A Novel Spectrophotometric Method for Determination of Percarbonate by Using N, N-Diethyl-P-Phenylenediamine as an Indicator and Its Application in Activated Percarbonate Degradation of Ibuprofen
Abstract
:1. Introduction
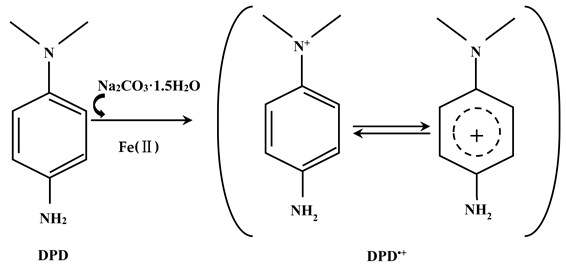
2. Results
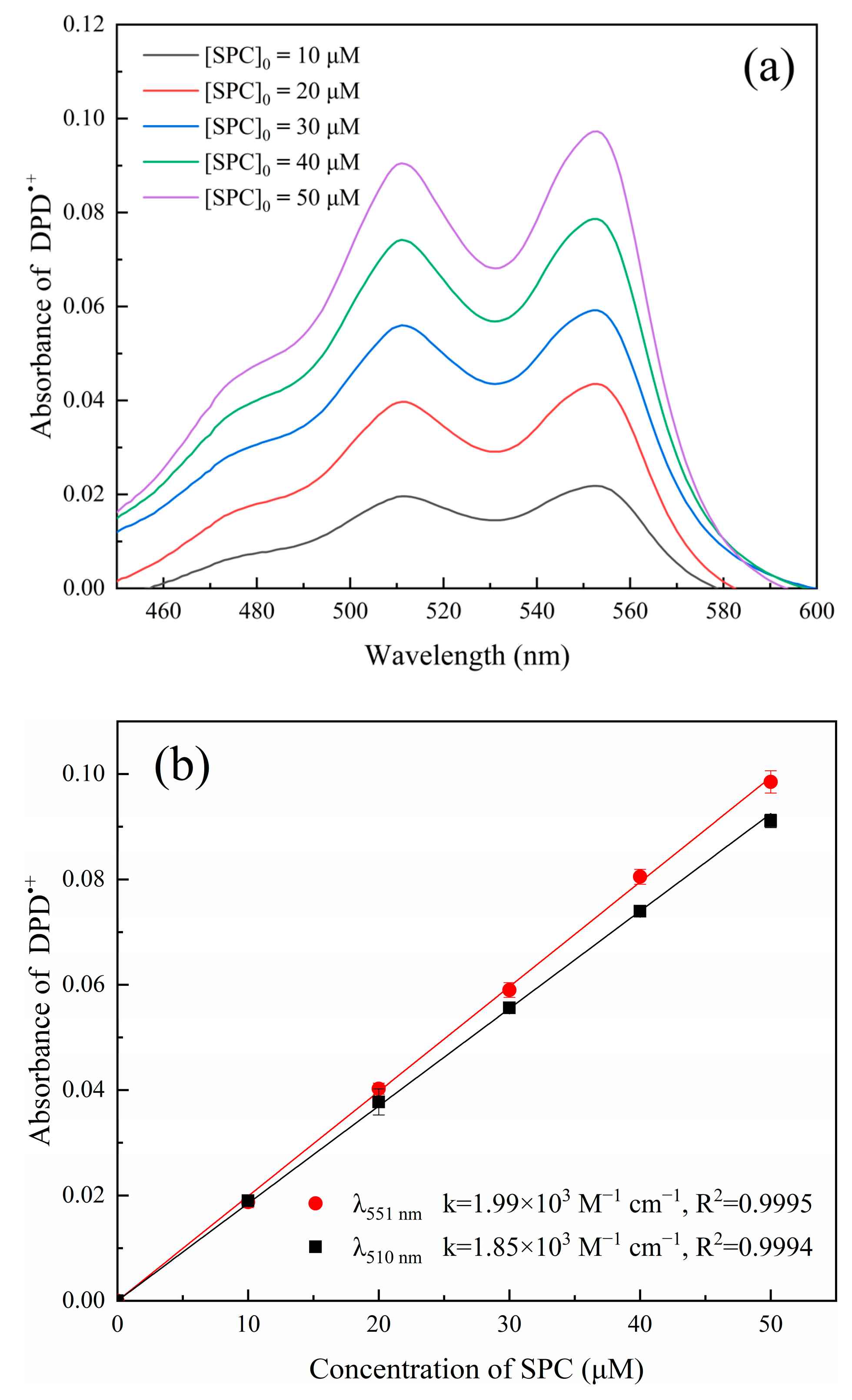
2.1. UV-Visible Absorption Spectra of DPD•+
2.2. Optimization of SPC Determination Conditions
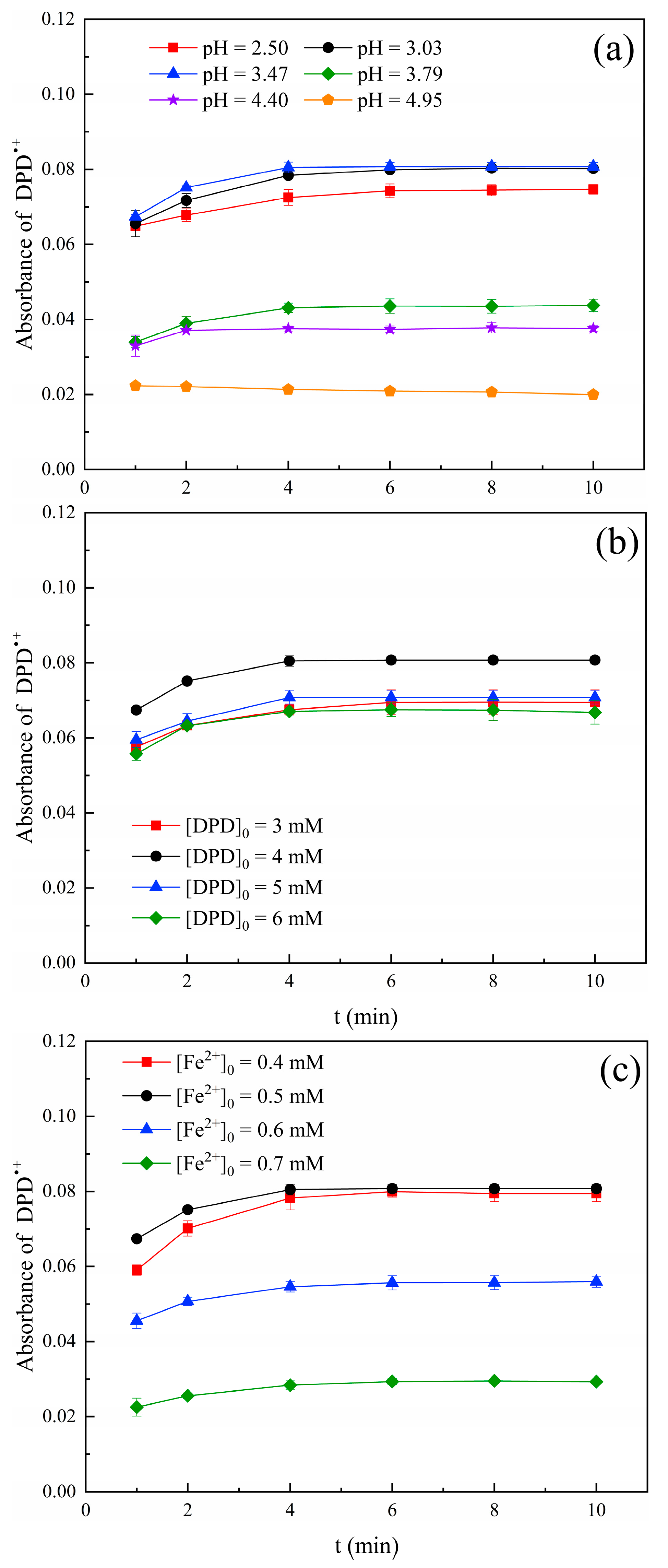
2.2.1. pH Value
2.2.2. Concentration of DPD
2.2.3. Dosage of Fe2+
2.3. Method Evaluation
2.3.1. Calibration Curve of SPC
2.3.2. DL and QL
2.3.3. Recovery Rate
2.3.4. Assessment of the Stability of DPD•+
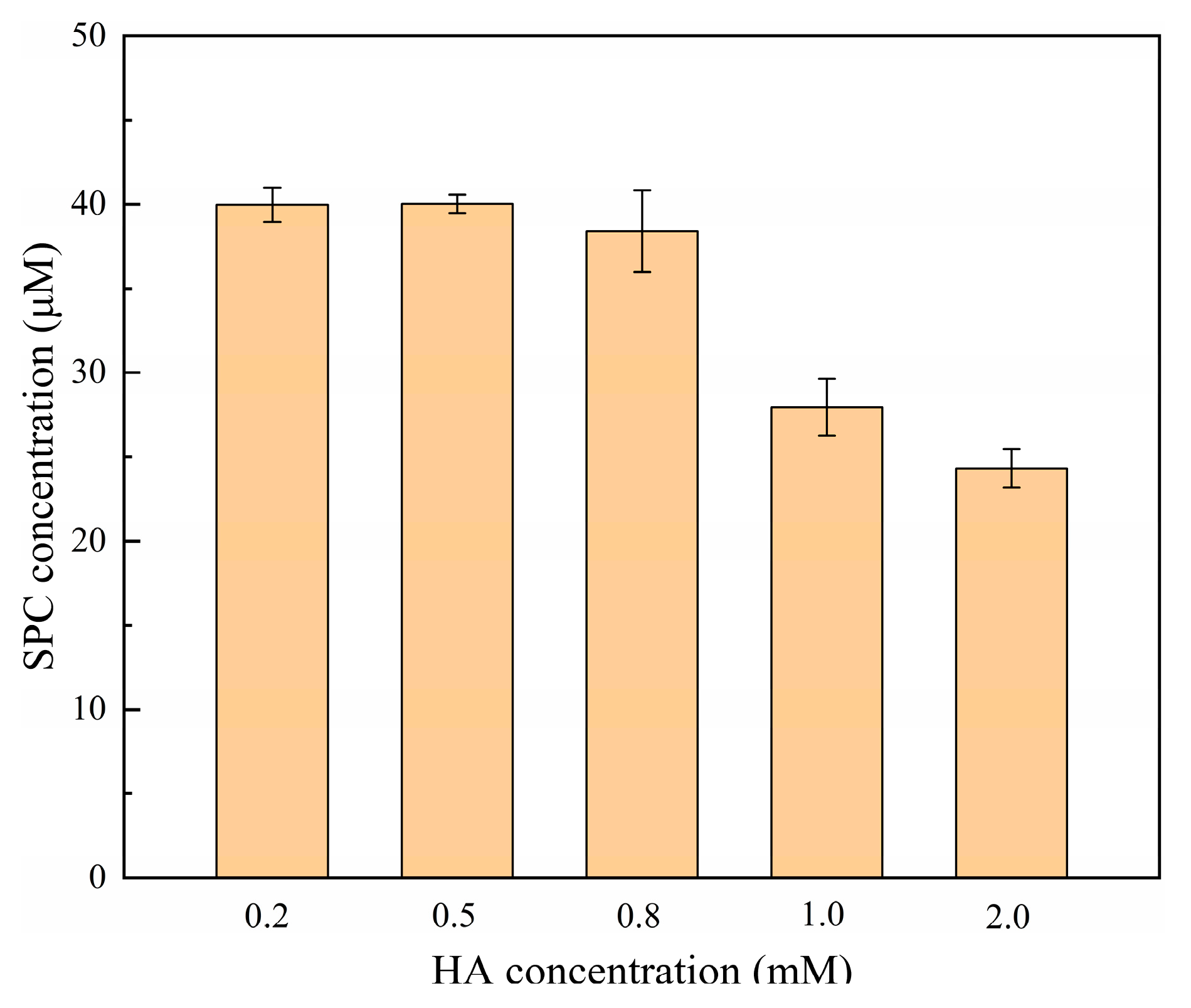
2.4. Effect of Hydroxylamine
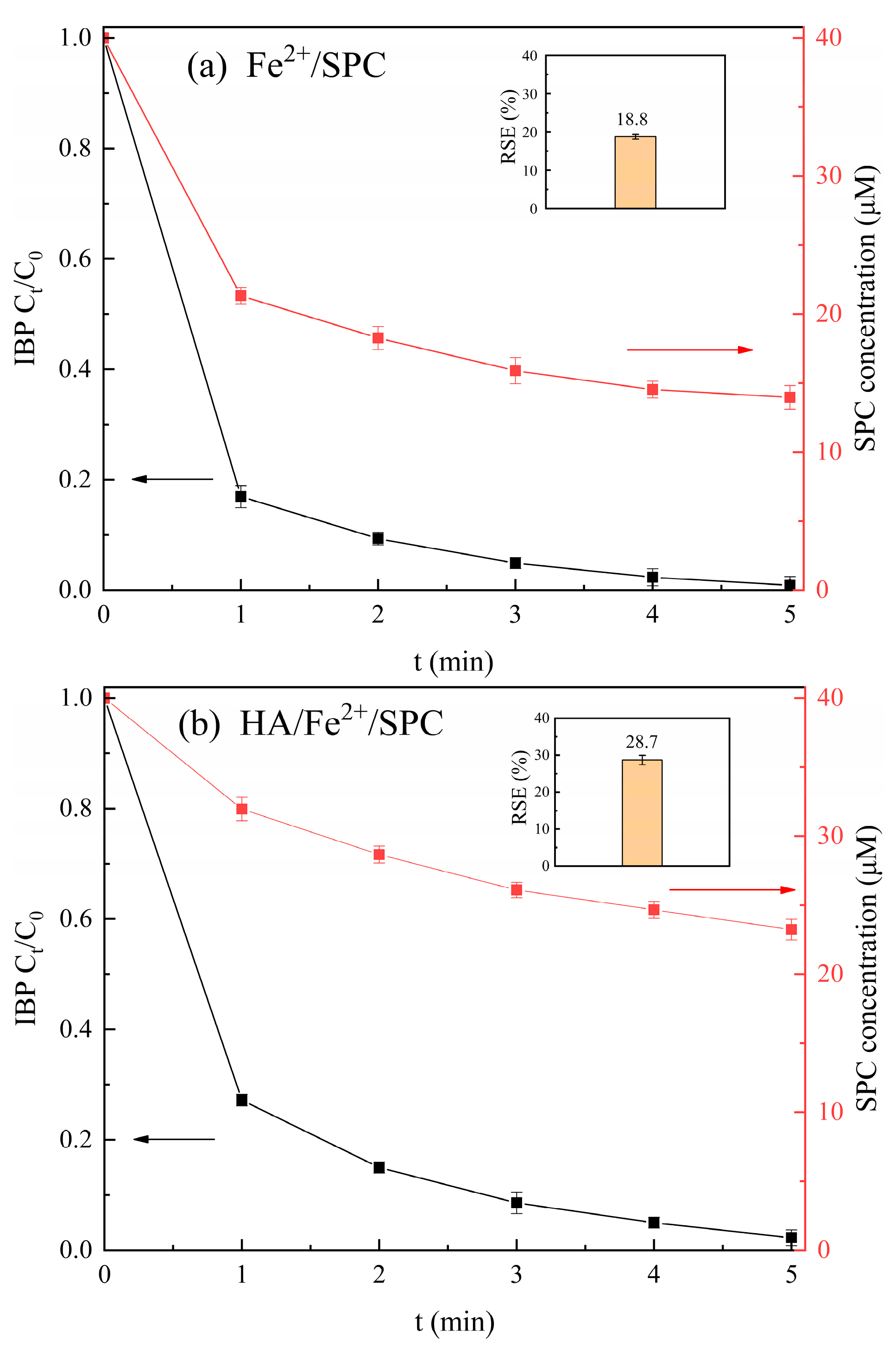
2.5. Utilization Rate of SPC in SPC-Based AOPs
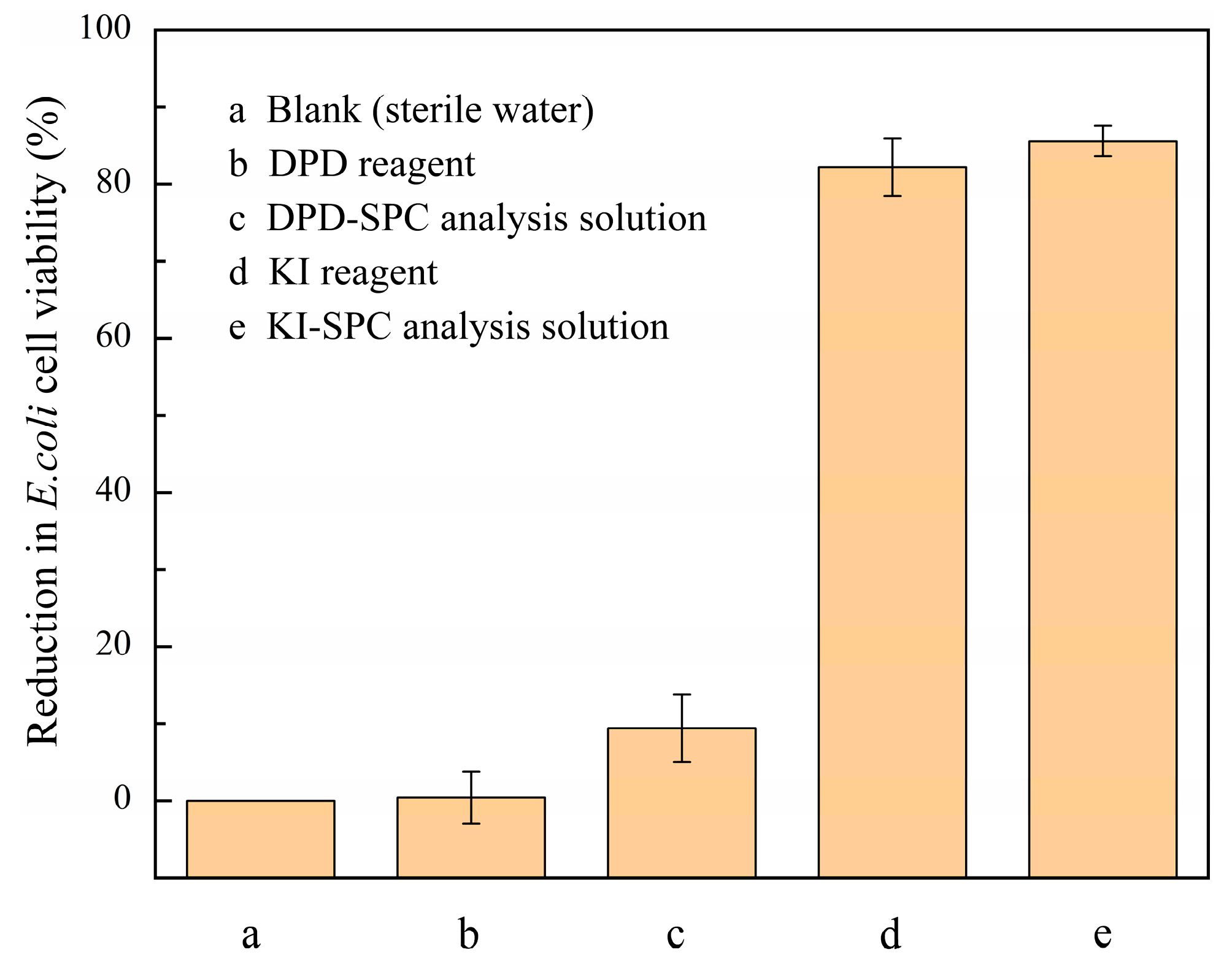
2.6. Toxicity Assessment
3. Materials and Methods
3.1. Experimental Material
3.2. Experimental Methods
3.2.1. SPC Calibration Curve
3.2.2. Detection Limit and Quantitative Limit
3.2.3. Recovery Rate
3.2.4. DPD•+ Stability Evaluation
3.3. Degradation of Ibuprofen by SPC-Based AOPs
3.4. E. coli Cell Activity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Can-Güven, E.; Daniser, Y.; Guvenc, S.Y.; Ghanbari, F.; Varank, G. Effective removal of furfural by ultraviolet activated persulfate, peroxide, and percarbonate oxidation: Focus on influencing factors, kinetics, and water matrix effect. J. Photochem. Photobiol. A Chem. 2022, 433, 114139. [Google Scholar] [CrossRef]
