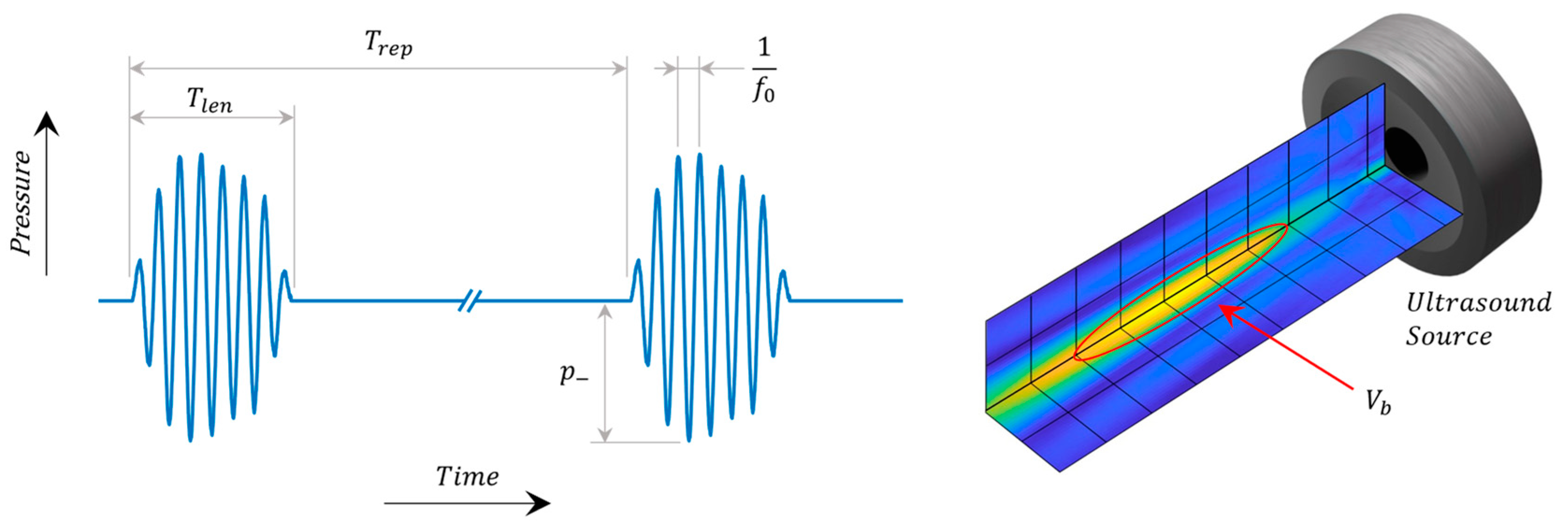
3.1. Ultrasound Parameters
3.2. Cavitation Monitoring
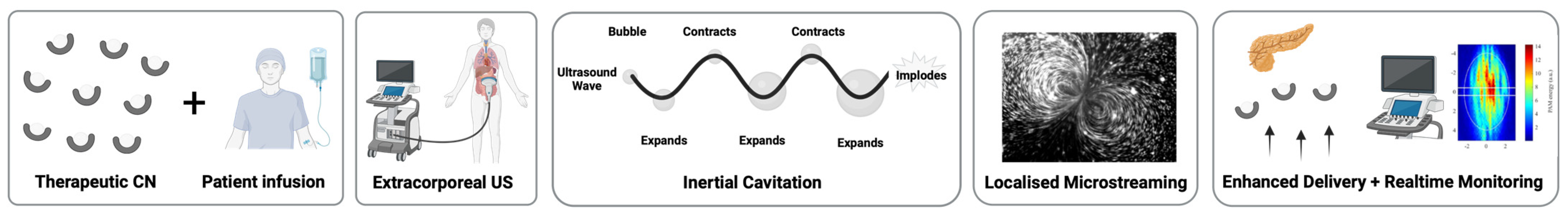
4. Therapeutic Applications
4.1. Drug Delivery to Solid Tumors
Clinical Progress
4.2. Transdermal Vaccine Delivery
4.3. Wound Healing
4.4. Biofilms
4.5. Blood–Brain Barrier
4.6. Gastrointestinal Drug Delivery
5. Future Directions of the Field
Funding
Acknowledgments
Conflicts of Interest
References
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef] [PubMed]
- May, M. Why drug delivery is the key to new medicines. Nat. Med. 2022, 28, 1100–1102. [Google Scholar] [CrossRef]
- Surapaneni, M.S.; Das, S.K.; Das, N.G. Designing Paclitaxel drug delivery systems aimed at improved patient outcomes: Current status and challenges. Int. Sch. Res. Not. 2012, 2012, 623139. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug deliery in oncology. Amer. Assoc. Canc. Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef]
- Garello, F.; Svenskaya, Y.; Parakhonskiy, B.; Filippi, M. On the Road to Precision Medicine: Magnetic Systems for Tissue Regeneration, Drug Delivery, Imaging, and Theranostics. Pharmaceutics 2023, 15, 1812. [Google Scholar] [CrossRef] [PubMed]
- Coussios, C.C.; Roy, R.A. Applications of Acoustics and Cavitation to Noninvasive Therapy and Drug Delivery. Annu. Rev. Fluid Mech. 2008, 40, 395–420. [Google Scholar] [CrossRef]
- Stride, E.; Coussios, C. Nucleation, mapping and control of cavitation for drug delivery. Nat. Rev. Phys. 2019, 1, 495–509. [Google Scholar] [CrossRef]
- Elder, S.A. Cavitation microstreaming. J. Acoust. Soc. Am. 1959, 31, 54–64. [Google Scholar] [CrossRef]
- Tho, P.; Manasseh, R.; Ooi, A. Cavitation microstreaming patterns in single and multiple bubble systems. J. Fluid Mech. 2007, 576, 191–233. [Google Scholar] [CrossRef]
- Tzu-Yin, W.; Wilson, K.E.; Machtaler, S.; Willmann, J.K. Ultrasound and microbubble guided drug delivery: Mechanistic understanding and clinical implications. Curr. Pharm. Biotechnol. 2013, 14, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wan, J.M.F.; Yu, A.C.H. Membrane Perforation and Recovery Dynamics in Microbubble-Mediated Sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Suslick, K.S. Plasma formation and temperature measurement during single-bubble cavitation. Nature 2005, 434, 52–55. [Google Scholar] [CrossRef]
- Gaitan, D.F.; Crum, L.A.; Church, C.C.; Roy, R.A. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J. Acoust. Soc. Am. 1992, 91, 3166–3183. [Google Scholar] [CrossRef]
- Marinesco, N. Action des ultrasons sur les plaques photographiques. Proc. R. Acad. Sci. Amst. 1933, 196, 858–860. [Google Scholar]
- Hilgenfeldt, S.; Grossmann, S.; Lohse, D. A simple explanation of light emission in sonoluminescence. Nature 1999, 398, 402–405. [Google Scholar] [CrossRef]
- Pecha, R.; Gompf, B. Microimplosions: Cavitation Collapse and Shock Wave Emission on a Nanosecond Time Scale. Phys. Rev. Lett. 2000, 84, 1328–1330. [Google Scholar] [CrossRef]
- Holzfuss, J.; Rüggeberg, M.; Billo, A. Shock Wave Emissions of a Sonoluminescing Bubble. Phys. Rev. Lett. 1998, 81, 5434–5437. [Google Scholar] [CrossRef]
- Suslick, K.S.; Doktycz, S.J.; Flint, E.B. On the origin of sonoluminescence and sonochemistry. Ultrasonics 1990, 28, 280–290. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (.cntdot.OH and .cntdot.H) by spin trapping. J. Am. Chem. Soc. 1982, 104, 3537–3539. [Google Scholar] [CrossRef]
- Maardalen, M.; Carlisle, R.; Coussios, C. Cavitation-Mediated Immunomodulation and Its Use with Checkpoint Inhibitors. Pharmaceutics 2023, 15, 2110. [Google Scholar] [CrossRef] [PubMed]
- Edsall, C.; Ham, E.; Holmes, H.; Hall, T.L.; Vlaisavljevich, E. Effects of frequency on bubble-cloud behavior and ablation efficiency in intrinsic threshold histotripsy. Phys. Med. Biol. 2021, 66, 225009. [Google Scholar] [CrossRef]
- Apfel, R.E. Acoustic cavitation inception. Ultrasonics 1984, 22, 167–173. [Google Scholar] [CrossRef]
- Karamanou, M.; Papaioannou, T.G.; Stefanadis, C.; Androutsos, G. Genesis of ultrasonic microbubbles: A quick historical overview. Curr. Pharm. Des. 2012, 18, 2115–2117. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Yusefi, H.; Helfield, B. Ultrasound Contrast Imaging: Fundamentals and Emerging Technology. Front. Phys. 2022, 10, 791145. [Google Scholar] [CrossRef]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Dev. Ther. 2013, 7, 375–388. [Google Scholar] [CrossRef]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control. Release 2015, 203, 51–56. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.J.; Borden, M.A. Lipid monolayer collapse and microbubble stability. Adv. Colloid Interface Sci. 2012, 184, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A. Intermolecular Forces Model for Lipid Microbubble Shells. Langmuir 2019, 35, 10042–10051. [Google Scholar] [CrossRef] [PubMed]
- Pellow, C.; Abenojar, E.C.; Exner, A.A.; Zheng, G.; Goertz, D.E. Concurrent visual and acoustic tracking of passive and active delivery of nanobubbles to tumors. Theranostics 2020, 10, 11690. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wang, J.; Ke, H.; Zhao, B.; Yue, X.; Dai, Z.; Liu, J. The fabrication of novel nanobubble ultrasound contrast agent for potential tumor imaging. Nanotechnology 2010, 21, 145607. [Google Scholar] [CrossRef]
- Myers, J.Z.; Navarro-Becerra, J.A.; Borden, M.A. Nanobubbles are non-echogenic for fundamental-mode contrast-enhanced ultrasound imaging. Bioconjugate Chem. 2022, 33, 1106–1113. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V.; Gupta, G.; Khanna, N. Effect of PEGylation on performance of protein microbubbles and its comparison with lipid microbubbles. Mater. Sci. Eng. C 2017, 71, 425–430. [Google Scholar] [CrossRef]
- Rudakovskaya, P.G.; Barmin, R.A.; Kuzmin, P.S.; Fedotkina, E.P.; Sencha, A.N.; Gorin, D.A. Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems. Pharmaceutics 2022, 14, 1236. [Google Scholar] [CrossRef]
