(Bio)active Compounds in Daisy Flower (Bellis perennis)
Abstract
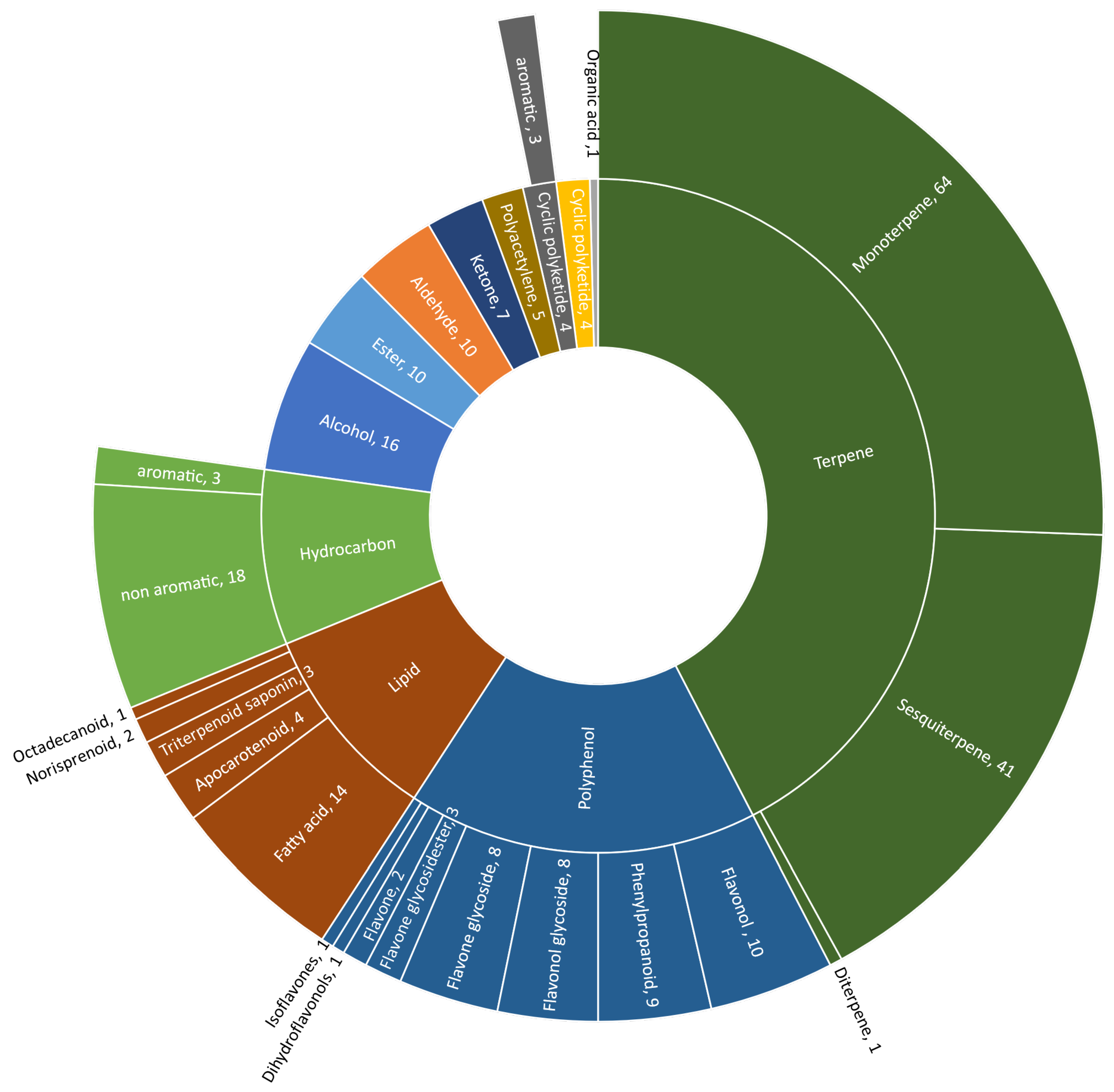
:1. Introduction
2. (Bio)activity
2.1. Antioxidative Activity
2.2. Antimicrobial Effects
2.3. Effects on Enzymes
2.4. Anticancerogenic Effects
2.5. Effects on Skin and Wound Healing
2.6. Antidepressive/Anxiolytic Effects
2.7. Effects on Lipid Metabolism
2.8. Effects on Blood Glucose Levels
2.9. Nephro- and Hematoprotective Effects
3. Homeopathy
3.1. Postpartum Bleeding
3.2. Neuroprotective Effect
3.3. Seroma Reduction
| Name | Substance Class | Organ | Extract | Ref. |
|---|---|---|---|---|
| Hexanol | Alcohol | leaves, flowers | essential oil | [3] |
| Heptanol | Alcohol | leaves, flowers | essential oil | [3] |
| Octanol | Alcohol | leaves, flowers | essential oil | [3] |
| 2-Ethyl-l-Hexenol | Alcohol | leaves, flowers | essential oil | [3] |
| trans-2-Hexenol | Alcohol | leaves, flowers | essential oil | [3] |
| cis-3-Hexenol | Alcohol | leaves, flowers | essential oil | [3] |
| Oct-l-en-3-ol | Alcohol | leaves, flowers | essential oil | [3] |
| Benzylalcohol | Alcohol | leaves, flowers | essential oil | [3] |
| 2-Phenylethanol | Alcohol | leaves, flowers | essential oil | [3] |
| Phytol | Alcohol | leaves, flowers, herb | essential oil | [3,6] |
| 1-Hexadecanol | Alcohol | herb, flowers | essential oil | [6] |
| 1-Octadecanol | Alcohol | herb, flowers | essential oil | [6] |
| 1-Octen-3-ol | Alcohol | herb, flowers | essential oil | [6] |
| 1-Dodecanol | Alcohol | herb, flowers | essential oil | [6] |
| 1-Tetradecanol | Alcohol | herb, flowers | essential oil | [6] |
| Isophytol | Alcohol | herb, flowers | essential oil | [6] |
| Hexanal | Aldehyde | leaves, flowers, herb | essential oil | [3,6] |
| Heptanal | Aldehyde | leaves, flowers | essential oil | [3] |
| Nonanal | Aldehyde | leaves, flowers, herb | essential oil | [3,6] |
| Decanal | Aldehyde | leaves, flowers, herb | essential oil | [3,6] |
| trans-2-Hexenal | Aldehyde | leaves, flowers | essential oil | [3] |
| 2,4-Hexadienal | Aldehyde | leaves, flowers | essential oil | [3] |
| Heptadienal | Aldehyde | leaves, flowers | essential oil | [3] |
| Decadienal | Aldehyde | leaves, flowers | essential oil | [3] |
| Benzaldehyde | Aldehyde | leaves, flowers | essential oil | [3] |
| Phenylacet-aldehyde | Aldehyde | leaves, flowers, herb | essential oil | [3,6] |
| Tetradecanal | Aldehyde | herb, flowers | essential oil | [6] |
| Pentadecanal | Aldehyde | herb, flowers | essential oil | [6] |
| (Z)-3-Hexenal | Aldehyde | herb, flowers | essential oil | [6] |
| (E,Z)-2,4-Heptadienal | Aldehyde | herb, flowers | essential oil | [6] |
| (E,E)-2,4-Heptadienal | Aldehyde | herb, flowers | essential oil | [6] |
| (E)-2-Nonenal | Aldehyde | herb, flowers | essential oil | [6] |
| (E,Z)-Nonadienal | Aldehyde | herb, flowers | essential oil | [6] |
| (E,Z)-2,4-Decadienal | Aldehyde | herb, flowers | essential oil | [6] |
| p-Vinylguaiacol | Aroma compound | leaves, flowers | essential oil | [3] |
| 2,3-Dihydrobenzofurane | Cyclic polyketides | leaves, flowers | essential oil | [3] |
| 2-Pentylfuran | Cyclic polyketides | herb, flowers | essential oil | [6] |
| 3,4-Dimethyl-5-penthylidene-2(5H)furanone | Cyclic polyketides | herb, flowers | essential oil | [6] |
| 3,4-Dimethyl-5-pentyl-5H-furan-2-one | Cyclic polyketides | herb, flowers | essential oil | [6] |
| cis-3-Hexenylacetate | Ester | leaves, flowers | essential oil | [3] |
| cis-3-Hexenyl-2-methylbutanoate | Ester | leaves, flowers | essential oil | [3] |
| Octen-l-ol | Ester | leaves, flowers | essential oil | [3] |
| Methyl palmitate | Ester | leaves, flowers | essential oil | [3] |
| Isopropyl palmitate | Ester | leaves, flowers | essential oil | [3] |
| Methyl linoleate | Ester | leaves, flowers, herb | essential oil | [3,6] |
| Methyl linoleate | Ester | leaves, flowers | essential oil | [3] |
| 9,12-Hexadecadienoic acid methylester | Ester | herb, flowers | essential oil | [6] |
| Ethyl linoleate | Ester | herb, flowers | essential oil | [6] |
| Methyl ethyl hexadeconoate | Ester | herb, flowers | essential oil | [6] |
| 1,2,3-Trimethylbenzene | Hydrocarbon (aromatic) | leaves, flowers | essential oil | [3] |
| Naphthalene | Hydrocarbon (aromatic) | leaves, flowers, herb | essential oil | [3,6] |
| Naphthalene-l,2-dihydro-l,l,6-trimethyl | Hydrocarbon (aromatic) | leaves, flowers | essential oil | [3] |
