Abstract
An efficient and direct approach to pyrroles was successfully developed by employing 3-formylchromones as decarboxylative coupling partners, and facilitated by microwave irradiation. The protocol utilizes easily accessible feedstocks, a catalytic amount of DBU without any metals, resulting in high efficiency and regioselectivity. Notably, all synthesized products were evaluated against five different cancer cell lines and compound 3l selectively inhibited the proliferation of HCT116 cells with an IC50 value of 10.65 μM.
1. Introduction
Substituted pyrroles are a valuable class of five-membered nitrogen heterocycles and are considered as “privileged scaffolds” found in natural products and bioactive compounds (Figure 1) [1,2,3,4]. Molecules containing the pyrrole unit have been demonstrated to have a broad range of biological properties, including being a tubulin polymerization inhibitor [5], a Cdc7 kinase inhibitor [6], a RTK inhibitor (Sunitinib) [7,8,9], a potassium-competitive acid blocker (P-CAB) [10], a natural antibiotic (pyoluteorin) [11,12], a dual PPARα/γ agonist (saroglitazar) [13,14], and marineosin [15].
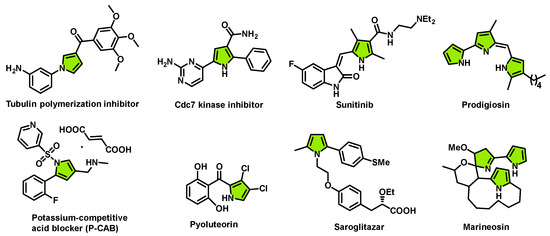
Figure 1.
Representative examples of substituted pyrrole derivatives.
Decarboxylative cyclization reactions are highly efficient strategies for synthesizing high-value organic molecules through the formation of C-C bond to form heterocycles, which serve as crucial structural motifs in a wide range of natural products and biologically active compounds [16,17]. Recently, a palladium-catalyzed three-component decarboxylation of propargylic carbonate used to produce pyrroles was described by Zhu and co-workers [18]. Cao et al. reported decarboxylative cycloaddition of β-ketoacids for synthesis of pyrrole by employing iron as catalysts through a Mannich/decarboxylative process [19]. However, the majority of representative decarboxylative cascades for synthesizing heterocyclic molecules have encountered at least one drawback: (1) a metal catalysis was required; (2) a large excess of additive was necessary; or (3) a highly activated substrate was involved (Scheme 1a). Therefore, a method capable of operating with a broad functional group tolerance and employing a catalytic amount of additive without transitional metals would be highly desirable.
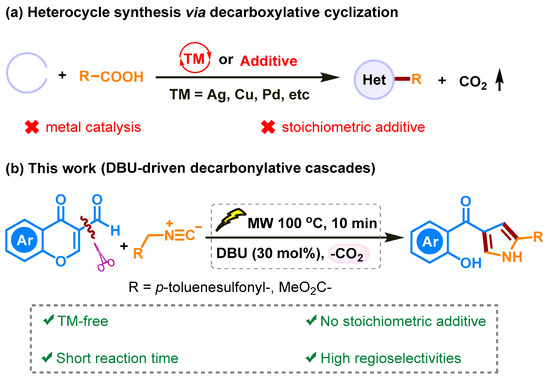
Scheme 1.
Synthesis of heterocyclic molecules by decarboxylative cyclization.
Pyrrole plays a crucial role in heterocyclic compounds due to its biological significance. The synthesis of substituted pyrroles is a significant area of research for rapidly expanding the diversity of pyrroles. Recently, Yang and colleagues reported the Ag2CO3-catalyzed cycloaddition for the synthesis of pyrroles under mild reaction conditions [20]. Furthermore, Yang’s group observed the silver-promoted cascade cyclization of 3-I-substituted chromones and ethyl isocyanoacetate, leading to the formation of chromeno[2,3-b]pyrrol-4(1H)-ones in good yields [21]. Zhao and co-workers presented Ag-catalyzed unusual transformations that enabled the synthesis of polysubstituted pyrroles through three-component reactions involving isocyanoacetates, amines, and 3-formylchromones [22]. In terms of the powerful and versatile synthon of 3-formylchromone, great efforts for synthesis of pyrroles have been made by Sliva and Ryabukhin’s group [23,24]. Recently, Kamal explored the combination of chromones and phenacyl azides using an aza-Wittig reaction [25]. The mixture of 3-formylchromones and N-phenyl glycines delivered functionalized chromeno[2,3-b]pyrrol-4(1H)-ones with CuBr as the catalyst [26]. To date, no methodology involving decarboxylation of 3-formylchromones for the synthesis of substituted pyrroles has been reported.