- Gao, J.; Song, J.; Ye, J.; Duan, X.; Dionysiou, D.D.; Yadav, J.S.; Nadagouda, M.N.; Yang, L.; Luo, S. Comparative toxicity reduction potential of UV/sodium percarbonate and UV/hydrogen peroxide treatments for bisphenol A in water: An integrated analysis using chemical, computational, biological, and metabolomic approaches. Water Res. 2021, 190, 116755. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2022, 442, 130014. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xia, X.; Ma, Y.; Luo, Y.; Zhong, Y. Stability of dissolved percarbonate and its implications for groundwater remediation. Chemosphere 2018, 205, 41–44. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Sun, J.; Meng, X.; Luo, J.; Zhou, D.; Crittenden, J.C. Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ. Sci. Technol. 2018, 52, 7380–7389. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Li, L.; Huangfu, X.; Xu, Y.; Qin, Y. Activation of sodium percarbonate with ferrous ions for degradation of chlorobenzene in aqueous solution: Mechanism, pathway and comparison with hydrogen peroxide. Environ. Chem. 2018, 14, 486–494. [Google Scholar] [CrossRef]
- Tang, P.; Jiang, W.; Lu, S.; Zhang, X.; Xue, Y.; Qiu, Z.; Sui, Q. Enhanced degradation of carbon tetrachloride by sodium percarbonate activated with ferrous ion in the presence of ethyl alcohol. Environ. Technol. 2019, 40, 356–364. [Google Scholar] [CrossRef]
- Wada, T.; Koga, N. Chemical composition of sodium percarbonate: An inquiry-based laboratory exercise. J. Chem. Educ. 2013, 90, 1048–1052. [Google Scholar] [CrossRef]
- Lee, K.M.; Park, K.Y.; Kim, N.Y.; Yoo, J.H.; Gil Choi, M.; Ahn, S.; Chang, S.-K. Colorimetric and fluorescence analysis of percarbonate and oxygen bleach by perhydrolysis of resorufin ester. Dye. Pigment. 2021, 184, 108804. [Google Scholar] [CrossRef]
- Zuo, S.; Li, D.; Xu, H.; Xia, D. An integrated microwave-ultraviolet catalysis process of four peroxides for wastewater treatment: Free radical generation rate and mechanism. Chem. Eng. J. 2020, 380, 122434. [Google Scholar] [CrossRef]
- Pang, Z.; Cai, Y.; Xiong, W.; Xiao, J.; Zou, J. A spectrophotometric method for measuring permanganate index (CODMn) by N, N-diethyl-p-phenylenediamine (DPD). Chemosphere 2021, 266, 128936. [Google Scholar] [CrossRef]
- Zou, J.; Cai, H.; Wang, D.; Xiao, J.; Zhou, Z.; Yuan, B. Spectrophotometric determination of trace hydrogen peroxide via the oxidative coloration of DPD using a Fenton system. Chemosphere 2019, 224, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Bader, H.; Sturzenegger, V.; Hoigne, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N, N-diethyl-p-phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Palin, A.T. The determination of free and combined chlorine in water by the use of diethyl-p-phenylene diamine. J. Am. Water Work. Assoc. 1957, 49, 873–880. Available online: https://www.jstor.org/stable/41254900 (accessed on 2 November 2022). [CrossRef]
- Gokulakrishnan, S.; Mohammed, A.; Prakash, H. Determination of persulphates using N, N-diethyl-p-phenylenediamine as colorimetric reagent: Oxidative coloration and degradation of the reagent without bactericidal effect in water. Chem. Eng. J. 2016, 286, 223–231. [Google Scholar] [CrossRef]
- Liu, X.; Cai, H.; Zou, J.; Pang, Z.; Yuan, B.; Zhou, Z.; Cheng, Q. Spectrophotometric determination of trace permanganate in water with N, N-diethyl-p-phenylenediamine (DPD). Chemosphere 2018, 212, 604–610. [Google Scholar] [CrossRef]
- Chang, Q.; Deng, K.; Zhu, L.; Jiang, G.; Yu, C.; Tang, H. Determination of hydrogen peroxide with the aid of peroxidase-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim. Acta 2009, 165, 299–305. [Google Scholar] [CrossRef]