| trans-Decahydronaphthalene | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| cis-Cyclododecene | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| Undecane | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| Dodecane | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| Tridecane | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| Tetradecane | Hydrocarbon (non-aromatic) | leaves, flowers, herb | essential oil | [3,6] |
| Hexadecane | Hydrocarbon (non-aromatic) | leaves, flowers, herb | essential oil | [3,6] |
| Heptadecane | Hydrocarbon (non-aromatic) | leaves, flowers, herb | essential oil | [3,6] |
| Octadecane | Hydrocarbon (non-aromatic) | leaves, flowers | essential oil | [3] |
| Nonacosan | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Heptacosan | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Dimethyl tetradecane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Pentadecane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Nonadecane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Eicosane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Heneicosane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Tricosan | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| Pentacosane | Hydrocarbon (non-aromatic) | herb, flowers | essential oil | [6] |
| 6-Methyl-5-hepten-2-one | Ketone | leaves, flowers | essential oil | [3] |
| Oct-3-en-2-one | Ketone | leaves, flowers | essential oil | [3] |
| Nonan-2-one | Ketone | leaves, flowers | essential oil | [3] |
| Pentadecan-2-one | Ketone | leaves, flowers, herb | essential oil | [3,6] |
| Heptadecan-2-one | Ketone | leaves, flowers | essential oil | [3] |
| Pentadecan-2-one-6,10,14-trimethyl | Ketone | leaves, flowers | essential oil | [3] |
| Acetophenone | Ketone | leaves, flowers | essential oil | [3] |
| β-Damascenone | Lipid (Apocarotenoid) | leaves, flowers | essential oil | [3] |
| β-Ionone | Lipid (Apocarotenoid) | leaves, flowers, herb | essential oil | [3,6] |
| Dihydroactinidiolide | Lipid (Apocarotenoid) | leaves, flowers | essential oil | [3] |
| Cyclocitral | Lipid (Apocarotenoid) | herb, flowers | essential oil | [6] |
| Octanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Nonanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Decanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Undecanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Lauric acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Tridecanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Myristic acid | Lipid (Fatty acids) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| Pentadecanoic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Palmitic acid | Lipid (Fatty acids) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| Heptadecanoic acid | Lipid (Fatty acids) | leaves, flowers, herb | essential oil | [3,6] |
| Stearic acid | Lipid (Fatty acids) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| Palmitoleic acid | Lipid (Fatty acids) | leaves, flowers | essential oil | [3] |
| Linoleic acid | Lipid (Fatty acids) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| Linolenic acid | Lipid (Fatty acids) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| (E)-Theaspirane | Lipid (Norisprenoid) | herb, flowers | essential oil | [6] |
| (Z)-Theaspirane | Lipid (Norisprenoid) | herb, flowers | essential oil | [6] |
| cis-Jasmone | Lipid (Octadecanoids) | herb, flowers | essential oil | [6] |
| Perennisaponin A | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin B | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin C | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin D | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin E | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin F | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisaponin G | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin H | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin I | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin J | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin K | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin L | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin M | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,29] |