Over the past few years, we have been focusing on developing diverse heterocycle synthesis using a 3-formylchromone core [27,28,29]. Chromone, an undoubtedly activated Michael acceptor and a high-value starting material, has been the subject of our interest. We hypothesized that 3-formylchromone could serve as a precursor to chromone-3-carboxylic acid and directly yield substituted pyrroles. In this work, we will report on the DBU-driven [3+2] cycloaddition sequence promoting the synthesis of substituted pyrrole through oxidation, Michael reaction, and decarboxylation steps. These reactions were performed under microwave reaction conditions (Scheme 1b). The significance of this chemistry can be summarized as follows: (1) no metal catalysts; (2) no stoichiometric additives; (3) high regioselectivities; and (4) the generation of pyrroles can be achieved in just 10 min, affording moderate to good yield of products.
2. Results
To test our hypothesis, we selected the reaction between 3-formylchromone 1a and toluenesulphonylmethyl isocyanide (TosMIC) 2a as the model substrates. The reaction was carried out with DCE (1,2-dichloroethane) as the solvent. Microwave technology is a highly efficient heating technique for the preparation of bioactive compounds in organic chemistry, owing to its short reaction times, higher yields, and good regioselectivity [30]. Moreover, taking inspiration from the fact that microwave-promoted decarboxylative reactions have been successfully applied to various substrates under optimized conditions [31,32], we performed the reaction under microwave assistance (Table 1, entry 1). The results showed that 1.0 equiv. of K2CO3 produced the desired product 3a with a yield of 27%. However, most of the starting materials were left, which forced us to try other bases. After a brief screening of other basic conditions (entries 1–7), we were pleased to find that DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) resulted in the formation of 3a with a yield of 45%. These results suggested that a transition metal was not required for the decarboxylation of 3-formylchromones to produce substituted pyrroles.

Table 1.
Optimization of the reaction conditions a.
Encouraged by these promising results, we examined the solvents DMSO (dimethyl sulfoxide), DMF (N,N-dimethylformamide), toluene, THF (tetrahydrofuran), MeCN (acetonitrile), EtOH (ethanol), and 1,4-dioxane to enhance the conversion of starting materials (entries 8–15). Following the GSK solvent sustainability guide [33], 1-hexanol and N-ethylpyrrolidone were additionally employed in our method. No satisfactory yield of 3a was obtained (entries 16-17). Among the screened the solvents, MeCN obtained the substituted pyrroles with the higher yields (entry 13). When the model reaction was treated with reaction conditions using a catalytic amount of DBU, the higher yield of 3a was obtained (entry 20). This result indicated the decarboxylation reaction did not require a transition metal and the large excess of base was not essential to directly generate substituted pyrroles. Under otherwise identical conditions, by elevating reaction temperature from 80 °C to 120 °C, it was found that base-triggered oxidation/decarboxylation of 3-formylchromones worked efficiently under microwave irradiation at 100 °C (entries 18–20). Additionally, prolonged and shortened reaction times did not afford satisfactory yields (entries 21 and 22). To further confirm the powerful microwave irradiation technology, the model reaction was conducted with conventional heating at 100 °C for 1 h, resulting in a 51% yield of the desired product 3a. Therefore, the optimized reaction conditions were determined to be: 3-formylchromone (0.3 mmol), isocyanide (1.0 equiv.), DBU (30 mol%), MeCN (3.0 mL), microwave irradiation at 100 °C for 10 min.