- Luo, W.; Abbas, M.; Zhu, L.; Deng, K.; Tang, H. Rapid quantitative determination of hydrogen peroxide by oxidation decolorization of methyl orange using a Fenton reaction system. Anal. Chim. Acta 2008, 629, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, J.A.I.; Dong, C.-D.; Garcia-Segura, S.; Abarca, R.R.M.; Chen, C.-W.; de Luna, M.D.G. Degradation of tetracycline antibiotics by Fe2+-catalyzed percarbonate oxidation. Sci. Total Environ. 2021, 781, 146411. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Treatment of phenol-containing wastewater by photoelectro-Fenton method using supported nanoscale zero-valent iron. Environ. Sci. Pollut. Res. 2013, 20, 1596–1605. [Google Scholar] [CrossRef]
- Walling, C.; Goosen, A. Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J. Am. Chem. Soc. 1973, 95, 2987–2991. [Google Scholar] [CrossRef]
- Yang, R.; Zeng, G.; Xu, Z.; Zhou, Z.; Huang, J.; Fu, R.; Lyu, S. Comparison of naphthalene removal performance using H2O2, sodium percarbonate and calcium peroxide oxidants activated by ferrous ions and degradation mechanism. Chemosphere 2021, 283, 131209. [Google Scholar] [CrossRef]
- Miao, Z.; Gu, X.; Lu, S.; Brusseau, M.L.; Yan, N.; Qiu, Z.; Sui, Q. Enhancement effects of reducing agents on the degradation of tetrachloroethene in the Fe (II)/Fe (III) catalyzed percarbonate system. J. Hazard. Mater. 2015, 300, 530–537. [Google Scholar] [CrossRef]
- Fu, X.; Dionysiou, D.D.; Brusseau, M.L.; Zaman, W.Q.; Zang, X.; Lu, S.; Qiu, Z.; Sui, Q. Enhanced effect of EDDS and hydroxylamine on Fe (II)-catalyzed SPC system for trichloroethylene degradation. Environ. Sci. Pollut. Res. 2018, 25, 15733–15742. [Google Scholar] [CrossRef]
