Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum
Abstract
:1. Introduction
2. Results
2.1. Colouration
2.2. Extract Content
2.3. Volatile Oil Content
2.4. Total Flavonoids Content
2.5. High-Performance Liquid Chromatography (HPLC) Determination of Six Flavonoids
2.6. Analysis of Variance (ANOVA) and Correlation Analysis
2.7. Principal Component Analysis
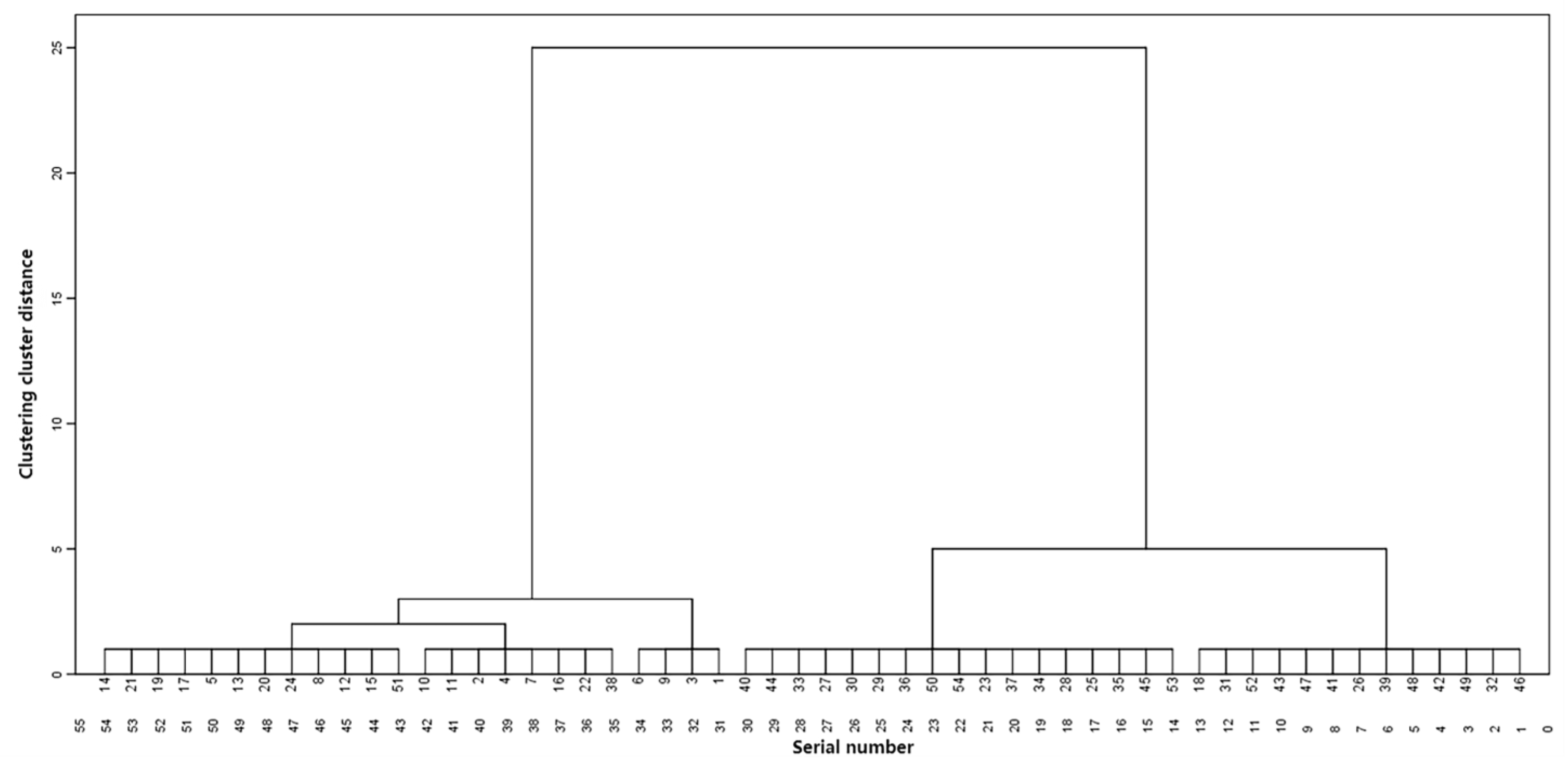
2.8. Hierarchical Cluster Analysis
2.9. K-Means Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Instruments and Reagents
4.2. Standard Substances Selection
4.3. Sample Materials
4.4. Colour Test
4.5. Determination of the Extract
4.6. Determination of Volatile Oil
4.7. Determination of Total Flavonoids
4.7.1. Preparation of the Test Solution
4.7.2. Preparation of the Standard Curve
4.7.3. Determination of the Test Solution
4.8. HPLC Determination of Six Flavonoids in Dalbergiae Odoriferae Lignum
4.8.1. Preparation of the Test Solution
4.8.2. Preparation of the Control Solution
4.8.3. Chromatographic Conditions
4.9. Data Processing and Classification Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: A Part, 2020th ed.; Pharmaceutical Science and Technology Press: Beijing, China, 2020; p. 240. [Google Scholar]
- Dai, H.F.; Mei, W.L. Modern Research on Hainan Medicinal Plants; Science and Technology Press: Beijing, China, 2007; pp. 103–104. [Google Scholar]
- Yu, H.C.; Wu, J.; Zhang, X.C.; Peng, M. Research advances on quality criteria of Dalbergia odorifera and its preparations. J. Trop. Crops. 2008, 29, 260–263. [Google Scholar]
- Zhao, X.S.; Wang, C.H.; Meng, H.; Yu, Z.X.; Yang, M.H.; Wei, J.H. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Mei, W.L.; Gong, M.F.; Bai, H.J.; Dai, H.F. Study on chemical compositions and antibacterial activity of the volatile oil from Dalbergiae Odoriferae. Guangdong Agric. Sci. 2012, 3, 95–99. [Google Scholar]
- Wang, W.; Weng, X.C.; Cheng, D.L. Antioxidant activities of natural phenolic components from Dalbergia odorifera T. Chen. Food Chem. 2000, 71, 45–49. [Google Scholar] [CrossRef]
- Zhou, W.P.; Yan, L.J.; Rong, Y.P.; Liang, D. Survey of studies on Dalbergia odorifera. Anhui Agric. Sci. 2010, 38, 14354–14356. [Google Scholar]
- Zhu, G.X.; Wu, C.W.; Liang, S.W.; Wang, S.M. Adance research of digitalization in quality control of Chinese medicine. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 225–229. [Google Scholar]