- Grinstaff, M.W.; Suslick, K.S. Air-filled proteinaceous microbubbles: Synthesis of an echo-contrast agent. Proc. Natl. Acad. Sci. USA 1991, 88, 7708–7710. [Google Scholar] [CrossRef] [PubMed]
- Koczera, P.; Appold, L.; Shi, Y.; Liu, M.; Dasgupta, A.; Pathak, V.; Ojha, T.; Fokong, S.; Wu, Z.; van Zandvoort, M.; et al. PBCA-based polymeric microbubbles for molecular imaging and drug delivery. J. Control. Release 2017, 259, 128–135. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, F.; Shi, M.; Yang, H.; Liu, Y. Polymeric Microbubbles for Ultrasonic Molecular Imaging and Targeted Therapeutics. J. Biomater. Sci. Polym. Ed. 2011, 22, 417–428. [Google Scholar] [CrossRef]
- Bloch, S.H.; Wan, M.; Dayton, P.A.; Ferrara, K.W. Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents. Appl. Phys. Lett. 2004, 84, 631–633. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Shiina, T. A comparative study of microbubble generation by mechanical agitation and sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 489–494. [Google Scholar] [CrossRef]
- Bjerknes, K.; Sontum, P.C.; Smistad, G.; Agerkvist, I. Preparation of polymeric microbubbles: Formulation studies and product characterisation. Int. J. Pharm. 1997, 158, 129–136. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Thomson, L.M.; Polizzotti, B.D.; McGowan, F.X.; Kheir, J.N. Manufacture of concentrated, lipid-based oxygen microbubble emulsions by high shear homogenization and serial concentration. J. Vis. Exp. 2014, 87, e51467. [Google Scholar] [CrossRef]
- Stride, E.; Edirisinghe, M. Novel microbubble preparation technologies. Soft Matter 2008, 4, 2350–2359. [Google Scholar] [CrossRef]
- Pancholi, K.P.; Farook, U.; Moaleji, R.; Stride, E.; Edirisinghe, M.J. Novel methods for preparing phospholipid coated microbubbles. Eur. Biophys. J. 2008, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Joscelyne, S.M.; Trägårdh, G. Membrane emulsification—A literature review. J. Membr. Sci. 2000, 169, 107–117. [Google Scholar] [CrossRef]
- Jerri, H.A.; Dutter, R.A.; Velegol, D. Fabrication of stable anisotropic microcapsules. Soft Matter 2009, 5, 827–834. [Google Scholar] [CrossRef]
- Farook, U.; Zhang, H.B.; Edirisinghe, M.J.; Stride, E.; Saffari, N. Preparation of microbubble suspensions by co-axial electrohydrodynamic atomization. Med. Eng. Phys. 2007, 29, 749–754. [Google Scholar] [CrossRef]
- Xia, Y.; Pack, D.W. Uniform biodegradable microparticle systems for controlled release. Chem. Eng. Sci. 2015, 125, 129–143. [Google Scholar] [CrossRef]
- Lee, M.; Lee, E.Y.; Lee, D.; Park, B.J. Stabilization and fabrication of microbubbles: Applications for medical purposes and functional materials. Soft Matter 2015, 11, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Y.; Abd Shukor, S.; Vijayakumaran, A.; Vlatakis, S.; Wright, M.; Thanou, M. Phase-shift nanodroplets as an emerging sonoresponsive nanomaterial for imaging and drug delivery applications. Nanoscale 2022, 14, 2943–2965. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, K.; Grishenkov, D.; Ghorbani, M. Review on Acoustic Droplet Vaporization in Ultrasound Diagnostics and Therapeutics. BioMed. Res. Int. 2019, 2019, 9480193. [Google Scholar] [CrossRef]
- Choudhury, S.A.B.S.; Xie, F.M.D.; Dayton, P.A.P.; Porter, T.R.M.D. Acoustic Behavior of a Reactivated, Commercially Available Ultrasound Contrast Agent. J. Am. Soc. Echocardiogr. 2017, 30, 189–197. [Google Scholar] [CrossRef]
- Reznik, N.; Lajoinie, G.; Shpak, O.; Gelderblom, E.C.; Williams, R.; de Jong, N.; Versluis, M.; Burns, P.N. On the Acoustic Properties of Vaporized Submicron Perfluorocarbon Droplets. Ultrasound Med. Biol. 2014, 40, 1379–1384. [Google Scholar] [CrossRef]
- Keipert, P.E.; Otto, S.; Flaim, S.F.; Weers, J.G.; Schutt, E.A.; Pelura, T.J.; Klein, D.H.; Yaksh, T.L. Influence of Perflubron Emulsion Particle Size on Blood Half-Life and Febrile Response in Rats. Artif. Cells Blood Substit. Biotechnol. 1994, 22, 1169–1174. [Google Scholar] [CrossRef]
- Navarro-Becerra, J.A.; Song, K.H.; Martinez, P.; Borden, M.A. Microbubble Size and Dose Effects on Pharmacokinetics. ACS Biomater. Sci. Eng. 2022, 8, 1686–1695. [Google Scholar] [CrossRef]
- Borden, M.A.; Shakya, G.; Upadhyay, A.; Song, K.-H. Acoustic nanodrops for biomedical applications. Curr. Opin. Colloid Interface Sci. 2020, 50, 101383. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Aydin, O.; Durmaz, Y.Y.; Lin, K.W.; Fowlkes, B.; Xu, Z.; ElSayed, M.E. Effects of Droplet Composition on Nanodroplet-Mediated Histotripsy. Ultrasound Med. Biol. 2016, 42, 931–946. [Google Scholar] [CrossRef]
- Mountford, P.A.; Borden, M.A. On the thermodynamics and kinetics of superheated fluorocarbon phase-change agents. Adv. Colloid Interface Sci. 2016, 237, 15–27. [Google Scholar] [CrossRef]
- Lee, J.Y.; Carugo, D.; Crake, C.; Owen, J.; de Saint Victor, M.; Seth, A.; Coussios, C.; Stride, E. Nanoparticle-Loaded Protein–Polymer Nanodroplets for Improved Stability and Conversion Efficiency in Ultrasound Imaging and Drug Delivery. Adv. Mater. 2015, 27, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Bau, L.; Wu, Q.; Smith, C.A.B.; Reimer, K.; Tang, M.; Ovenden, N.; Stride, E. Predicting the spontaneous vaporisation of nanodroplets. In Proceedings of the 28th European Symposium on Ultrasound Contrast Imaging, Rotterdam, The Netherlands, 19–20 January 2023; pp. 85–88. [Google Scholar]
- Kim, J.; DeRuiter, R.M.; Goel, L.; Xu, Z.; Jiang, X.; Dayton, P.A. A Comparison of Sonothrombolysis in Aged Clots between Low-Boiling-Point Phase-Change Nanodroplets and Microbubbles of the Same Composition. Ultrasound Med. Biol. 2020, 46, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Mannaris, C.; Bau, L.; Grundy, M.; Gray, M.; Lea-Banks, H.; Seth, A.; Teo, B.; Carlisle, R.; Stride, E.; Coussios, C.C. Microbubbles, nanodroplets and gas-stabilizing solid particles for ultrasound-mediated extravasation of unencapsulated drugs: An exposure parameter optimization study. Ultrasound Med. Biol. 2019, 45, 954–967. [Google Scholar] [CrossRef]