- Chen, L.; Ma, J.; Li, X.; Zhang, J.; Fang, J.; Guan, Y.; Xie, P. Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles. Environ. Sci. Technol. 2011, 45, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, S.; Yang, H.; Huang, Y.; Dai, L.; Zhang, B.; Zou, J. Spectrophotometric determination of hydrogen peroxide in water with peroxidase-catalyzed oxidation of potassium iodide and its applications to hydroxylamine-involved Fenton and Fenton-like systems. Chemosphere 2021, 270, 129448. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Ghauch, A.; Ayoub, G.; Naim, S. Degradation of sulfamethoxazole by persulfate assisted micrometric Fe0 in aqueous solution. Chem. Eng. J. 2013, 228, 1168–1181. [Google Scholar] [CrossRef]
- Miao, Z.; Gu, X.; Lu, S.; Zang, X.; Wu, X.; Xu, M.; Ndong, L.B.B.; Qiu, Z.; Sui, Q.; Fu, G.Y. Perchloroethylene (PCE) oxidation by percarbonate in Fe2+-catalyzed aqueous solution: PCE performance and its removal mechanism. Chemosphere 2015, 119, 1120–1125. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, L.; Liu, Y.-L.; Zhao, Q.; Ma, J. Unraveling the interaction of hydroxylamine and Fe (III) in Fe (II)/Persulfate system: A kinetic and simulating study. Water Res. 2020, 168, 115093. [Google Scholar] [CrossRef]