- Li, J.Y.; Wei, X.J.; Wan, G.H.; Yang, X.; Shi, J.L. Historical evolution and modern research progress of quality evaluation based on character identification of traditional Chinese medicinal materials. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 189–196. [Google Scholar]
- Fu, Y.; Liu, B.; Yang, Y.; Ye, Y.Q. Research and application of quality evaluation of traditional Chinese medicine through character identification based on modern biomimetic technology. Guangming J. Chin. Med. 2023, 38, 1819–1822. [Google Scholar]
- Yang, X.Y.; Xiao, X.; Xiong, Y.; Lin, H.; Zhou, H.Q.; Yan, Y.H. Correlation analysis between colour and effective constituents’ contents of Lonicera japonica. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 92–95. [Google Scholar]
- He, W.W.; Zhang, J.K.; Li, Y.J.; Kang, T.G. Correlation between colour traits of Menispermi Rhizoma powder and its effective components. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 57–62. [Google Scholar]
- Ma, T.T.; Gong, M.X.; Wang, Z.M.; He, R.; Wu, S.; Zhang, C.; Li, J.; Li, Z.X.; Lu, Y.; Xu, Y.S. Study on correlation between colour and effective components contents of Glycyrrhiza uralensis. Chin. J. Tradit. Chin. 2017, 42, 3776–3785. [Google Scholar]
- Li, D.H.; Wang, L.Y.; Wu, H.W.; Zhang, S.J.; Zhang, Y.G.; Li, Y.F. Quality analysis of traditional Chinese medicine based on spectroscopic colourometer. Med. Pharm. J. Chin. People’s Lib. Army 2020, 32, 109–112. [Google Scholar]
- Diao, Y.Y.; Qiu, Z.D.; Zhang, X.S.; Yang, J.Y.; Gao, H.; Zhang, Y.H.; Jia, A.L. Correlation between HPLC fingerprint and colour and chemical identification pattern analysis of Cinnamomi Cortex. Lishizhen Med. Mater. Med. Res. 2021, 32, 2676–2680. [Google Scholar]
- Zheng, X.Q.; Xu, C.; Jin, C.S.; Liu, J.L.; Liu, C.F.; Li, L. Research on relationship between processing degree and internal and external quality of Polygonatum cyrtonema processed by “nine-steaming and nine-suncuring” based on colour change. Chin. Tradit. Herb. Drugs 2022, 53, 1719–1729. [Google Scholar]
- Zhou, Z.Z.; Lv, S.; Jin, H.X.; Yang, H.J.; Wu, L.J.; Wu, H.X.; Rao, Y. Study on quality grade standard of Eucommiae Cortex based on “quality evaluation through morphological identification” combined with HPLC fingerprint. Tradit. Chin. Drug Res. Clin. Pharmacol. 2023, 34, 668–674. [Google Scholar]
- Xu, Z.Z.; Shi, X.X.; Fan, X.L.; Wang, S.M. Correlation between effective components content and colour values of Aucklandiae Radix based on colour difference principle. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 17–21. [Google Scholar]
