Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia)
Abstract
:1. Introduction
2. Results and Discussion
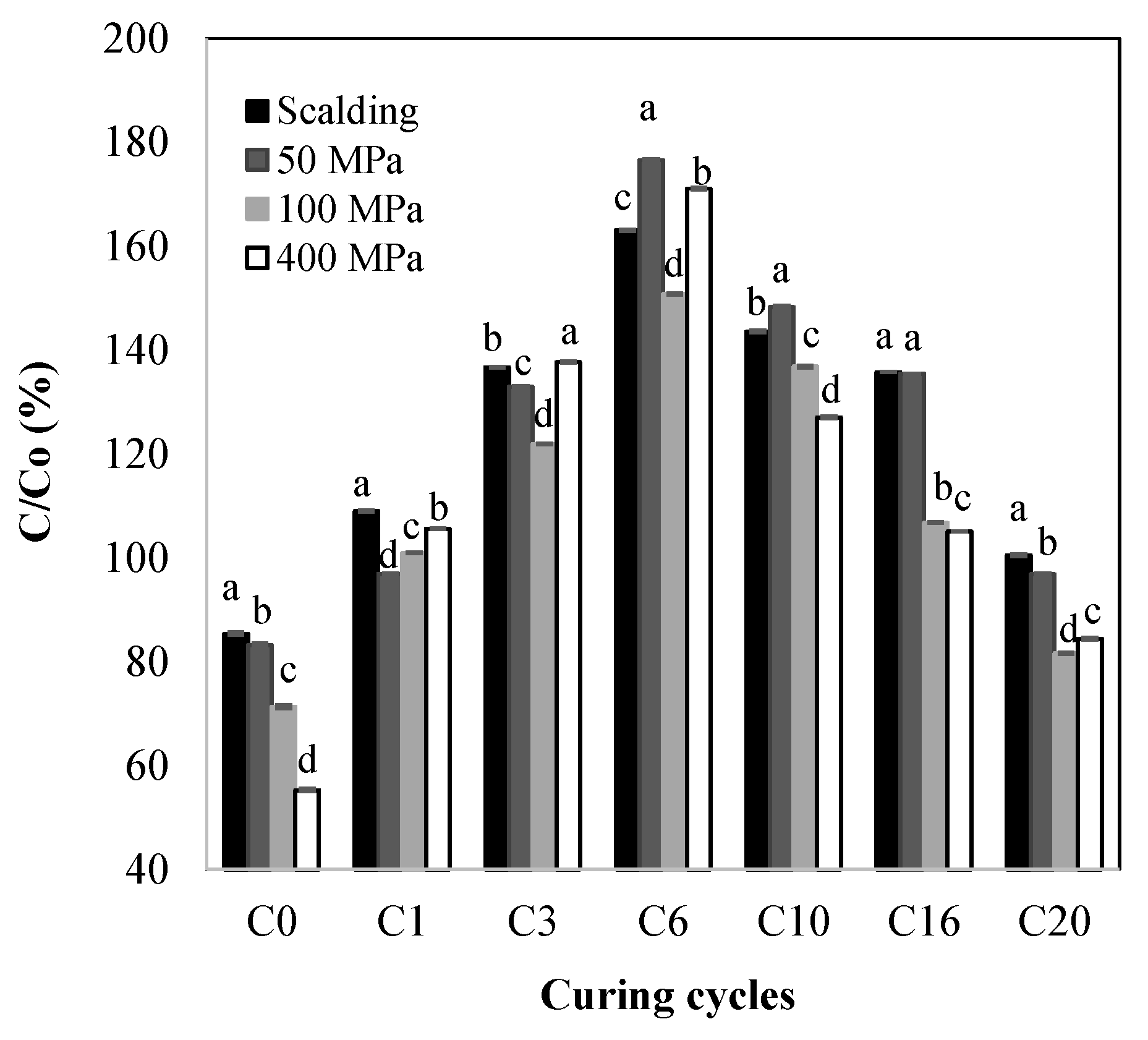
2.1. Total Phenolic Content (TPC)
2.2. Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity
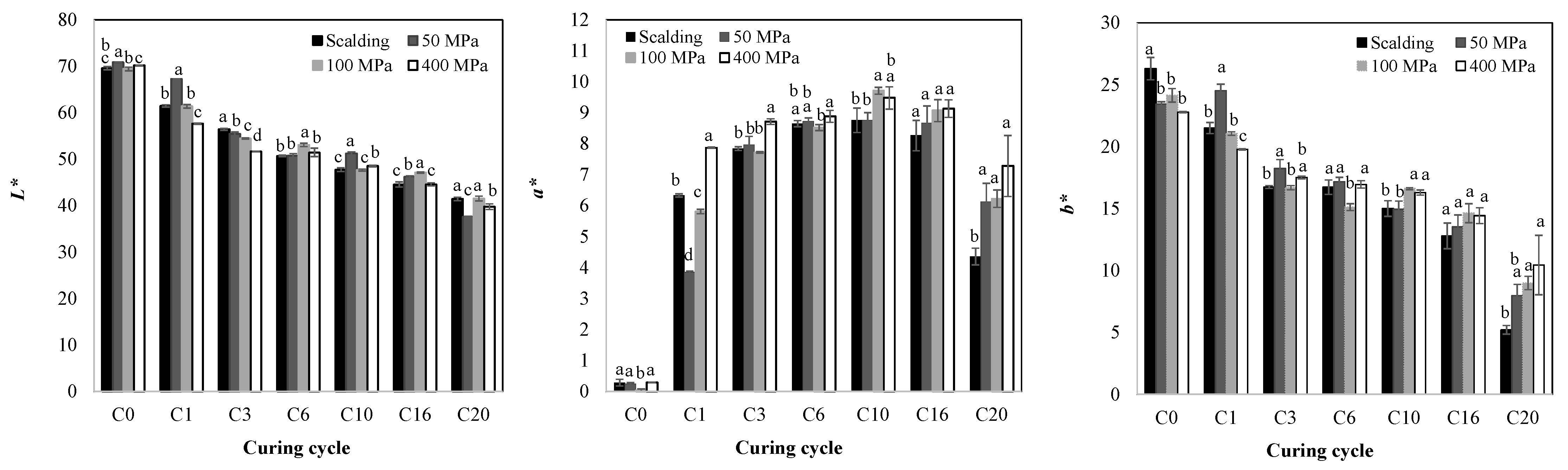
2.3. Color Parameters
3. Materials and Methods
3.1. Vanilla Beans
3.2. High Hydrostatic Pressure (HHP)-Assisted Curing
3.3. Freeze Drying
3.4. Total Phenolic Content (TPC)
3.5. Polyphenol Oxidase (PPO) Activity
3.6. Peroxidase (POD) Activity
3.7. Color Parameter Measurement
3.8. Kinetic Analysis
3.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.; Gu, F.; Hong, Y.; Chen, Y.; Xu, F.; An, K. Metabolite transformation and enzyme activities of Hainan vanilla beans during curing to improve flavor formation. Molecules 2019, 24, 2781. [Google Scholar] [CrossRef] [PubMed]
- Peña-Barrientos, A.; de Jesús Perea-Flores, M.; Martínez-Gutiérrez, H.; Patrón-Soberano, A.; González-Jiménez, F.E.; Vega-Cuellar, M.Á.; Davila-Ortiz, G. Physicochemical, microbiological, and structural relationship of vanilla beans (Vanilla planifolia, Andrews) during traditional curing process and use of its waste. J. Appl. Res. Med. Plants 2022, 32, 100445. [Google Scholar] [CrossRef]
- Odoux, E.; Escoute, J.; Verdeil, J.L. The relation between glucovanillin, β-D-glucosidase activity and cellular compartmentation during the senescence, freezing and traditional curing of vanilla beans. Ann. Appl. Biol. 2006, 149, 43–52. [Google Scholar] [CrossRef]
- Márquez, O.; Waliszewski, K.N. The effect of thermal treatment on β-glucosidase inactivation in vanilla bean (Vanilla planifolia Andrews). Int. J. Food Sci. 2008, 43, 1993–1999. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.J. Metabolite transformation and ß-D-glucosidase activity during the high hydrostatic pressure assisted curing process of vanilla beans (Vanilla planifolia) to improve phenolic compounds formation. Food Chem. 2022, 384, 132497. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Buitimea-Cantúa, G.V.; de Jesús Rostro-Alanis, M.; Gutierrez-Sánchez, A.; Navarro-Baez, J.; Welti-Chanes, J. High Hydrostatic Pressure Processing. In Smart Food Industry: The Blockchain for Sustainable Engineering; CRC Press: Boca Raton, FL, USA, 2023; pp. 248–261. [Google Scholar]
- Frenkel, C.; Ranadive, A.S.; Vázquez, J.T.; Havkin-Frenkel, D. (Eds.) Curing of vanilla. Handbook of Vanilla Science and Technology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 191–221. [Google Scholar]
- Pardío, V.T.; Flores, A.; López, K.M.; Martínez, D.I.; Márquez, O.; Waliszewski, K.N. Effect of endogenous and exogenous enzymatic treatment of green vanilla beans on extraction of vanillin and main aromatic compounds. J. Food Sci. Technol. 2018, 55, 2059–2067. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. Chemical deterioration and physical instability of foods and beverages. In The Stability and Shelf Life of Food; Woodhead Publishing: Sawston, UK, 2016; pp. 43–76. [Google Scholar]