- Dai, L.; Xu, J.; Lin, J.; Wu, L.; Cai, H.; Zou, J.; Ma, J. Iodometric spectrophotometric determination of peroxydisulfate in hydroxylamine-involved AOPs: 15 min or 15 s for oxidative coloration? Chemosphere 2021, 272, 128577. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, X.; Zou, J.; Xiao, J.; Yuan, B.; Li, F.; Cheng, Q. Multi-wavelength spectrophotometric determination of hydrogen peroxide in water with peroxidase-catalyzed oxidation of ABTS. Chemosphere 2018, 193, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, Y.; Zhu, L.; Cui, Z.; Yuan, B. Multi-wavelength spectrophotometric measurement of persulfates using 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate)(ABTS) as indicator. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 214–220. [Google Scholar] [CrossRef] [PubMed]
 ) and 0–5 μM (
) and 0–5 μM ( ); (b) calibration curves for the determination of SPC in natural waters. ([DPD]0 = 4 mM, [Fe2+]0 = 0.5 mM, pH value = 3.50, t = 4 min, and T = 25 °C).
); (b) calibration curves for the determination of SPC in natural waters. ([DPD]0 = 4 mM, [Fe2+]0 = 0.5 mM, pH value = 3.50, t = 4 min, and T = 25 °C).
 ) and 0–5 μM (
) and 0–5 μM ( ); (b) calibration curves for the determination of SPC in natural waters. ([DPD]0 = 4 mM, [Fe2+]0 = 0.5 mM, pH value = 3.50, t = 4 min, and T = 25 °C).