- Mountford, P.A.; Sirsi, S.R.; Borden, M.A. Condensation phase diagrams for lipid-coated perfluorobutane microbubbles. Langmuir 2014, 30, 6209–6218. [Google Scholar] [CrossRef]
- Sheeran, P.S.; Matsunaga, T.O.; Dayton, P.A. Phase-transition thresholds and vaporization phenomena for ultrasound phase-change nanoemulsions assessed via high-speed optical microscopy. Phys. Med. Biol. 2013, 58, 4513–4534. [Google Scholar] [CrossRef] [PubMed]
- Welch, P.J.; Li, D.S.; Forest, C.R.; Pozzo, L.D.; Shi, C. Perfluorocarbon nanodroplet size, acoustic vaporization, and inertial cavitation affected by lipid shell composition in vitro. J. Acoust. Soc. Am. 2022, 152, 2493. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Haworth, K.J.; Fakhri, N.H.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1006–1017. [Google Scholar] [CrossRef]
- Kripfgans, O.D.; Fowlkes, J.B.; Miller, D.L.; Eldevik, O.P.; Carson, P.L. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound. Med. Biol. 2000, 26, 1177–1189. [Google Scholar] [CrossRef]
- Vasiukhina, A.; Eshraghi, J.; Ahmadzadegan, A.; Goergen, C.J.; Vlachos, P.P.; Solorio, L. Stable Thermally-Modulated Nanodroplet Ultrasound Contrast Agents. Nanomaterials 2021, 11, 2225. [Google Scholar] [CrossRef]
- Huang, Y.; Vezeridis, A.M.; Wang, J.; Wang, Z.; Thompson, M.; Mattrey, R.F.; Gianneschi, N.C. Polymer-Stabilized Perfluorobutane Nanodroplets for Ultrasound Imaging Agents. J. Am. Chem. Soc. 2017, 139, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Yuksel Durmaz, Y.; Vlaisavljevich, E.; Xu, Z.; ElSayed, M. Development of Nanodroplets for Histotripsy-Mediated Cell Ablation. Mol. Pharm. 2014, 11, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, F.; Chegeni, M.; Moztarzadeh, F.; Hadian-Ghazvini, S.; Raz, M. Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: Curcumin. Mater. Sci. Eng. C 2017, 74, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lea-Banks, H.; O’Reilly, M.A.; Hynynen, K. Ultrasound-responsive droplets for therapy: A review. J. Control. Release 2019, 293, 144–154. [Google Scholar] [CrossRef]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Schneewind, S.; Bruce, M.; Khaing, Z.; O’Donnell, M.; Pozzo, L. Spontaneous Nucleation of Stable Perfluorocarbon Emulsions for Ultrasound Contrast Agents. Nano Lett. 2019, 19, 173–181. [Google Scholar] [CrossRef]
- Sheeran, P.S.; Rojas, J.D.; Puett, C.; Hjelmquist, J.; Arena, C.B.; Dayton, P.A. Contrast-Enhanced Ultrasound Imaging and in Vivo Circulatory Kinetics with Low-Boiling-Point Nanoscale Phase-Change Perfluorocarbon Agents. Ultrasound Med. Biol. 2015, 41, 814–831. [Google Scholar] [CrossRef]
- Wallqvist, V.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A. Interaction forces between talc and hydrophobic particles probed by AFM. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 183–190. [Google Scholar] [CrossRef]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-propelled nanocups for drug delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Thomas, R.G.; Bharatula, L.D.; Kwan, J.J. Remote targeted implantation of sound-sensitive biodegradable multi-cavity microparticles with focused ultrasound. Sci. Rep. 2019, 9, 9612. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, J.K.; Lyons, B.; Balkaran, J.; Rijal, P.; Dunn-Lawless, D.; Caproni, L.; Gray, M.; Suslick, K.S.; Coussios, C.C.; Carlisle, R.C. Cavitation-mediated Transcutaneous Delivery of Protein and Nucleotide-based Antigen for Rapid High-level Immune Responses. Adv. Ther. 2023. [Google Scholar] [CrossRef]
- Zhang, P.; He, J.; Ma, X.; Gong, J.; Nie, Z. Ultrasound assisted interfacial synthesis of gold nanocones. Chem. Commun. 2013, 49, 987–989. [Google Scholar] [CrossRef]
- Oxley, J.D.; Prozorov, T.; Suslick, K.S. Sonochemistry and sonoluminescence of room-temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 11138–11139. [Google Scholar] [CrossRef]
- Suslick, K.S.; Doctycz, S. Interparticle collisions driven by ultrasound. Science 1990, 247, 946. [Google Scholar]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Su, X.; Jonnalagadda, U.S.; Bharatula, L.D.; Kwan, J.J. Unsupported gold nanocones as sonocatalytic agents with enhanced catalytic properties. Ultrason. Sonochem. 2021, 79, 105753. [Google Scholar] [CrossRef]
- Sazgarnia, A.; Shanei, A.; Taheri, A.R.; Meibodi, N.T.; Eshghi, H.; Attaran, N.; Shanei, M.M. Therapeutic effects of acoustic cavitation in the presence of gold nanoparticles on a colon tumor model. J. Ultrasound Med. 2013, 32, 475–483. [Google Scholar] [CrossRef]
- Kresge, A.C.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Ultrasound responsive mesoporous silica nanoparticles for biomedical applications. Chem. Commun. 2019, 55, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Hah, H.J.; Kim, J.S.; Jeon, B.J.; Koo, S.M.; Lee, Y.E. Simple preparation of monodisperse hollow silica particles without using templates. Chem. Commun. 2003, 14, 1712–1713. [Google Scholar] [CrossRef]
- Paris, J.L.; Mannaris, C.; Cabañas, M.V.; Carlisle, R.; Manzano, M.; Vallet-Regí, M.; Coussios, C.C. Ultrasound-mediated cavitation-enhanced extravasation of mesoporous silica nanoparticles for controlled-release drug delivery. Chem. Eng. J. 2018, 340, 2–8. [Google Scholar] [CrossRef]
- Sviridov, A.; Tamarov, K.; Fesenko, I.; Xu, W.; Andreev, V.; Timoshenko, V.; Lehto, V.P. Cavitation induced by Janus-like mesoporous silicon nanoparticles enhances ultrasound hyperthermia. Front. Chem. 2019, 7, 393. [Google Scholar] [CrossRef]
- Kim, H.J.; Matsuda, H.; Zhou, H.; Honma, I. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)-mesoporous silica composite. Adv. Mater. 2006, 18, 3083–3088. [Google Scholar] [CrossRef]
- Lee, S.F.; Zhu, X.M.; Wang, Y.X.J.; Xuan, S.H.; You, Q.; Chan, W.H.; Wong, C.H.; Wang, F.; Yu, J.C.; Cheng, C.H.K.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Milgroom, A.; Intrator, M.; Madhavan, K.; Mazzaro, L.; Shandas, R.; Liu, B.; Park, D. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surf. B Biointerfaces 2014, 116, 652–657. [Google Scholar] [CrossRef]