- Gallagher, M.S.; Mahajan, P.V.; Yan, Z. Modelling chemical and physical deterioration of foods and beverages. In Food and Beverage Stability and Shelf Life; Woodhead Publishing: Sawston, UK, 2011; pp. 459–481. [Google Scholar]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2010, 97, 574–581. [Google Scholar] [CrossRef]
- Bai, J.W.; Gao, Z.J.; Xiao, H.W.; Wang, X.T.; Zhang, Q. Polyphenol oxidase inactivation and vitamin C degradation kinetics of F uji apple quarters by high humidity air impingement blanching. Int. J. Sci. Technol. 2013, 48, 1135–1141. [Google Scholar]
- Chen, C.R.; Ramaswamy, H.S. Color and texture change kinetics in ripening bananas. LWT-Food Sci. Technol. 2002, 35, 415–419. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.; Silva, C.L. Modelling the kinetics of peroxidase inactivation, colour and texture changes of pumpkin (Cucurbita maxima L.) during blanching. J. Food Eng. 2007, 81, 693–701. [Google Scholar] [CrossRef]
- Huang, W.; Ji, H.; Liu, S.; Zhang, C.; Chen, Y.; Guo, M.; Hao, J. Inactivation effects and kinetics of polyphenol oxidase from Litopenaeus vannamei by ultra-high pressure and heat. Innov. Food Sci. Emerg. Technol. 2014, 26, 108–115. [Google Scholar] [CrossRef]
- Shinwari, K.J.; Rao, P.S. Enzyme inactivation and its kinetics in a reduced-calorie sapodilla (Manilkara zapota L.) jam processed by thermal-assisted high hydrostatic pressure. Food Bioprod Proc. 2021, 126, 305–316. [Google Scholar] [CrossRef]
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Thao, B.T.T.; Vo, T.T.K.; Tran, T.Y.N.; Le, D.T.; Tran, T.T.; Bach, L.G.; Dao, T.P. Application of mathematical techniques to study the moisture loss kinetics and polyphenol degradation kinetics of mango (Mangifera indica L.) slices during heat pump drying by pilot equipment. LWT-Food Sci. Tech. 2023, 176, 114454. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Terefe, N.S. The inactivation kinetics of soluble and membrane-bound polyphenol oxidase in pear during thermal and high-pressure processing. Food Bioprocess Technol. 2018, 11, 1039–1049. [Google Scholar] [CrossRef]
- Manso, M.C.; Oliveira, F.A.; Oliveira, J.C.; Frías, J.M. Modelling ascorbic acid thermal degradation and browning in orange juice under aerobic conditions. Int. J. Food Sci. Technol. 2001, 36, 303–312. [Google Scholar] [CrossRef]
- Tapia-Ochoategui, A.P.; Camacho-Diaz, B.H.; Perea-Flores, M.J.; Ordóñez-Ruíz, I.M.; Gutierrez-Lopez, G.F.; Davila-Ortiz, G. Cambios morfométricos durante el beneficio tradicional de las vainas de vainilla (Vanilla planifolia; Orchidaceae) en México. Rev. Mex. Ing. Química 2011, 10, 105–115. [Google Scholar]