| Perennisaponin N | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin O | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin P | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin Q | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin R | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin S | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Perennisaponin T | Lipid (Triterpene saponin) | flowers | methanolic extract | [23] |
| Bellissaponin BS1 | Lipid (Triterpene saponin) | flowers | methanolic extract | [23,36] |
| Perennisoside I | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside II | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside III | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside IV | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside V | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside VI | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside VII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Besysaponin UD2 | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28] |
| Bellidioside A | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Asterbatanoside D | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Bernardioside B2 | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Bellissaponin BS6 | Lipid (Triterpene saponin) | flowers | methanolic extract | [25,28,36] |
| Perennisoside VIII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside IX | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside X | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XI | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XIII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XIV | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XV | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XVI | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XVII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XVIII | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Perennisoside XIX | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Bellisoside D | Lipid (Triterpene saponin) | flowers, roots | methanolic extract | [18,25,36] |
| Bellisoside E | Lipid (Triterpene saponin) | flowers, roots | methanolic extract | [18,25,36] |
| Bellisoside F | Lipid (Triterpene saponin) | flowers, roots | methanolic extract | [18,25,36] |
| Bellissaponin BS5 | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Bellissaponin BS9 | Lipid (Triterpene saponin) | flowers | methanolic extract | [25] |
| Polygalacin D | Lipid (Triterpene saponin) | whole plant | ethanolic extract | [13] |
| Bellissaponin 1 | Lipid (Triterpene saponin) | whole plant | methanolic extract | [14] |
| Bellissaponin 2 | Lipid (Triterpene saponin) | whole plant | methanolic extract | [14] |
| Bellisoside A | Lipid (Triterpenoid saponin) | roots | methanolic extract | [18] |
| Bellisoside B | Lipid (Triterpenoid saponin) | roots | methanolic extract | [18] |
| Bellisoside C | Lipid (Triterpenoid saponin) | roots | methanolic extract | [18] |
| Hexandicarboxilyc acid | Organic acids | leaves, flowers | essential oil | [3] |
| Benzoic acid | Organic acids (aromatic carboxylic acid) | leaves, flowers | essential oil | [3] |
| Phenylacetic acid | Organic acids (aromatic carboxylic acid) | leaves, flowers | essential oil | [3] |
| 3,5-Dimethylphenol | Organic acids (aromatic carboxylic acid) | leaves, flowers | essential oil | [3] |
| Methyldeca-4,6-diynoate | Polyacetylenes | leaves, flowers, aerial organs | essential oil | [3,15] |
| Lachnophyllum ester | Polyacetylenes | leaves, flowers | essential oil | [3] |
| Matricaria ester | Polyacetylenes | leaves, flowers | essential oil | [3] |
| Deca-4,6-diynoic acid | Polyacetylenes | leaves, flowers, aerial organs | essential oil | [3,15] |
| Lachophyllum acid | Polyacetylenes | leaves, flowers | essential oil | [3] |
| Gallic acid monohydrate | Polyphenol | aerial parts | methanolic extract, DCM extract | [10] |
| Taxifolin hydrate | Polyphenol (Dihydroflavonols) | aerial parts | methanolic extract, DCM extract | [10] |
| Apigenin-7-glucoside | Polyphenol (Flavone glycosides) | plants | ethanolic extract | [31] |
| Apigenin-7-glucuronide | Polyphenol (Flavone glycosides) | plants | ethanolic extract | [31] |
| Apigenin-7-O-glucopyranoside | Polyphenol (Flavone glycosides) | flowers | ethanolic extract | [9] |