Using the optimized reaction conditions, we evaluated the generalizability of DBU-mediated direct oxidation/decarboxylation for accessing substituted pyrroles by utilizing various 3-formylchromones and isocyanides, as shown in Scheme 2. In contrast, DBU-driven oxidation/decarboxylative [3+2] cycloaddition exhibited high chemoselectivity, and no 2-substituted chromanones were observed [34]. Under the standard reaction conditions, the yields of the 2-tosylxy-4-benzolyoxy pyrroles 3a–3p ranged from 60% to 78%. When TosMIC was used as one of the starting materials, the 3-formylchromones containing electron-donating groups (H, Me, Et, i-Pr, OCOMe) were efficiently converted into the desired compounds 3a–3e. Furthermore, the 3-formylchromones containing electron-withdrawing groups (F, Cl, di-Cl, Br) were also tolerated and yielded the final products in moderate to good yields under the optimized reaction conditions. Specifically, compound 3g (CCDC 2282072, see Figure 2) was isolated and its structure was determined by X-ray analysis, as shown in Scheme 2. However, using 6-nitrochromone-3-carboxaldehyde for the synthesis of compound 3k afforded a diminished yield. To our surprise, the reported protocols for pyrrole synthesis commonly utilize either methyl isocyanoacetate or TosMIC. To the best of our knowledge, isocyanoacetate exhibits similar properties to TosMIC in producing pyrrole analogues. In our protocol, isocyanoacetate served as an effective synthon for generating the 2-methoxycarbonyl-3-benzoyloxy pyrroles through decarboxylative [3+2] cycloaddition (3l–3p). The different substitutions on the phenyl ring of chromone-3-carboxaldehyde have little or no effect on the yield. Regrettably, DBU-mediated oxidation/decarboxylative [3+2] cycloaddition using benzyl isocyanide with inactive methylene as a substrate for obtaining compound 3q was found to be unfeasible, as shown in Scheme 2.
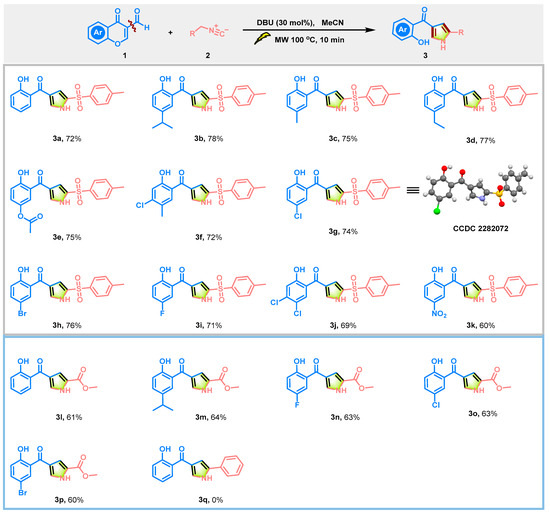
Scheme 2.
Scope of pyrroles.
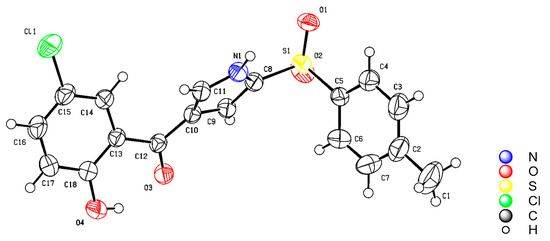
Figure 2.
X-ray of compound 3g.