); (b) calibration curves for the determination of SPC in natural waters. ([DPD]0 = 4 mM, [Fe2+]0 = 0.5 mM, pH value = 3.50, t = 4 min, and T = 25 °C).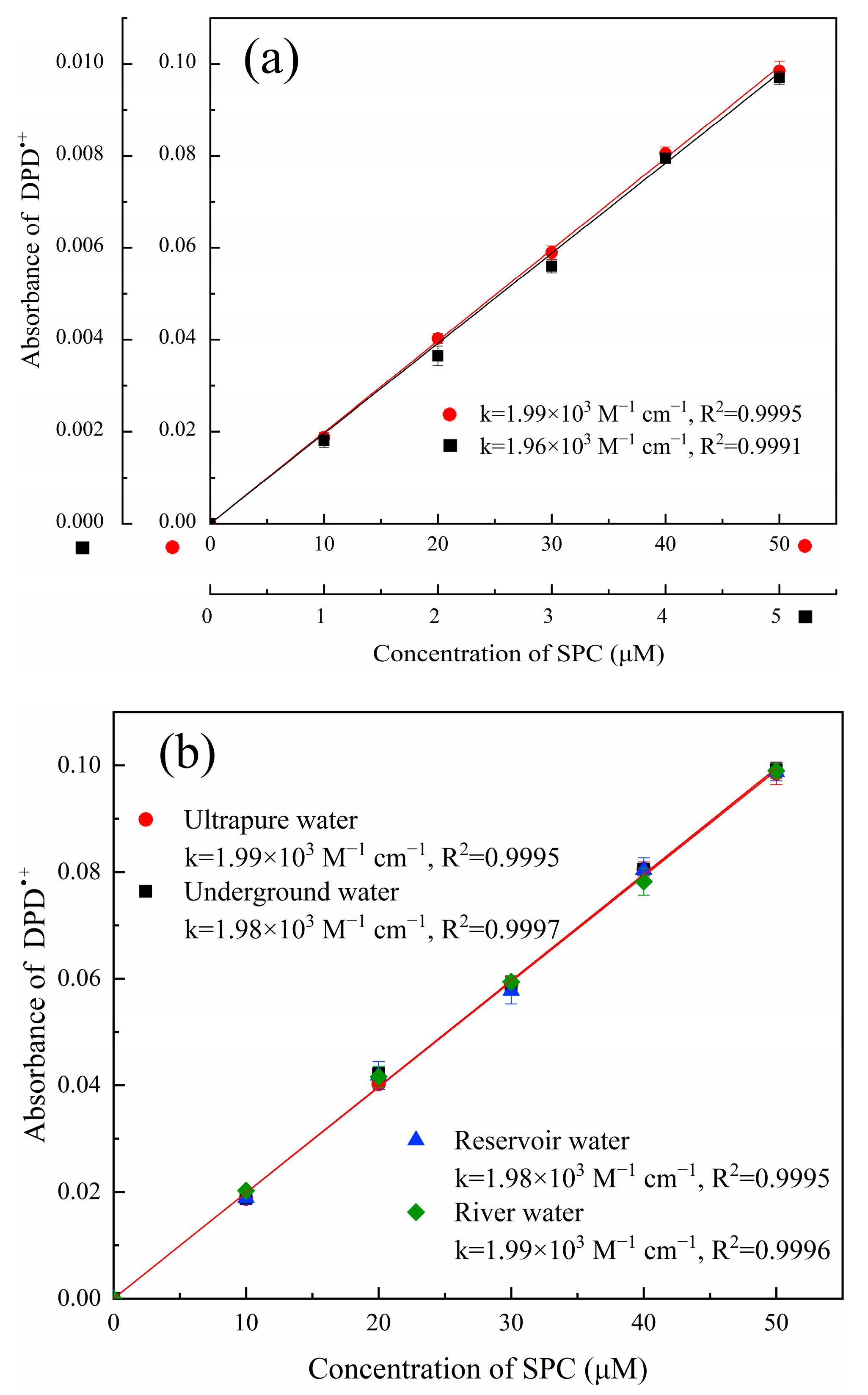
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, A.; Meng, Q.; Xue, H.; Yuan, B. A Novel Spectrophotometric Method for Determination of Percarbonate by Using N, N-Diethyl-P-Phenylenediamine as an Indicator and Its Application in Activated Percarbonate Degradation of Ibuprofen. Molecules 2023, 28, 7732. https://doi.org/10.3390/molecules28237732
Li J, Chen A, Meng Q, Xue H, Yuan B. A Novel Spectrophotometric Method for Determination of Percarbonate by Using N, N-Diethyl-P-Phenylenediamine as an Indicator and Its Application in Activated Percarbonate Degradation of Ibuprofen. Molecules. 2023; 28(23):7732. https://doi.org/10.3390/molecules28237732
Chicago/Turabian StyleLi, Jinying, Aoxue Chen, Qingling Meng, Honghai Xue, and Baoling Yuan. 2023. "A Novel Spectrophotometric Method for Determination of Percarbonate by Using N, N-Diethyl-P-Phenylenediamine as an Indicator and Its Application in Activated Percarbonate Degradation of Ibuprofen" Molecules 28, no. 23: 7732. https://doi.org/10.3390/molecules28237732
APA StyleLi, J., Chen, A., Meng, Q., Xue, H., & Yuan, B. (2023). A Novel Spectrophotometric Method for Determination of Percarbonate by Using N, N-Diethyl-P-Phenylenediamine as an Indicator and Its Application in Activated Percarbonate Degradation of Ibuprofen. Molecules, 28(23), 7732. https://doi.org/10.3390/molecules28237732