- Wen, T.N.; Prasad, K.N.; Yang, B.; Ismail, A. Bioactive substance contents and antioxidant capacity of raw and blanched vegetables. Innov. Food Sci. Emerg. Technol. 2010, 11, 464–469. [Google Scholar] [CrossRef]
- Oboh, G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Sci. Technol. 2005, 38, 513–517. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nut. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009, 114, 263–269. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pinheiro, J.; Alegria, C.; Abreu, M.; Brandão, T.R.; Silva, C.L. Degradation kinetics of peroxidase enzyme, phenolic content, and physical and sensorial characteristics in broccoli (Brassica oleracea L. ssp.) during blanching. J. Agric. Food Chem. 2009, 57, 5370–5375. [Google Scholar] [CrossRef] [PubMed]
- Liang, D. A salutary role of reactive oxygen species in intercellular tunnel-mediated communication. Front. Cell Dev. Biol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High hydrostatic pressure to increase the biosynthesis and extraction of phenolic compounds in food: A review. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef]
- Rux, G.; Gelewsky, R.; Schlüter, O.; Herppich, W.B. High hydrostatic pressure effects on membrane-related quality parameters of fresh radish tubers. Postharvest Biol. Tech. 2019, 151, 1–9. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Welti-Chanes, J.; Cano, M.P. Release mechanisms of bioactive compounds in fruits submitted to high hydrostatic pressure: A dynamic microstructural analysis based on prickly pear cells. Int. Food Res. J. 2020, 130, 108909. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzym. Microb. Tech. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Hendrickx, M.; Ludikhuyze, L.; Van den Broeck, I.; Weemaes, C. Effect of high pressure on enzymes related to food quality. Trends Food Sci. Technol. 1998, 9, 197–203. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Márquez, O.; Pardio, V.T. Quantification and characterisation of polyphenol oxidase from vanilla bean. Food Chem. 2009, 117, 196–203. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Gutiérrez, J.L.; Hernando, I.; Quiles, A. Changes in tannin solubility and microstructure of high hydrostatic pressure–treated persimmon cubes during storage at 4 C. Eur. Food Res. Tech. 2013, 237, 9–17. [Google Scholar] [CrossRef]
- Márquez, O.; Waliszewski, K.N.; Oliart, R.M.; Pardio, V.T. Purification and characterization of cell wall-bound peroxidase from vanilla bean. LWT-Food Sci. Tech. 2008, 41, 1372–1379. [Google Scholar] [CrossRef]
- Ganjloo, A.; Rahman, R.A.; Osman, A.; Bakar, J.; Bimakr, M. Kinetics of crude peroxidase inactivation and color changes of thermally treated seedless guava (Psidium guajava L.). Food Bioprocess Tech. 2011, 4, 1442–1449. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Gu, F.; Huang, F.; Wu, G.; Zhu, H. Contribution of polyphenol oxidation, chlorophyll and vitamin C degradation to the blackening of Piper nigrum L. Molecules 2018, 23, 370. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Sánchez-Ortiz, A.; Pérez, A.G. Role of polyphenol oxidase and peroxidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2011, 44, 629–635. [Google Scholar] [CrossRef]