| Luteolin-7-O-β-d-glucoside | Polyphenol (Flavone glycosides) | aerial parts | DCM extract | [10] |
| Apigenin-7-O-β-d glucoside | Polyphenol (Flavone glycosides) | flowers, leaves | chloroformic extract | [37] |
| Apigenin-7-O-β-d-glucuronide | Polyphenol (Flavone glycosides) | flowers, leaves | chloroformic extract | [37] |
| Apigenin-7-O-β-d-glucopyranoside | Polyphenol (Flavone glycosides) | flowers | methanolic extract | [36] |
| Apigenin-7-O-β-d-glucuronpyranoside | Polyphenol (Flavone glycosides) | flowers | methanolic extract | [36] |
| Apigenin-7-O-β-d-glucuronpyranoside methyl ester | Polyphenol (Flavone glycosidoester) | flowers | methanolic extract | [36] |
| Apigenin-7-O-β-d-methylglucuronide | Polyphenol (Flavoneglycosidoester) | flowers, leaves | Petrol/chloroform, chloroformic extract | [37,38] |
| Apigenin-7-O-(6′′-E-caffeoyl)-β-d-glucoside | Polyphenol (Flavoneglycosidoester) | flowers, leaves | Petrol/chloroform, chloroformic extract | [37,38] |
| Apigenin | Polyphenol (Flavone) | plants, flowers, leaves | ethanolic extract, methanolic extract, chloroformic extract | [10,31,36,37] |
| Myricetin | Polyphenol (Flavone) | aerial parts | methanolic extract, DCM extract | [10,22] |
| Isorhamnetin-3-O-β-d-galactoside | Polyphenol (Flavonol glycosides) | flowers, leaves | chloroformic extract | [37,39] |
| Isorhamnetin-3-O-β-d-(6′′-acetyl)-galactoside | Polyphenol (Flavonol glycosides) | flowers, leaves | chloroformic extract | [37,39] |
| Kaempferol-3-O-β-d-glucoside | Polyphenol (Flavonol glycosides) | flowers, leaves | chloroformic extract | [37,39] |
| Kaempferol 3-β-d-glucopyranoside | Polyphenol (Flavonol glycosides) | aerial parts | methanolic extract, DCM extract | [10] |
| Isorhamnetin-3-O-β-d-glucopyranoside | Polyphenol (Flavonol glycosides) | flowers | methanolic extract | [36] |
| Isorhamnetin-3-O-β-d-glucuronpyranoside | Polyphenol (Flavonol glycosides) | flowers | methanolic extract | [36] |
| Isorhamnetin-3-O-rutinoside | Polyphenol (Flavonol glycosides) | flowers | methanolic extract | [36] |
| Isorhamnetin-3-O-robinobioside | Polyphenol (Flavonol glycosides) | flowers | methanolic extract | [36] |
| isorhamnetin 3-O-β-d-(6′′-acetyl)-galactopyranoside | Polyphenol (Flavonol glycosides) | flowers | ethanolic extract | [17] |
| Rutin hydrate | Polyphenol (Flavonol) | aerial parts | methanolic extract, DCM extract | [10] |
| Rutin | Polyphenol (Flavonol) | plants, flowers | ethanolic extract, methanolic extract | [31,36] |
| Hyperoside | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Isoquercitrin | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Guaijaverin | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Avicularin | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Quercitrin | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Quercetin | Polyphenol (Flavonol) | plants, flowers, leaves | ethanolic extract, chloroformic extract | [10,31,37] |
| Kaempferol | Polyphenol (Flavonol) | plants, flowers, leaves | ethanolic extract, chloroformic extract | [31,37] |
| Luteolin | Polyphenol (Flavonol) | plants | ethanolic extract | [31] |
| Genistein | Polyphenol (Isoflavones) | aerial parts | methanolic extract, DCM extract | [10] |
| Coumaric acid | Polyphenol (Phenylpropanoid) | leaves, flowers | essential oil | [3] |
| Anethole | Polyphenol (Phenylpropanoid) | leaves, flowers | essential oil | [3] |
| Eugenol | Polyphenol (Phenylpropanoid) | leaves, flowers | essential oil | [3] |
| Neochlorogenic acid | Polyphenol (Phenylpropanoid) | plants | ethanolic extract | [31] |
| Chlorogenic acid | Polyphenol (Phenylpropanoid) | plants | ethanolic extract | [31] |
| Caffeic acid | Polyphenol (Phenylpropanoid) | plants | ethanolic extract | [10,31] |
| Vanillic acid | Polyphenol (Phenylpropanoid) | aerial parts | methanolic extract, DCM extract | [10] |
| p-Coumaric acid | Polyphenol (Phenylpropanoid) | aerial parts | methanolic extract, DCM extract | [10] |
| Coumarin | Polyphenol (Phenylpropanoid) | aerial parts | DCM extract | [10] |
| Abietatriene | Terpene (Diterpene) | leaves, flowers | essential oil | [3] |