- Ma, M.; Xu, H.; Chen, H.; Jia, X.; Zhang, K.; Wang, Q.; Zheng, S.; Wu, R.; Yao, M.; Cai, X.; et al. A drug–perfluorocarbon nanoemulsion with an ultrathin silica coating for the synergistic effect of chemotherapy and ablation by high-intensity focused ultrasound. Adv. Mater. 2014, 26, 7378–7385. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Holland, C.K.; McPherson, D.D. Echogenic Lipsomes for Targeted Drug Delivery. Proc. IEEE Int. Symp. Biomed. Imaging 2009, 2009, 755–758. [Google Scholar] [PubMed]
- Wei, M.; Lai, M.; Zhang, J.; Pei, X.; Yan, F. Biosynthetic Gas Vesicles from Halobacteria NRC-1: A Potential Ultrasound Contrast Agent for Tumor Imaging. Pharmaceutics 2022, 14, 1198. [Google Scholar] [CrossRef]
- Park, D.; Jung, H.C.; Park, J.; Bae, S.; Shin, U.; Kim, S.W.; Kim, C.W.; Lee, Y.H.; Seo, J. Synthesis of echogenic liposomes for sonoporation. Micro Nano Lett. 2022, 17, 276–285. [Google Scholar] [CrossRef]
- Bar-Zion, A.; Nourmahnad, A.; Mittelstein, D.R.; Shivaei, S.; Yoo, S.; Buss, M.T.; Hurt, R.C.; Malounda, D.; Abedi, M.H.; Lee-Gosselin, A.; et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nat. Nanotechnol. 2021, 16, 1403–1412. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sun, T.; Palomba, R.; Rama, E.; Zhang, Y.; Power, C.; Moeckel, D.; Liu, M.; Sarode, A.; Weiler, M.; et al. Nonspherical ultrasound microbubbles. Proc. Natl. Acad. Sci. USA 2023, 120, e2218847120. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin Delivery into Tumor Cells with Ultrasound and Microbubbles. Mol. Pharm. 2011, 8, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Dimcevski, G.; Helge Gilja, O.; Hoem, D.; Postema, M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med. Phys. 2013, 40, 072902. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Luo, Y.; Zhang, Y.; Cui, W.; Zhang, D.; Wu, J.; Zhang, J.; Tu, J. The correlation between acoustic cavitation and sonoporation involved in ultrasound-mediated DNA transfection with polyethylenimine (PEI) in vitro. J. Control. Release 2010, 145, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.G.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Forsberg, F.; Minimo, C.; Liu, J.B.; Merton, D.A.; Claudio, P.P. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J. Cell. Physiol. 2006, 209, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Coviello, C.; Erbs, P.; Foloppe, J.; Rowe, C.; Kwan, J.; Crake, C.; Finn, S.; Jackson, E.; Balloul, J.M.; et al. Polymeric Cups for Cavitation-mediated Delivery of Oncolytic Vaccinia Virus. Mol. Ther. 2016, 24, 1627–1633. [Google Scholar] [CrossRef]
- Carlisle, R.; Choi, J.; Bazan-Peregrino, M.; Laga, R.; Subr, V.; Kostka, L.; Ulbrich, K.; Coussios, C.C.; Seymour, L.W. Enhanced tumor uptake and penetration of virotherapy using polymer stealthing and focused ultrasound. J. Natl. Cancer Inst. 2013, 105, 1701–1710. [Google Scholar] [CrossRef]
- Jin, L.F.; Li, F.; Wang, H.P.; Wei, F.; Qin, P.; Du, L.F. Ultrasound targeted microbubble destruction stimulates cellular endocytosis in facilitation of adeno-associated virus delivery. Int. J. Mol. Sci. 2013, 14, 9737–9750. [Google Scholar] [CrossRef]
- Rychak, J.J.; Klibanov, A.L. Nucleic acid delivery with microbubbles and ultrasound. Adv. Drug Deliv. Rev. 2014, 72, 82–93. [Google Scholar] [CrossRef]
- Bao, S.; Thrall, B.D.; Miller, D.L. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med. Biol. 1997, 23, 953–959. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Wu, C.-H.; Chen, C.-C.; Li, P.-C. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound Med. Biol. 2006, 32, 1931–1941. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release Off. J. Control. Release Soc. 2016, 243, 172–181. [Google Scholar] [CrossRef]
- Zhou, B.; Lian, Q.; Jin, C.; Lu, J.; Xu, L.; Gong, X.; Zhou, P. Human clinical trial using diagnostic ultrasound and microbubbles to enhance neoadjuvant chemotherapy in HER2-negative breast cancer. Front. Oncol. 2022, 12, 992774. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. From mechanism to mouse: A tale of two bioorthogonal reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, N.; Francis, M.B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 2011, 7, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.J.; Hsu, M.J.; Schutt, C.E.; Hall, D.J.; Mattrey, R.F.; Esener, S.C. Phospholipid/carbocyanine dye-shelled microbubbles as ultrasound-modulated fluorescent contrast agents. Soft Matter 2013, 9, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A.; Caskey, C.F.; Little, E.; Gillies, R.J.; Ferrara, K.W. DNA and polylysine adsorption and multilayer construction onto cationic lipid-coated microbubbles. Langmuir 2007, 23, 9401–9408. [Google Scholar] [CrossRef]
- Dwivedi, P.; Kiran, S.; Han, S.; Dwivedi, M.; Khatik, R.; Fan, R.; Mangrio, F.A.; Du, K.; Zhu, Z.; Yang, C.; et al. Magnetic Targeting and Ultrasound Activation of Liposome-Microbubble Conjugate for Enhanced Delivery of Anticancer Therapies. ACS Appl. Mater. Interfaces 2020, 12, 23737–23751. [Google Scholar] [CrossRef]
- Owen, J.; Rademeyer, P.; Chung, D.; Cheng, Q.; Holroyd, D.; Coussios, C.; Friend, P.; Pankhurst, Q.A.; Stride, E. Magnetic targeting of microbubbles against physiologically relevant flow conditions. Interface Focus 2015, 5, 20150001. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Wong, M.; Suslick, K.S. Sonochemically produced hemoglobin microbubbles. MRS Online Proc. Libr. 1994, 372, 89. [Google Scholar] [CrossRef]
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Appl. Mater. Interfaces 2012, 4, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Möhwald, H.; Shchukin, D. Self-Healing and antifouling multifunctional coatings based on pH and sulfide ion sensitive nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Chapla, R.; Huynh, K.T.; Schutt, C.E. Microbubble–Nanoparticle Complexes for Ultrasound-Enhanced Cargo Delivery. Pharmaceutics 2022, 14, 2396. [Google Scholar]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Fix, S.M.; Papadopoulou, V.; Velds, H.; Kasoji, S.K.; Rivera, J.N.; Borden, M.A.; Chang, S.; Dayton, P.A. Oxygen microbubbles improve radiotherapy tumor control in a rat fibrosarcoma model–A preliminary study. PLoS ONE 2018, 13, e0195667. [Google Scholar] [CrossRef]