- Dunphy, P.; Bala, K. Optimizing the traditional curing of vanilla beans. Perfum. Flavorist 2015, 40, 22–27. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1-1. [Google Scholar]
- Vásquez-Caicedo, A.L.; Schilling, S.; Carle, R.; Neidhart, S. Effects of thermal processing and fruit matrix on β-carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chem. 2007, 102, 1172–1186. [Google Scholar] [CrossRef]
- Dignum, M.J.; Kerler, J.; Verpoorte, R. β-Glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochem. Anal. 2001, 12, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.T.; Singh, R.K.; Kong, F. Fundamentals of Food Process Engineering, 4th ed.; Springer: New York, NY, USA, 2018; pp. 183–194. [Google Scholar]


| Blanched | 50 MPa | 100 MPa | 400 MPa | ||
|---|---|---|---|---|---|
| TPC increment (C0–C6) | k | 1.02 × 10−1 ± 1.79 × 10−4 d | 1.26 × 10−1 ± 2.06 × 10−4 b | 1.13 × 10−1 ± 2.43 × 10−4 c | 1.64 × 10−1 ± 4.20 × 10−4 a |
| R2 | 0.92 | 0.98 | 0.88 | 0.78 | |
| TPC decrement (C6–C20) | k | 3.07 × 10−2 ± 6.93 × 10−5 d | 3.86 × 10−2 ± 7.87 × 10−5 c | 4.35 × 10−2 ± 9.72 × 10−5 b | 4.76 × 10−2 ± 1.01 × 10−4 a |
| R2 | 0.86 | 0.91 | 0.97 | 0.97 |
| Blanched | 50 MPa | 100 MPa | 400 MPa | ||
|---|---|---|---|---|---|
| PPO (C1–C20) | k | 0.106 ± 0.012 a | 0.083 ± 0.011 a,b | 0.090 ± 0.007 a,b | 0.064 ± 0.019 b |
| R2 | 0.82 | 0.83 | 0.66 | 0.88 | |
| POD (C1–C20) | k | 0.034 ± 0.001 a | 0.029 ± 0.001 a,b | 0.025 ± 0.003 b | 0.014 ± 0.002 c |
| R2 | 0.81 | 0.93 | 0.72 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitimea-Cantúa, G.V.; Chávez-Leal, V.; Soto-Caballero, M.C.; Tellez-Medina, D.I.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia). Molecules 2023, 28, 7606. https://doi.org/10.3390/molecules28227606
Buitimea-Cantúa GV, Chávez-Leal V, Soto-Caballero MC, Tellez-Medina DI, Welti-Chanes J, Escobedo-Avellaneda Z. Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia). Molecules. 2023; 28(22):7606. https://doi.org/10.3390/molecules28227606
Chicago/Turabian StyleBuitimea-Cantúa, Génesis V., Viridiana Chávez-Leal, Mayra C. Soto-Caballero, Dario I. Tellez-Medina, Jorge Welti-Chanes, and Zamantha Escobedo-Avellaneda. 2023. "Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia)" Molecules 28, no. 22: 7606. https://doi.org/10.3390/molecules28227606
APA StyleBuitimea-Cantúa, G. V., Chávez-Leal, V., Soto-Caballero, M. C., Tellez-Medina, D. I., Welti-Chanes, J., & Escobedo-Avellaneda, Z. (2023). Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia). Molecules, 28(22), 7606. https://doi.org/10.3390/molecules28227606