| α-Pinene | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| β-Pinene | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| β-Myrcene | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| Alloocimene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| 3-Carene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| 1,4-Cineole | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| α-Terpinene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| p-Cymene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Limonene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| β-Phellandrene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| 1,8-Cineole | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Ocimene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Ocimene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Linalooloxide | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Linalooloxide | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| α-Terpinolene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Linalool | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| 1,3,8-p-Menthatriene | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Pinenehydrate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Pinocarveol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Pinenehydrate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Camphor | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Pinocarvone | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Citronellal | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Lavandulol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| 4-Terpineol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| α-Terpineol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Dihydrocarveol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Piperitol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Piperitol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Carvenol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Nerol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Carveol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Isogeraniol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Pulegone | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Neral | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Piperitone | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Sabinenehydrate acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Geraniol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Linalyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Verbenyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Geranial | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Geranic acid methyl ester | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Thymol | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Verbenyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| cis-Pinocarveyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| trans-Pinocarveyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Sabinyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Dihydrocarveyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Lavandulyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| α-Terpinenyl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Neryl acetate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Geranyl acetate | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| Geranylacetone | Terpene (Monoterpene) | leaves, flowers, herb | essential oil | [3,6] |
| Neryl propionate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| propionate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Neryl angelate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Geranyl angelate | Terpene (Monoterpene) | leaves, flowers | essential oil | [3] |
| Carvacrol | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| (Z)-β-Ocimene | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| trans-p-Menth-2-en-1-ol | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| cis-Piperitone oxide | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| Piperitenone oxide | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| Nerylacetate | Terpene (Monoterpene) | herb, flowers | essential oil | [6] |