In order to validate the mechanistic details, a series of control experiments was conducted. Firstly, the model substrates 1a and 2a were reacted in the presence of 3.0 equiv. of TEMPO, resulting in the isolation of the cycloaddition adduct 3a with a yield of 68%. This observation suggests that the radical pathway for the synthesis of 3a could be excluded (Scheme 3a). To elucidate the mechanism of the aldehyde group removed from 3-formylchromone, a control experiment was performed using chromone-3-carboxylic acid in Scheme 3b. Compound 4 led to the formation of the desired product 3a with a yield of 64%. Therefore, we concluded that the oxidation of the aldehyde could be followed by decarboxylative [3+2] cycloaddition. As shown in Scheme 3c, aerobic oxidation is a crucial step in obtaining the final products. The yield of final product 3a was increased to 76% under atmosphere, which proved that the oxidant for the conversion of 1a to 4 is oxygen in air. Subsequently, in order to elucidate the decarboxylation pathway, the reaction between chromone 5 and TosMIC 2a was conducted under standard reaction conditions, resulting in the detection of a trace amount of 3a (Scheme 3d). Therefore, chromone is not a key intermediate in this process.
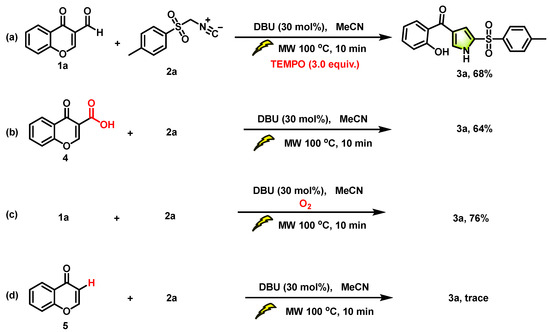
Scheme 3.
Control experiments.
Based on the experimental results presented above and relevant reports in the literature, a possible mechanistic pathway for the formation of pyrroles is proposed in Scheme 4. 3-Formylchromone is oxidized using microwave irradiation with air as the exclusive oxidant. Under basic reaction conditions, deprotonation of 2a gives rise to the nucleophilic carbanion 6. Intermediate 7 is formed through a retro-Michael addition reaction, followed by γ-pyrone ring opening to yield intermediate 8 [35]. This structure 8 further undergoes deprotonation for intermediate 9 and decarboxylation to yield intermediate 10. To the best of our knowledge, isocyanide is a well-known zwitterionic compound that can act as a nucleophile trap. The isocyano group of 10 undergoes intramolecular α-nucleophilic addition to yield the pyrrole precursor 11 [36]. Finally, the 1,2-H shift process of compound 11 leads to the aromatization and the formation of the desired product 3a.
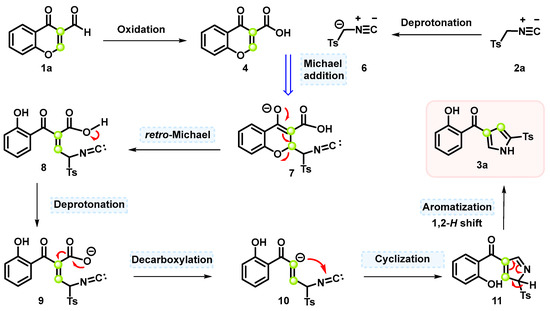
Scheme 4.
A possible mechanistic pathway.
Pyrroles are significant scaffolds in various bioactive compounds and have diverse biological applications. To assess the potential of developing a drug candidate from all the synthesized pyrroles, we selected a panel of human tumor cell lines (A549, DU145, H8, PC3, and HCT116), which are classified as difficult-to-inhibit cell lines in the National Cancer Institute’s panel of 60 human cancer cell lines (Figure 3). To our delight, 3l exhibited potent antitumor activities in vitro, with an IC50 value of 10.65 μM against HCT116 cells. Also, colony formation assay confirmed the results that smaller and lesser colonies were formed in PC3 cells treated with compound 3l, compared with a control group after exposure to inhibitors for 10 days. These assays suggest that compound 3l inhibited the proliferation and growth of HCT116 cell lines in vitro at micromolar concentrations. Moreover, 3l could be regarded as a lead compound for further modification in the discovery of an anticancer agent.