- Corro, R.; Urquijo, C.F.; Aguila, O.; Villa, E.; Santana, J.; Rios, A.; Escalante, B. Use of nitric oxide donor-loaded microbubble destruction by ultrasound in thrombus treatment. Molecules 2022, 27, 7218. [Google Scholar] [CrossRef]
- Abbott, J.G. Rationale and derivation of MI and TI—A review. Ultrasound Med. Biol. 1999, 25, 431–441. [Google Scholar] [CrossRef]
- Apfel, R.E.; Holland, C.K. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 1991, 17, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Rifai, B.; Carlisle, R.C.; Choi, J.; Arvanitis, C.D.; Seymour, L.W.; Coussios, C.C. Cavitation-enhanced delivery of a replicating oncolytic adenovirus to tumors using focused ultrasound. J. Control. Release 2013, 169, 40–47. [Google Scholar] [CrossRef] [PubMed]
- DeImran, K.M.; Tintera, B.; Morrison, H.A.; Tupik, J.D.; Nagai-Singer, M.A.; Ivester, H.; Council-Troche, M.; Edwards, M.; Coutermarsh-Ott, S.; Byron, C.; et al. Improved Therapeutic Delivery Targeting Clinically Relevant Orthotopic Human Pancreatic Tumors Engrafted in Immunocompromised Pigs Using Ultrasound-Induced Cavitation: A Pilot Study. Pharmaceutics 2023, 15, 1585. [Google Scholar] [CrossRef]
- ISRCTN. Testing If the Sonotran Platform Can Enhance Drug Delivery in Metastatic Colorectal Cancer. 2021. Available online: https://www.isrctn.com/ISRCTN17598292 (accessed on 1 August 2023).
- Huang, Y.; Meng, Y.; Pople, C.B.; Bethune, A.; Jones, R.M.; Abrahao, A.; Hamani, C.; Kalia, S.K.; Kalia, L.V.; Lipsman, N.; et al. Cavitation Feedback Control of Focused Ultrasound Blood-Brain Barrier Opening for Drug Delivery in Patients with Parkinson’s Disease. Pharmaceutics 2022, 14, 2607. [Google Scholar] [CrossRef]
- Morse, S.V.; Pouliopoulos, A.N.; Chan, T.G.; Copping, M.J.; Lin, J.; Long, N.J.; Choi, J.J. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology 2019, 291, 458–465. [Google Scholar] [CrossRef]
- Edsall, C.; Khan, Z.M.; Mancia, L.; Hall, S.; Mustafa, W.; Johnsen, E.; Klibanov, A.L.; Durmaz, Y.Y.; Vlaisavljevich, E. Bubble cloud behavoir and ablation capacity for histotripsy generated from intrinsic or artificial cavitation nuclei. Ultrasound Med. Biol. 2021, 47, 620–639. [Google Scholar] [CrossRef]
- Li, T.; Khokhlova, T.D.; Sapozhnikov, O.A.; O’Donnell, M.; Hwang, J.H. A New Active Cavitation Mapping Technique for Pulsed HIFU Applications-Bubble Doppler. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 1698–1708. [Google Scholar] [CrossRef]
- Gyöngy, M.; Coussios, C.C. Passive cavitation mapping for localization and tracking of bubble dynamics. J. Acoust. Soc. Am. 2010, 128, EL175–EL180. [Google Scholar] [CrossRef]
- Haworth, K.J.; Bader, K.B.; Rich, K.T.; Holland, C.K.; Mast, T.D. Quantitative Frequency-Domain Passive Cavitation Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 177–191. [Google Scholar] [CrossRef]
- Coviello, C.; Kozick, R.; Choi, J.; Gyöngy, M.; Jensen, C.; Smith, P.P.; Coussios, C.-C. Passive acoustic mapping utilizing optimal beamforming in ultrasound therapy monitoring. J. Acoust. Soc. Am. 2015, 137, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Polichetti, M.; Varray, F.; Gilles, B.; Bera, J.C.; Nicolas, B. Use of the Cross-Spectral Density Matrix for Enhanced Passive Ultrasound Imaging of Cavitation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 910–925. [Google Scholar] [CrossRef]
- Telichko, A.V.; Lee, T.; Jakovljevic, M.; Dahl, J.J. Passive Cavitation Mapping by Cavitation Source Localization From Aperture-Domain Signals-Part I: Theory and Validation Through Simulations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.T.; Apostolakis, I.; Konofagou, E.E. Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles. Phys. Med. Biol. 2018, 63, 11. [Google Scholar] [CrossRef]
- Grundy, M.; Bau, L.; Hill, C.; Paverd, C.; Mannaris, C.; Kwan, J.; Crake, C.; Coviello, C.; Coussios, C.; Carlisle, R. Improved therapeutic antibody delivery to xenograft tumors using cavitation nucleated by gas-entrapping nanoparticles. Nanomedicine 2020, 16, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sukovich, J.R.; Macoskey, J.J.; Lundt, J.E.; Gerhardson, T.I.; Hall, T.L.; Xu, Z. Real-Time Transcranial Histotripsy Treatment Localization and Mapping Using Acoustic Cavitation Emission Feedback. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Pan, E.; Bogumil, D.; Cortessis, V.; Yu, S.; Nieva, J. A Systematic Review of the Efficacy of Preclinical Models of Lung Cancer Drugs. Front. Oncol. 2020, 10, 591. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Sun, G.; Rong, D.; Li, Z.; Sun, G.; Wu, F.; Li, X.; Cao, H.; Cheng, Y.; Tang, W.; Sun, Y. Role of Small Molecule Targeted Compounds in Cancer: Progress, Opportunities, and Challenges. Front. Cell Dev. Biol. 2021, 9, 694363. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M. Targeted therapies for cancer. BMC Med. 2022, 20, 1–3. [Google Scholar] [CrossRef]
- Haslam, A.; Kim, M.S.; Prasad, V. Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006–2020. Ann. Oncol. 2021, 32, 926–932. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Xu, A.; Lythgoe, M.P.; Prasad, V. Cancer Drug Approvals That Displaced Existing Standard-of-Care Therapies, 2016–2021. JAMA Netw. Open 2022, 5, e222265. [Google Scholar] [CrossRef]
- Chaudhry, M.; Lyon, P.; Coussios, C.; Carlisle, R. Thermosensitive liposomes: A promising step toward localised chemotherapy. Expert Opin. Drug Deliv. 2022, 19, 899–912. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Tours University Hospital. Targeted Delivery of Chemotherapy with Ultrasound and Microbubbles. Available online: https://clinicaltrials.gov/ct2/show/NCT03458975 (accessed on 15 January 2023).