| δ-Elemene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Cubebene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| Naphthalene-1,2,3,4,4a,7-hexahydro-1,6-Dimethyl-4-(1-methylethyl) | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Ylangene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Copaene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| β-Patchoulene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| β-Bourbonene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| β-Cubebene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| β-Caryophyllene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| Aromadendrene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Himachalene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| α-Humulene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| cis-β-Farnesene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| allo-Aromadendrene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| γ-Muurolene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| Curcumene-ar | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| β-Selinene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| δ-Selinene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Selinene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| Germacrene B | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Longipinene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Muurolene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| α-Farnesene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| γ-Cadinene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| 2,4,6-Trimethylazulene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| δ-Cadinene | Terpene (Sesquiterpene) | leaves, flowers, herb | essential oil | [3,6] |
| 4,5,9,10-Dehydroisolongifolene | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| Neophytadiene | Terpene (Sesquiterpene) | leaves, flowers, aerial parts | essential oil, methanolic extract | [3,11] |
| Torreyol | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| cis-Chyrsanthenylacetat | Terpene (Sesquiterpene) | leaves, flowers | essential oil | [3] |
| γ-Himachalene | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Germacrene-d | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Hexahydrofarnesyl acetone | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Copaborneol | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Cyclosativene | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Eremophylene | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Bicyclogermacrene | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Epi-cubebol | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Cubebol | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Caryophyllene oxide | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| 1-epi-Cubenol | Terpene (Sesquiterpene) | herb, flowers | essential oil | [6] |
| Decylbutirate | herb, flowers | essential oil | [6] | |
| Stigmasta-7,22-dien-3-ol | aerial parts | methanolic extract | [11] | |
| 2-Phytene | aerial parts | methanolic extract | [11] | |
| Methyl syringate 4-O-β-d-glycopyranoside | flowers | methanolic extract | [36] | |
| (Z)-3-Hexenyl β-d-glucopyranoside | flowers | methanolic extract | [36] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lüder, R. Grundkurs Pflanzenbestimmung: Eine Praxisanleitung für Anfänger und Fortgeschrittene, 8th ed.; Quelle & Meyer: Wiebelsheim, Germany, 2017. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; Volume 7, pp. 204–212. [Google Scholar] [CrossRef]
- Avato, P.; Tava, A. Acetylenes and terpenoids of Bellis perennis. Phytochemistry 1995, 1, 141–147. [Google Scholar] [CrossRef]
- Wray, V.; Kunath, A.; Schöpke, T.; Hiller, K. Bayogenin and asterogenic acid glycosides from Bellis perennis. Phytochemistry 1992, 31, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Karakas, F.P.; Şöhretoğlu, D.; Liptaj, T.; Štujber, M.; Ucar Turker, A.; Marák, J.; Çalış, İ.; Yalçın, F.N. Isolation of an oleanane-type saponin active from Bellis perennis through antitumor bioassay-guided procedures. Pharm. Biol. 2014, 52, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Kavalcioğrlu, N.; Acik, L.; Demirci, F.; Demirci, B.; Demir, H.; Baser, K.H.C. Biological activities of Bellis perennis volatiles and extracts. Nat. Prod. Commun. 2010, 5, 147–150. [Google Scholar] [CrossRef]
- Siatka, T.; Kašparová, M. Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules 2010, 15, 9450–9461. [Google Scholar] [CrossRef]
- Marques, T.H.C.; De Melo, C.H.S.; De Freitas, R.M. In vitro evaluation of antioxidant, anxiolytic and antidepressant-like effects of the Bellis perennis extract. Braz. J. Pharmacogn. 2012, 22, 1044–1052. [Google Scholar] [CrossRef]