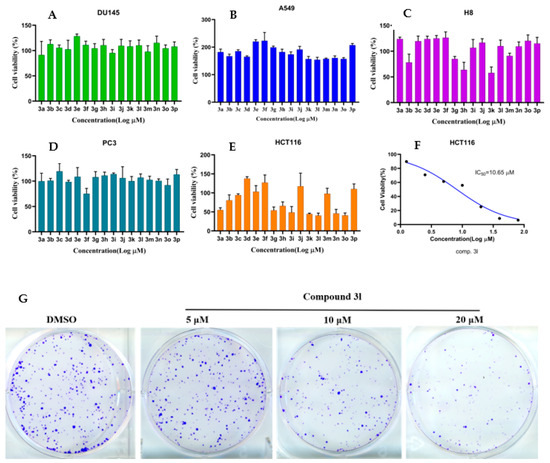
Figure 3.
Compound 3l showed excellent inhibitory activity against different cancer cell lines (A–E). Proliferation inhibition activity of 3a–3p was assessed by MTT in lung cancer cell (A549), prostate cancer cell (DU145), cervical epithelial cell (H8), prostate cancer cell (PC3), and colon cell (HCT116). These cells were treated with compounds 3a–3p at 10 μM for 48h respectively, then cell viability was measured. (F) IC50 values of compound 3l in HCT116 colon cancer cell lines. (G) Colony formation assay was conducted to evaluate the effects in vitro on the clonogenicity of PC3 cell after treating with different concentrations (0, 5, 10, and 20 μM) of compound 3l for 10 days. The colonies were visualized with the images.
3. Experimental Section
3.1. General Information
Each component of laboratory glassware was oven-dried, then used for carrying out the general experimental procedures. 1H and 13C NMR were recorded on a Bruker 400 spectrometer, frequency: 6–440 MHz; frequency resolution: ≤0.005 Hz; sensitivity: 1H ≥ 500:1, 13C ≥ 220:1, 19F ≥ 500:1, probe: 5 mm BBFO Z-gradient. 1H NMR data are reported as follows: chemical shift in ppm (δ), multiplicity (s = singlet, d = doublet, t= triplet, m = multiplet), coupling constant (Hz), relative intensity. 13C NMR data are reported as follows: chemical shift in ppm (δ). HPLC-MS analyses were performed on a Shimadzu-2020 LC-MS instrument using the following conditions: Shim-pack VP-ODS C18 column (reverse phase, 150 × 2.0 mm); 90% acetonitrile and 10% water over 6.0 min; flow rate of 0.5 mL/min; UV photodiode array detection from 200 to 400 nm. High-resolution mass spectra (HRMS) were recorded on a Q Exactive hybrid quadrupole-Orbitrap mass spectrometer (Bremen, Germany, Thermo Fisher Scientific) with an ESI source of 140,000 fwhm, the AGC target set to 1 × 106, and a scan range of 100–1000 m/z. The raw data were deconvoluted using Xcalibur 4.1. The products were purified by Biotage Isolera™ Spektra Systems and Hexane/EtOAc solvent systems. All reagents and solvents were obtained from commercial sources and used without further purification.
3.2. Microwave Irradiation Experiments
All microwave irradiation experiments were carried out in a Biotage Initiator Classic microwave apparatus with continuous irradiation power from 0 to 400 W with utilization of the standard absorbance level of 250 W maximum power. The reactions were carried out in 10 mL glass tubes, sealed with a microwave cavity. The reaction was irradiated at the required ceiling temperature (the reaction temperature was monitored by an external surface sensor using the Biotage Initiator reactor) using maximum power for the stipulated time. Then, it was cooled to 50 °C with gas jet cooling.
3.3. Cell Lines and Culture and Viability Assay
Cell lines and culture. The human lung cancer cell A549, prostate cancer cell DU145, cervical epithelial cell H8, prostate cancer cell PC3, and colon carcinoma cells HCT116 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cancer cells were cultured in high-glucose DMEM (Hyclone, SH30022.01, Logan, UT, USA) medium supplemented with 10% fetal bovine serum (FBS, Gibco, 10099, Thomastown, Australia origin). The cells were maintained in the incubator at 37 °C and 5% CO2 with humidified atmosphere.