- University of Oxford. CEeDD: Sonosensitive Particles and Ultrasound to Enhance Drug Delivery in Metastatic Colorectal Cancer. 2022. Available online: https://www.oncology.ox.ac.uk/clinical-trials/oncology-clinical-trials-office-octo/current-trials/ceedd (accessed on 15 January 2023).
- Banerji, U.; Tiu, C.D.; Curcean, A.; Gurung, S.; O’Leary, M.; Bush, N.; Kotopoulis, S.; Healey, A.; Kvåle, S.; McElwaine-Johnn, H.H.; et al. Phase I trial of acoustic cluster therapy (ACT) with chemotherapy in patients with liver metastases of gastrointestinal origin (ACTIVATE study). J. Clin. Oncol. 2021, 39, TPS3145. [Google Scholar] [CrossRef]
- Dillon, P. Focused Ultrasound with Low-Dose Gemcitabine to Augment Immune Control of Early Stage Breast Cancer. Report No. NCT04796220. 2022. Available online: https://clinicaltrials.gov/study/NCT04796220 (accessed on 15 January 2023).
- St. Olavs Hospital. Ultrasound-Enhanced Delivery of Chemotherapy to Patients with Liver Metastasis from Breast—And Colorectal Carcinoma—A Randomized Trial. Report No. study/NCT03477019. 2022. Available online: https://clinicaltrials.gov/study/NCT03477019 (accessed on 15 January 2023).
- Yuan, Y. A Single-Arm, Single-Center Exploratory Study of the Safety and Effectiveness of High-Intensity Focused Ultrasound Therapy Combined with REGOTORI for Metastatic Colorectal Cancer. Report No. NCT04819516. 2021. Available online: https://clinicaltrials.gov/study/NCT04819516 (accessed on 15 January 2023).
- Lee, J.Y. Therapeutic Efficacy and Safety of Concurrent FOLFIRINOX Plus HIFU for Locally Advanced/Borderline Resectable Pancreatic Cancer: A Prospective Single-Center, Single-Arm, Investigator-Initiated, Open-Labeled, Exploratory Clinical Trial. Report No. study/NCT05262452. 2022. Available online: https://clinicaltrials.gov/study/NCT05262452 (accessed on 15 January 2023).
- Thomas Jefferson University. Optimizing Ultrasound Enhanced Delivery of Therapeutics. Report No. NCT04821284. 2022. Available online: https://www.clinicaltrials.gov/study/NCT04821284 (accessed on 15 January 2023).
- St. Olavs Hospital. Ultrasound-Enhanced Uptake of Chemotherapy in Patients with Inoperable Pancreatic Ductal Adenocarcinoma—A Randomized Controlled Trial. Report No. NCT04146441. 2021. Available online: https://clinicaltrials.gov/study/NCT04146441 (accessed on 15 January 2023).
- Kim, A. A Phase I Study of Lyso-Thermosensitive Liposomal Doxorubicin (LTLD, ThermoDox®) and Magnetic Resonance-Guided High Intensity Focused Ultrasound (MR-HIFU) for Relapsed or Refractory Solid Tumors in Children, Adolescents, and Young Adults. Report No. NCT02536183. 2021. Available online: https://clinicaltrials.gov/study/NCT02536183 (accessed on 15 January 2023).
- Gao, Y.; Du, L.; Li, Q.B.; Li, Q.B.; Zhu, L.; Yang, M.; Wang, X.; Zhao, B.B.; Ma, S. How physical techniques improve the transdermal permeation of therapeutics: A review. Medicine 2022, 101, e29314. [Google Scholar] [CrossRef]
- Finnin, B.C.; Morgan, T.M. Transdermal penetration enhancers: Applications, limitations, and potential. J. Pharm. Sci. 1999, 88, 955–958. [Google Scholar] [CrossRef]
- Ita, K. Recent progress in transdermal sonophoresis. Pharm. Dev. Technol. 2017, 22, 458–466. [Google Scholar] [CrossRef]
- Paliwal, S.; Menon, G.K.; Mitragotri, S. Low-Frequency Sonophoresis: Ultrastructural Basis for Stratum Corneum Permeability Assessed Using Quantum Dots. J. Investig. Dermatol. 2006, 126, 1095–1101. [Google Scholar] [CrossRef]
- Tezel, A.; Mitragotri, S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Sens, A.; Tuchscherer, J.; Mitragotri, S. Frequency dependence of sonophoresis. Pharm. Res. 2001, 18, 1694–1700. [Google Scholar] [CrossRef]
- Wolloch, L.; Kost, J. The importance of microjet vs shock wave formation in sonophoresis. J. Control. Release 2010, 148, 204–211. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Schiffter, H.; Coussios, C.-C. Exploitation of Acoustic Cavitation-Induced Microstreaming to Enhance Molecular Transport. J. Pharm. Sci. 2014, 103, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Kushner, J.; Blankschtein, D.; Langer, R. Experimental demonstration of the existence of highly permeable localized transport regions in low-frequency sonophoresis. J. Pharm. Sci 2004, 93, 2733–2745. [Google Scholar] [CrossRef]
- Kushner, J.; Kim, D.; So PT, C.; Blankschtein, D.; Langer, R.S. Dual-Channel Two-Photon Microscopy Study of Transdermal Transport in Skin Treated with Low-Frequency Ultrasound and a Chemical Enhancer. J. Investig. Dermatol. 2007, 127, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Zimon, R.L.; Lerman, G.; Elharrar, E.; Meningher, T.; Barzilai, A.; Masalha, M.; Chintakunta, R.; Hollander, E.; Goldbart, R.; Traitel, T.; et al. Ultrasound targeting of Q-starch/miR-197 complexes for topical treatment of psoriasis. J. Control. Release 2018, 284, 103–111. [Google Scholar] [CrossRef]
- Tandara, A.A.; Mustoe, T.A. Oxygen in Wound Healing—More than a Nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for Wound Healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Cates, N.K.; Kim, P.J. Topical Oxygen Therapy for Wound Healing: A Critical Evaluation. Surg. Technol. Int. 2022, 40, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Oropallo, A.; Andersen, C.A. Topical Oxygen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Roe, D.F.; Gibbins, B.L.; Ladizinsky, D.A. Topical dissolved oxygen penetrates skin: Model and method. J. Surg. Res. 2010, 159, e29–e36. [Google Scholar] [CrossRef]
- Castro, C.I.; Briceno, J.C. Perfluorocarbon-based oxygen carriers: Review of products and trials. Artif. Organs 2010, 34, 622–634. [Google Scholar] [CrossRef]
- Davis, S.C.; Cazzaniga, A.L.; Ricotti, C.; Zalesky, P.; Hsu, L.-C.; Creech, J.; Eaglstein, W.H.; Mertz, P.M. Topical Oxygen Emulsion: A Novel Wound Therapy. Arch. Dermatol. 2007, 143, 1252–1256. [Google Scholar] [CrossRef]
- Gold, M.H.; Nestor, M.S. A Supersaturated Oxygen Emulsion for Wound Care and Skin Rejuvenation. J. Drugs Dermatol. 2020, 19, 250–253. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Albala, L.; Kramer, M.R.; Daroshefski, N.; Brown, D.; Liu, J.-B.; Stanczak, M.; O’kane, P.; Forsberg, F.; Wheatley, M.A. Development of an ultrasound sensitive oxygen carrier for oxygen delivery to hypoxic tissue. Int. J. Pharm. 2015, 478, 361–367. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Hsu, H.-C.; Wu, B.-H.; Lin, Y.-C.; Liao, L.-D.; Yeh, C.-K. Preventing ischemia-reperfusion injury by acousto-mechanical local oxygen delivery. J. Control. Release 2023, 356, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Y.; Hu, Y.; Zhou, Y.; Chen, Y.; Huang, X.; Chen, J.; Deng, Q.; Cao, S.; Hu, B.; et al. Ultrasound-controlled nano oxygen carriers enhancing cell viability in 3D GelMA hydrogel for the treatment of myocardial infarction. Int. J. Biol. Macromol. 2023, 244, 125139. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M. Ultrasound-Assisted Ice Nucleation, Fibroblast Migration and Oxygen Delivery in Collagen Scaffolds for Tissue Engineering. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2022. [Google Scholar]