- Marques, T.H.C.; De Melo, C.H.S.; De Carvalho, R.B.F.; Costa, L.M.; De Souza, A.A.; David, J.M.; De Lima David, J.P.; De Freitas, R.M. Phytochemical profile and qualification of biological activity of an isolated fraction of Bellis perennis. Biol. Res. 2013, 46, 231–238. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U.; Karakas, A.; Mshvildadze, V.; Pichette, A.; Legault, J. In vitro cytotoxic, antibacterial, anti-inflammatory and antioxidant activities and phenolic content in wild-grown flowers of common daisy—A medicinal plant. J. Herb. Med. 2017, 8, 31–39. [Google Scholar] [CrossRef]
- Marrelli, M.; Russo, N.; Chiocchio, I.; Statti, G.; Poli, F.; Conforti, F. Potential use in the treatment of inflammatory disorders and obesity of selected wild edible plants from Calabria region (Southern Italy). S. Afr. J. Bot. 2020, 128, 304–311. [Google Scholar] [CrossRef]
- Karić, E.; Horozić, E.; Pilipović, S.; Dautović, E.; Ibišević, M.; Džambić, A.; Čeliković, S.; Halilčević, A. Tyrosinase inhibition, antioxidant and antibacterial activity of commercial daisy extract (Bellis perennis). J. Pharm. Res. Int. 2023, 35, 13–19. [Google Scholar] [CrossRef]
- Desevedavy, C.; Amoros, M.; Girre, L.; Lavaud, C.; Massiot, G. Antifungal agents: In vitro and in vivo antifungal extract from the common daisy, Bellis perennis. J. Nat. Prod. 1989, 52, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Willigmann, I.; Schnelle, G.; Bodinet, C.; Beuscher, N. Antimycotic compounds from different Bellis perennis varieties. Planta Med. 1992, 58, 636–637. [Google Scholar] [CrossRef]
- Avato, P.; Vitali, C.; Mongelli, P.; Tava, A. Antimicrobial activity of polyacetylenes from Bellis perennis and their synthetic derivatives. Planta Med. 1997, 63, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Ellmann, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Marques, T.H.C.; Dos Santos, P.S.d.; De Freitas, R.M.; De Carvalho, R.B.F.; De Melo, C.H.S.; David, J.P.; David, J.M.; Lima, L.S. Atividade anticolinesterásica e perfil químico de uma fração cromatográfica ativa do extrato etanólico das flores Bellis perennis L. (Asteraceae). Quím. Nova 2013, 36, 549–553. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Koike, K.; Nikaido, T.; Furuya, T.; Yoshikawa, T. Bellisosides A–F, six novel acylated triterpenoid saponins from Bellis perennis (compositae). Tetrahedron 2005, 61, 2921–2929. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Chang, C.J.; Smith, D.L. Simple Bench-Top Bioassays (Brine Shrimp and Potato Discs) for the Discovery of Plant Antitumor Compounds: Review of Recent Progress; Kinghorn, A.D., Ed.; ACS: Washington, DC, USA, 1993; pp. 112–137. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Mclaughlin, J.L. Crown gall tumors on potato disks and brine shrimp lethality: Two simple bioassays for higher plant screening and fractionation. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; Volume 6, pp. 1–32. [Google Scholar]
- Karakas, F.P.; Yidirim, A.B.; Bayram, R.; Yavuz, M.Z.; Gepdiremen, A.; Turker, A.U. Antiproliferative activity of some medicinal plants on human breast and hepatocellular carcinoma cell lines and their phenolic contents. Trop. J. Pharm. Res. 2015, 14, 1787–1795. [Google Scholar] [CrossRef]
- Ninomiya, K.; Motai, C.; Nishida, E.; Kitagawa, N.; Yoshihara, K.; Hayakawa, T.; Muraoka, O.; Li, X.; Nakamura, S.; Yoshikawa, M.; et al. Acylated oleanane-type triterpene saponins from the flowers of Bellis perennis show anti-proliferative activities against human digestive tract carcinoma cell lines. J. Nat. Med. 2016, 70, 435–451. [Google Scholar] [CrossRef]
- Karakaş, F.P.; Karakaş, A.; Boran, Ç.; Turker, A.U.; Yalçin, F.N.; Bilensoy, E. The evaluation of topical administration of Bellis perennis fraction on circular excision wound healing in Wistar albino rats. Pharm. Biol. 2012, 50, 1031–1037. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Takamori, Y.; Nishida, E.; Yasue, M.; Hayakawa, T.; Muraoka, O.; Li, X.; Nakamura, S.; Yoshikawa, M.; et al. Oleanane-type triterpene saponins with collagen synthesis-promoting activity from the flowers of Bellis perennis. Phytochemistry 2015, 116, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Souza de Carvalho, V.M.; Covre, J.L.; Correia-Silva, R.D.; Lice, I.; Corrêa, M.P.; Leopoldino, A.M.; Gil, C.D. Bellis perennis extract mitigates UVA-induced keratinocyte damage: Photoprotective and immunomodulatory effects. J. Photochem. Photobiol. B 2021, 221, 112247. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, F.P.; Karakaş, A.; Coskun, H.; Turker, A.U. Effects of common daisy (Bellis perennis L.) aqueous extracts on anxiety-like behaviour and spatial memory performance in Wistar albino rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1378–1388. [Google Scholar] [CrossRef]