- Apetrei, C.; Apetrei, I.M.; Villanueva, S.; de Saja, J.A.; Gutierrez-Rosales, F.; Rodriguez-Mendez, M.L. Combination of an e-nose, an e-tongue and an e-eye for the characterisation of olive oils with different degree of bitterness. Anal. Chim. Acta 2010, 663, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yan, P.; Zhou, Y.; Zhao, T.; Ma, X.Y.; Zhan, R.T. Quality evaluation of Lignum Dalbergiae Odoriferae medicinal materials. J. Guangzhou Univ. Tradit. Chin. Med. 2015, 32, 1087–1090. [Google Scholar]
- Liu, X.C.; Lai, X.P.; Xu, H.H. Cell morphology differentiation of Dalbergiae Odoriferae Lignum cultivars. J. Guangzhou Univ. Tradit. Chin. Med. 1996, 13, 79–82. [Google Scholar]
- Chen, M.; Qin, R.A.; Yin, H. Differentiation of Dalbergiae Odoriferae Lignum and counterfeit Dalbergia hainanensis Merr. Guizhou Med.J. 2001, 25, 662. [Google Scholar]
- Dong, X.N.; Chen, F.F.; Lin, L.; Huang, C.T.; Zhang, Y.M.; Liu, B. Research progress on identification techniques of Dalbergia odorifera wood. J. Zhejiang For. Sci. Technol. 2022, 42, 112–118. [Google Scholar]
- Liu, R.X.; Wang, Q.; Guo, H.Z.; Li, C.; Bi, K.S.; Guo, D.A. Simultaneous determination of 10 major flavonoids in Dalbergia odorifera by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2005, 39, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Qi, L.K.; Lin, L.; Li, Z.; Kong, J.Y. Simultaneous determination of seven flavonoids in Dalbergiae Odoriferae Lignum by UPLC and principal component analysis. Chin. J. Pharm. Anal. 2019, 39, 240–248. [Google Scholar]
- Wang, L. Studies on the Chemical Constituents of Lignum Dalbergiae Odoriferae and Extraction and Isolation Technology of Total Flavonoids. Ph.D. Thesis, College of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, China, 2008. [Google Scholar]
- Wang, J.; Jiang, W.; Wang, Y. Anti-inflammation of flavonoid compounds from Dalbergia odorifera T. Chen in lipopolysaccharide stimulated RAW264.7 macrophages. J. Cell. Mol. Immunol. 2013, 29, 681–684. [Google Scholar]
- He, X.; Yang, Y.; Zhao, X.S.; Wang, C.H.; Wei, J.H.; Meng, H. Research progress on chemical constituents and pharmacological effects of Dalbergia odorifera. Chin. Modern Tradit. Med. 2022, 24, 1149–1166. [Google Scholar]
- Meng, X.W.; Zhu, Q.; Zhang, N.; Liu, T.T.; Deng, Y.H.; Liu, Y.; Xu, Z.J.; Shao, F.; Chen, L.Y.; Liu, R.H. Rapid identification on chemical components in Dalbergiae Odoriferae Lignum by UPLC-Q-TOF-MS /MS. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 107–118. [Google Scholar]