- Guo, R.; Xu, N.; Liu, Y.; Ling, G.; Yu, J.; Zhang, P. Functional ultrasound-triggered phase-shift perfluorocarbon nanodroplets for cancer therapy. Ultrasound Med. Biol. 2021, 47, 2064–2079. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Unger, E.C.; Porter, T.; Culp, W.; Labell, R.; Matsunaga, T.; Zutshi, R. Therapeutic applications of lipid-coated microbubbles. Adv. Drug Deliv. Rev. 2004, 56, 1291–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Memon, F.; Touma, G.; Baltsavias, S.; Jang, J.H.; Chang, C.; Rasmussen, M.F.; Olcott, E.; Jeffrey, R.B.; Arbabian, A. Capsule ultrasound device: Characterization and testing results. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; IEEE: New York, NY, USA; pp. 1–4. [Google Scholar]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3, 10–1128. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Kaplan, J.B. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs 2009, 32, 545–554. [Google Scholar] [CrossRef]
- Lattwein, K.R.; Shekhar, H.; Kouijzer, J.J.; van Wamel, W.J.; Holland, C.K.; Kooiman, K. Sonobactericide: An emerging treatment strategy for bacterial infections. Ultrasound Med. Biol. 2020, 46, 193–215. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Wang, Z.; Peng, N.; Yu, J. Synergy of ultrasound microbubbles and vancomycin against Staphylococcus epidermidis biofilm. J. Antimicrob. Chemother. 2013, 68, 816–826. [Google Scholar] [CrossRef]
- Kouijzer, J.J.; Lattwein, K.R.; Beekers, I.; Langeveld, S.A.; Leon-Grooters, M.; Strub, J.-M.; Oliva, E.; Mislin, G.L.; de Jong, N.; van der Steen, A.F.; et al. Vancomycin-decorated microbubbles as a theranostic agent for Staphylococcus aureus biofilms. Int. J. Pharm. 2021, 609, 121154. [Google Scholar] [CrossRef]
- LuTheryn, G.; Hind, C.; Campbell, C.; Crowther, A.; Wu, Q.; Keller, S.B.; Glynne-Jones, P.; Sutton, J.M.; Webb, J.S.; Gray, M.; et al. Bactericidal and anti-biofilm effects of uncharged and cationic ultrasound-responsive nitric oxide microbubbles on Pseudomonas aeruginosa biofilms. Front. Cell. Infect. Microbiol. 2022, 12, 956808. [Google Scholar] [CrossRef]
- Plazonic, F.; LuTheryn, G.; Hind, C.; Clifford, M.; Gray, M.; Stride, E.; Glynne-Jones, P.; Hill, M.; Sutton, J.M.; Carugo, D. Bactericidal effect of ultrasound-responsive microbubbles and sub-inhibitory gentamicin against Pseudomonas aeruginosa biofilms on substrates with differing acoustic impedance. Ultrasound Med. Biol. 2022, 48, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Lattwein, K.R.; Shekhar, H.; van Wamel, W.J.B.; Gonzalez, T.; Herr, A.B.; Holland, C.K.; Kooiman, K. An in vitro proof-of-principle study of sonobactericide. Sci. Rep. 2018, 8, 3411. [Google Scholar] [CrossRef] [PubMed]
- Horsley, H.; Owen, J.; Browning, R.; Carugo, D.; Malone-Lee, J.; Stride, E.; Rohn, J. Ultrasound-activated microbubbles as a novel intracellular drug delivery system for urinary tract infection. J. Control. Release 2019, 301, 166–175. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Li, P.; Yu, J. Ultrasound microbubbles enhance the activity of vancomycin against Staphylococcus epidermidis biofilms in vivo. J. Ultrasound Med. 2018, 37, 1379–1387. [Google Scholar] [CrossRef]
- Ronan, E.; Edjiu, N.; Kroukamp, O.; Wolfaardt, G.; Karshafian, R. USMB-induced synergistic enhancement of aminoglycoside antibiotics in biofilms. Ultrasonics 2016, 69, 182–190. [Google Scholar] [CrossRef]
- He, N.; Hu, J.; Liu, H.; Zhu, T.; Huang, B.; Wang, X.; Wu, Y.; Wang, W.; Qu, D. Enhancement of vancomycin activity against biofilms by using ultrasound-targeted microbubble destruction. Antimicrob. Agents Chemother. 2011, 55, 5331–5337. [Google Scholar] [CrossRef]
- Chua, S.L.; Liu, Y.; Yam, J.K.H.; Chen, Y.; Vejborg, R.M.; Tan, B.G.C.; Kjelleberg, S.; Tolker-Nielsen, T.; Givskov, M.; Yang, L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014, 5, 4462. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.C., III; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Anderl, J.N.; Zahller, J.; Roe, F.; Stewart, P.S. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. [Google Scholar] [CrossRef]
- Barna, J.; Williams, D. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 1984, 38, 339–357. [Google Scholar] [CrossRef]
- Barraud, N.; JKelso, M.; ARice, S.; Kjelleberg, S. Nitric oxide: A key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des. 2015, 21, 31–42. [Google Scholar] [CrossRef]
- Kelm, M. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 273–289. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Régina, A.; Jodoin, J.; Laplante, A.; Dagenais, C.; Berthelet, F.; Moghrabi, A.; Béliveau, R. Drug transport to the brain: Key roles for the efflux pump P-glycoprotein in the blood–brain barrier. Vasc. Pharmacol. 2002, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Reese, T.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Huang, Y.; Querbes, W.; Sah, D.W.; Hynynen, K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J. Control. Release 2012, 163, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, 1901935. [Google Scholar] [CrossRef] [PubMed]
- Smart, A.L.; Gaisford, S.; Basit, A.W. Oral peptide and protein delivery: Intestinal obstacles and commercial prospects. Expert Opin. Drug Deliv. 2014, 11, 1323–1335. [Google Scholar] [CrossRef]
- Varum FJ, O.; Hatton, G.B.; Basit, A.W. Food, physiology and drug delivery. Int. J. Pharm. 2013, 457, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Langer, R.; Traverso, G. Of microneedles and ultrasound: Physical modes of gastrointestinal macromolecule delivery. Tissue Barriers 2016, 4, e1150235. [Google Scholar] [CrossRef]
- Newman, M.K.; Kill, M.; Frampton, G. Effects of ultrasound alone and combined with hydrocortisone injections by needle or hypo-spray. Am. J. Phys. Med. 1958, 37, 206–209. [Google Scholar]