- Morikawa, T.; Li, X.; Nishida, E.; Ito, Y.; Matsuda, H.; Nakamura, S.; Muraoka, O.; Yoshikawa, M. Perennisosides I-VII, acylated triterpene saponins with antihyperlipidemic activities from the flowers of Bellis perennis. J. Nat. Prod. 2008, 71, 828–835. [Google Scholar] [CrossRef]
- Morikawa, T.; Li, X.; Nishida, E.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Oda, Y.; Muraoka, O.; Yoshikawa, M. Medicinal flowers. Part 29 †. Acylated oleanane-type triterpene bisdesmosides: Perennisaponins G, H, I, J, K, L, and M with pancreatic lipase inhibitory activity from the flowers of Bellis perennis. Helv. Chim. Acta 2010, 93, 573–586. [Google Scholar] [CrossRef]
- Haselgrübler, R.; Stübl, F.; Stadlbauer, V.; Lanzerstorfer, P.; Weghuber, J. An in ovo model for testing insulin-mimetic compounds. J. Vis. Exp. 2018, 134, e57237. [Google Scholar] [CrossRef]
- Haselgrübler, R.; Stadlbauer, V.; Stübl, F.; Schwarzinger, B.; Rudzionyte, I.; Himmelsbach, M.; Iken, M.; Weghuber, J. Insulin mimetic properties of extracts prepared from Bellis perennis. Molecules 2018, 23, 2605. [Google Scholar] [CrossRef]
- Zangeneh, M.M. Preclinical evaluation of hematoprotective and nephroprotective activities of Bellis perennis L. aqueous extract on CCl4-induced renal injury in mice. Comp. Clin. Pathol. 2018, 27, 1557–1566. [Google Scholar] [CrossRef]
- Oberbaum, M.; Galoyan, N.; Lerner-Geva, L.; Singer, S.R.; Grisaru, S.; Shashar, D.; Samueloff, A. The effect of the homeopathic remedies Arnica montana and Bellis perennis on mild postpartum bleeding—A randomized, double-blind, placebo-controlled study—Preliminary results. Complement. Ther. Med. 2005, 13, 87–90. [Google Scholar] [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Khan, M.; Tabassum, R.; Ahmed, M.; Raza, S.; Ashafaq, M.; Khuwaja, V. Neuroprotective effect of Bellis perennis and Hypericum perforatum on PC12 cells. Indian J. Res. Homoeopath. 2011, 5, 27–35. [Google Scholar] [CrossRef]
- Lotan, A.M.; Gronovich, Y.; Lysy, I.; Binenboym, R.; Eizenman, N.; Stuchiner, B.; Goldstein, O.; Babai, P.; Oberbaum, M. Arnica montana and Bellis perennis for seroma reduction following mastectomy and immediate breast reconstruction: Randomized, double-blind, placebo-controlled trial. Eur. J. Plast. Surg. 2020, 43, 285–294. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Li, X.; Nishida, E.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Morikawa, T. Medicinal flowers. XXI. Structures of perennisaponins A, B, C, D, E, and F, acylated oleanane-type triterpene oligoglycosides, from the flowers of Bellis perennis. Chem. Pharm. Bull. 2008, 56, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Gudej, J. Qualitative and quantitative chromatographic investigation of flavonoids in Bellis perennis L. Acta Pol. Pharm. 2001, 58, 401–404. [Google Scholar] [PubMed]
- Nazaruk, J.; Gudej, J. Apigenin glycosides from the flowers of Bellis perennis L. Acta Pol. Pharm. 2000, 57, 129–130. [Google Scholar] [PubMed]
- Gudej, J.; Nazaruk, J. Flavonol glycosides from the flowers of Bellis perennis. Fitoterapia 2001, 72, 839–840. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albien, A.-L.; Stark, T.D. (Bio)active Compounds in Daisy Flower (Bellis perennis). Molecules 2023, 28, 7716. https://doi.org/10.3390/molecules28237716
Albien A-L, Stark TD. (Bio)active Compounds in Daisy Flower (Bellis perennis). Molecules. 2023; 28(23):7716. https://doi.org/10.3390/molecules28237716
Chicago/Turabian StyleAlbien, Anna-Lena, and Timo D. Stark. 2023. "(Bio)active Compounds in Daisy Flower (Bellis perennis)" Molecules 28, no. 23: 7716. https://doi.org/10.3390/molecules28237716
APA StyleAlbien, A.-L., & Stark, T. D. (2023). (Bio)active Compounds in Daisy Flower (Bellis perennis). Molecules, 28(23), 7716. https://doi.org/10.3390/molecules28237716