- Liu, R.H.; Yin, X.X.; Meng, X.W.; Zhu, Q.; Liu, Y.; Yu, H.H.; Lin, L.H.; Shao, F.; Chen, L.Y. Quality evaluation of Dalbergiae Odoriferae Lignum by HPLC fingerprint and multi-component quantitative analysis. Chin. J. Tradit. Chin. Med. 2022, 47, 959–966. [Google Scholar]
- Ma, R.K.; Luo, J.; Qiao, M.J.; Fu, Y.L.; Zhu, P.C.; Wei, P.L.; Liu, Z.G. Chemical composition of extracts from Dalbergia odorifera heartwood and its correlation with colour. Ind. Crops Prod. 2022, 180, 114728. [Google Scholar] [CrossRef]
- Zhao, X.S.; Wei, J.H.; Meng, H.; Feng, J.D. Comparison of the content of liquiritigenin and fingerprint of commercial Dalbergia odorifera in different location of China. W. China J. Pharm. Sci. 2012, 27, 86–89. [Google Scholar]
| Indicator | Minimum Value | Maximum Value | Average Value | Standard Deviation | Variance | Coefficient of Variation |
|---|---|---|---|---|---|---|
| Extract/% | 14.81 | 42.74 | 27.38 | 6.92 | 47.9 | 25.28 |
| Volatile oil/% | 1.05 | 3.37 | 1.92 | 0.59 | 0.35 | 30.68 |
| Total flavonoids/% | 1.46 | 5.53 | 3.67 | 0.93 | 0.87 | 25.42 |
| Liquiritigenin/% | 0.06 | 0.55 | 0.27 | 0.08 | 0.01 | 28.58 |
| Naringenin/% | 0.16 | 1.97 | 0.73 | 0.36 | 0.13 | 49.41 |
| Formononetin/% | 0.02 | 0.16 | 0.07 | 0.03 | 0.00 | 46.26 |
| Pinocembrin/% | 0.01 | 0.29 | 0.09 | 0.06 | 0.00 | 63.39 |
| Isoliquiritigenin/% | 0.00 | 0.54 | 0.13 | 0.07 | 0.00 | 52.25 |
| Butein/% | 0.00 | 0.42 | 0.15 | 0.07 | 0.01 | 47.37 |
| L* | 38.98 | 52.91 | 44.59 | 3.29 | 10.80 | 7.37 |
| a* | 5.93 | 12.84 | 9.71 | 1.65 | 2.72 | 16.98 |
| b* | 3.83 | 16.88 | 9.44 | 2.93 | 8.61 | 31.09 |
| Indicator | Extract | Volatile Oil | Total Flavonoids | Liquiritigenin | Naringenin | Formononetin | Pinocembrin | Isoliquiritigenin | Butein | L* | a* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile oil | 0.661 ** | ||||||||||
| Total flavonoids | 0.800 ** | 0.502 ** | |||||||||
| Liquiritigenin | 0.158 | 0.168 | 0.342 * | ||||||||
| Naringenin | 0.522 ** | 0.476 ** | 0.505 ** | 0.390 ** | |||||||
| Formononetin | 0.562 ** | 0.333 * | 0.402 ** | 0.254 | 0.232 | ||||||
| Pinocembrin | 0.477 ** | 0.403 ** | 0.408 ** | 0.261 | 0.656 ** | 0.407 ** | |||||
| Isoliquiritigenin | −0.239 | −0.290 * | 0.105 | 0.682 ** | −0.075 | 0.009 | −0.059 | ||||
| Butein | −0.150 | 0.011 | −0.057 | 0.262 | 0.391 ** | −0.410 ** | 0.301 * | 0.032 | |||
| L* | −0.812 ** | −0.640 ** | -0.754 ** | −0.065 | −0.486 ** | −0.302 * | −0.340 * | 0.321 * | 0.077 | ||