- Polat, B.E.; Blankschtein, D.; Langer, R. Low-frequency sonophoresis: Application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin. Drug Deliv. 2010, 7, 1415–1432. [Google Scholar] [CrossRef]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Ultrasound-Mediated Transdermal Protein Delivery. Science 1995, 269, 850–853. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Polat, B.E.; Mendenhall, J.; Maa, R.; Jones, B.; Hart, D.P.; Langer, R.; Blankschtein, D. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J. Control. Release 2012, 163, 154–160. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Srinivasan, S.; Barman, R.; Mo, S.H.; Polat, B.E.; Langer, R.; Blankschtein, D. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J. Control. Release 2015, 202, 93–100. [Google Scholar] [CrossRef]
- Bawiec, C.R.; Sunny, Y.; Nguyen, A.T.; Samuels, J.A.; Weingarten, M.S.; Zubkov, L.A.; Lewin, P.A. Finite element static displacement optimization of 20-100 kHz flexural transducers for fully portable ultrasound applicator. Ultrasonics 2013, 53, 511–517. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Schroeder, A.; Maa, R.; Lauwers, G.Y.; Swiston, A.; Zervas, M.; Barman, R.; DiCiccio, A.M.; Brugge, W.R.; Anderson, D.G.; et al. Ultrasound-mediated gastrointestinal drug delivery. Sci. Transl. Med. 2015, 7, 310ra168. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Lauwers, G.Y.; Goettel, J.A.; Oberli, M.A.; Cleveland, C.; Park, J.Y.; Minahan, D.; Chen, Y.; Anderson, D.G.; Jaklenec, A.; et al. Ultrasound-Mediated Delivery of RNA to Colonic Mucosa of Live Mice. Gastroenterology 2017, 152, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Chen, Y.; Cleveland, C.; Minahan, D.; Bensel, T.; Park, J.Y.; Saxton, S.; Lee, Y.-A.L.; Booth, L.; Langer, R.; et al. Defining optimal permeant characteristics for ultrasound-mediated gastrointestinal delivery. J. Control. Release 2017, 268, 113–119. [Google Scholar] [CrossRef]
- France, M.M.; del Rio, T.; Travers, H.; Raftery, E.; Xu, K.; Langer, R.; Traverso, G.; Lennerz, J.K.; Schoellhammer, C.M. Ultra-rapid drug delivery in the oral cavity using ultrasound. J. Control. Release 2019, 304, 1–6. [Google Scholar] [CrossRef]
- France, M.M.; del Rio, T.; Travers, H.; Raftery, E.; Langer, R.; Traverso, G.; Schoellhammer, C.M. Platform for the Delivery of Unformulated RNA In Vivo. J. Pharm. Sci. 2022, 111, 1770–1775. [Google Scholar] [CrossRef]
- Stewart, F.; Verbeni, A.; Qiu, Y.; Cox, B.F.; Vorstius, J.; Newton, I.P.; Huang, Z.; Menciassi, A.; Näthke, I.; Cochran, S. A Prototype Therapeutic Capsule Endoscope for Ultrasound-Mediated Targeted Drug Delivery. J. Med. Robot. Res. 2018, 3, 1840001. [Google Scholar] [CrossRef]
- Stewart, F.R.; Qiu, Y.; Lay, H.S.; Newton, I.P.; Cox, B.F.; Al-Rawhani, M.A.; Beeley, J.; Liu, Y.; Huang, Z.; Cumming, D.R.S.; et al. Acoustic Sensing and Ultrasonic Drug Delivery in Multimodal Theranostic Capsule Endoscopy. Sensors 2017, 17, 1553. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.; Cummins, G.; Turcanu, M.V.; Cox, B.F.; Prescott, A.; Clutton, E.; Newton, I.P.; Desmulliez, M.P.Y.; Thanou, M.; Mulvana, H.; et al. Ultrasound mediated delivery of quantum dots from a proof of concept capsule endoscope to the gastrointestinal wall. Sci. Rep. 2021, 11, 2584. [Google Scholar] [CrossRef]
- Fix, S.M.; Koppolu, B.P.; Novell, A.; Hopkins, J.; Kierski, T.M.; Zaharoff, D.A.; Dayton, P.A.; Papadopoulou, V. Ultrasound-Stimulated Phase-Change Contrast Agents for Transepithelial Delivery of Macromolecules, Toward Gastrointestinal Drug Delivery. Ultrasound Med. Biol. 2019, 45, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
| Tumor Site | Study Design | n | Cavitation Agent | Anti-Cancer Agent | US Modality | ClinicalTrials.Gov or ISCRTN ID |
|---|---|---|---|---|---|---|
| Colorectal liver metastases | Phase 1–2 | 45 | SonoTran® Particles (OxsSonics) (nanocups) | FOLFIRI and Cetuximab | Focused ultrasound | ISRCTN17598292 [170] |
| Solid tumours with liver metastases (colon and pancreas) | Phase 1–2 | 37–43 | Microbubble–microdroplet clusters (PS101) | FOLFOX/FOLFIRI for colorectal cancer; Gemcitabine and nab-paclitaxel for pancreatic cancer | Dual-frequency ultrasound | NCT04021277 [171] |
| Breast | Window-of-opportunity study, randomized | 48 | No cavitation agent | Gemcitabine | Focused ultrasound | NCT04796220 [172] |
| Liver metastases from breast and colon/rectum | 2 liver metastases in each patient; 1 target lesion and the other control lesion | 60 | SonoVue® (Bracco) Microbubbles | Chemotherapy not otherwise specified | Focused ultrasound | NCT03477019 [173] |
| Metastatic colorectal cancer | Early phase, single arm | 10 | No cavitation agent | Toripalimab and regorafenib | HIFU | NCT04819516 [174] |
| Locally advanced pancreatic cancer | Early-phase, single-arm, exploratory clinical trial | 60 | No cavitation agent | FOLFIRINOX | HIFU | NCT05262452 [175] |
| Pancreas | Phase I/II, randomized | 120 | Sonazoid microbubbles | FOLFIRINOX | Focused ultrasound | NCT04821284 [176] |
| Inoperable pancreatic ductal adenocarcinoma | Early phase, randomized | 30 | SonoVue Microbubbles | Chemotherapy, not otherwise specified | Focused ultrasound | NCT04146441 [177] |
| Pediatric refractory solid tumor | Phase 1, non-randomized | 34 | No cavitation agent | ThermoDox | MR-HIFU | NCT02536183 [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyons, B.; Balkaran, J.P.R.; Dunn-Lawless, D.; Lucian, V.; Keller, S.B.; O’Reilly, C.S.; Hu, L.; Rubasingham, J.; Nair, M.; Carlisle, R.; et al. Sonosensitive Cavitation Nuclei—A Customisable Platform Technology for Enhanced Therapeutic Delivery. Molecules 2023, 28, 7733. https://doi.org/10.3390/molecules28237733
Lyons B, Balkaran JPR, Dunn-Lawless D, Lucian V, Keller SB, O’Reilly CS, Hu L, Rubasingham J, Nair M, Carlisle R, et al. Sonosensitive Cavitation Nuclei—A Customisable Platform Technology for Enhanced Therapeutic Delivery. Molecules. 2023; 28(23):7733. https://doi.org/10.3390/molecules28237733
Chicago/Turabian StyleLyons, Brian, Joel P. R. Balkaran, Darcy Dunn-Lawless, Veronica Lucian, Sara B. Keller, Colm S. O’Reilly, Luna Hu, Jeffrey Rubasingham, Malavika Nair, Robert Carlisle, and et al. 2023. "Sonosensitive Cavitation Nuclei—A Customisable Platform Technology for Enhanced Therapeutic Delivery" Molecules 28, no. 23: 7733. https://doi.org/10.3390/molecules28237733
APA StyleLyons, B., Balkaran, J. P. R., Dunn-Lawless, D., Lucian, V., Keller, S. B., O’Reilly, C. S., Hu, L., Rubasingham, J., Nair, M., Carlisle, R., Stride, E., Gray, M., & Coussios, C. (2023). Sonosensitive Cavitation Nuclei—A Customisable Platform Technology for Enhanced Therapeutic Delivery. Molecules, 28(23), 7733. https://doi.org/10.3390/molecules28237733