| a* | −0.457 ** | −0.241 | −0.497 ** | 0.087 | −0.216 | 0.145 | 0.020 | 0.318 * | −0.072 | 0.694 ** | |
| b* | −0.817 ** | −0.533 ** | −0.777 ** | 0.024 | −0.369 ** | −0.333 ** | −0.285 * | 0.297 * | 0.204 | 0.958 ** | 0.746 ** |
| Principal Component | Initial Eigenvalue | Extract the Sum of Squares and Load | ||||
|---|---|---|---|---|---|---|
| Summation | Variance Contribution/% | Cumulative Contribution/% | Summation | Variance Contribution/% | Cumulative Contribution/% | |
| 1 | 5.095 | 56.612 | 56.612 | 5.095 | 56.612 | 56.612 |
| 2 | 1.578 | 17.534 | 74.146 | 1.578 | 17.534 | 74.146 |
| 3 | 0.887 | 9.851 | 83.996 | |||
| 4 | 0.563 | 6.261 | 90.257 | |||
| 5 | 0.314 | 3.491 | 93.748 | |||
| 6 | 0.267 | 2.971 | 96.719 | |||
| 7 | 0.147 | 1.634 | 98.353 | |||
| 8 | 0.129 | 1.428 | 99.781 | |||
| 9 | 0.020 | 0.219 | 100.000 | |||
| Principal Component | Extract | Volatile Oil | Total Flavonoids | Naringenin | Formononetin | Pinocembrin | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.927 | 0.721 | 0.864 | 0.647 | 0.483 | 0.554 | −0.924 | −0.586 | −0.902 |
| 2 | 0.064 | 0.166 | −0.055 | 0.366 | 0.551 | 0.629 | 0.270 | 0.720 | 0.345 |
| Indicator | Clustering | Inaccuracies | ||||
|---|---|---|---|---|---|---|
| Mean Square | df | Mean Square | df | F | Sig. | |
| Extract | 744.925 | 3 | 4.685 | 49 | 158.989 | 0.000 |
| Volatile oil | 2.980 | 3 | 0.187 | 49 | 15.927 | 0.000 |
| Total flavonoids | 10.202 | 3 | 0.293 | 49 | 34.835 | 0.000 |
| Naringenin | 0.625 | 3 | 0.104 | 49 | 6.037 | 0.001 |
| L* | 147.018 | 3 | 2.404 | 49 | 61.144 | 0.000 |
| b* | 115.068 | 3 | 1.843 | 49 | 62.423 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Sun, Y.; Zhang, S.; Li, J.; Feng, J.; Yang, Y.; Meng, H.; Zhang, Z. Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum. Molecules 2023, 28, 7635. https://doi.org/10.3390/molecules28227635
He W, Sun Y, Zhang S, Li J, Feng J, Yang Y, Meng H, Zhang Z. Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum. Molecules. 2023; 28(22):7635. https://doi.org/10.3390/molecules28227635
Chicago/Turabian StyleHe, Wenjie, Ying Sun, Sai Zhang, Jiawen Li, Jixing Feng, Yun Yang, Hui Meng, and Zheng Zhang. 2023. "Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum" Molecules 28, no. 22: 7635. https://doi.org/10.3390/molecules28227635
APA StyleHe, W., Sun, Y., Zhang, S., Li, J., Feng, J., Yang, Y., Meng, H., & Zhang, Z. (2023). Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum. Molecules, 28(22), 7635. https://doi.org/10.3390/molecules28227635