MTT assay. Cancer cells were counted and seeded into the 96-well plate containing 100 µL complete medium; the density of the cells was 3 × 103 cells per well. After incubation for 24 h, another 100 µL complete medium containing 10 µM compounds 3 or equal amount of dimethyl sulfoxide (DMSO) was added; each treatment was triple replicated. The compound-treated cells were cultured for another 48 h, 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT, Beyotime, ST316, Shanghai, China) was added, and the plate was incubated for another 4 h. After incubation, the medium was removed and 150 μL DMSO was added into each well to dissolve the formazan. The optical density (OD) of each well was measured with a microplate reader (Bio-Tek, Winooski, VT, USA) at an absorbance wavelength of 570 nm. The viability of the compound-treated cells equals the ratio of ODcompound to ODDMSO.
Colony formation assay. HCT116 cells were harvested at a density of 80% and seeded onto the 6-well plates with 400 cells per well containing 1 mL medium. After 24 h incubation, HCT116 cells were treated with compound 3l with different concentrations (0, 5, 10, and 20 μM) for 48 h, then changed to a fresh medium without compound 3l and incubated for another 10 days. After that, HCT116 cells were washed with PBS three times and fixed with 4% paraformaldehyde for 25 min. Finally, the cells were further washed with PBS three times again, and stained with a 0.5% crystal violet staining solution (Beyotime, C0121, Shanghai, China).
3.4. Synthetic Procedures for the Synthesis of Compound 3
3-Formylchromone (52 mg, 0.3 mmol), isocyanide (0.3 mmol), and DBU (14 mg, 30 mol %) were dissolved in MeCN (3.0 mL) in a 5 mL microwave vial. Then, the mixture was sealed and heated under microwave irradiation at 100 °C for 10 min. The reaction mixture was then cooled to room temperature and concentrated under reduced pressure to give a residue, which was diluted with EtOAc (15.0 mL), before being washed sequentially with saturated brine. The organic solution was then dried over MgSO4 and concentrated to give a residue, which was purified by column chromatography over silica gel eluting with a gradient of ethyl acetate/hexane (0−50%) to afford the relative pyrrole compound 3.
(2-Hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3a), a white solid (72%), Rf = 0.25 (n-Hexane/EtOAc, 8:2), m.p. = 135–136 °C. 1H NMR (400 MHz, CDCl3) δ 11.80 (s, 1H), 10.22 (s, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.74 (dd, J = 8.0, 1.5 Hz, 1H), 7.48 (dd, J = 3.1, 1.6 Hz, 1H), 7.45 − 7.38 (m, 1H), 7.28 − 7.20 (m, 3H), 6.98 − 6.93 (m, 1H), 6.88 − 6.81 (m, 1H), 2.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 193.14, 162.55, 144.90, 138.24, 135.96, 131.65, 130.85, 130.18, 127.92, 127.19, 125.41, 119.76, 119.00, 118.43, 116.28, 21.64. HRMS (ESI) m/z: [M+H]+ Calcd for C18H16NO4S+ 342.0795; Found 342.0795.
(2-Hydroxy-5-isopropylphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3b), a white solid (78%), Rf = 0.25 (n-Hexane/EtOAc, 8:2), m.p. = 138–139 °C. 1H NMR (400 MHz, CDCl3) δ 11.56 (s, 1H), 9.78 (s, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.53 (d, J = 2.2 Hz, 1H), 7.45 (dd, J = 3.2, 1.6 Hz, 1H), 7.31 (dd, J = 8.6, 2.3 Hz, 1H), 7.26 (d, J = 8.1 Hz, 2H), 7.17 (dd, J = 2.5, 1.7 Hz, 1H), 6.90 (d, J = 8.6 Hz, 1H), 2.80 (dt, J = 13.8, 6.9 Hz, 1H), 2.36 (s, 3H), 1.15 (d, J = 6.9 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 193.16, 160.64, 144.85, 139.26, 138.33, 134.52, 131.02, 130.15, 128.84, 127.25, 125.70, 119.46, 118.23, 116.11, 33.22, 24.05, 21.64. HRMS (ESI) m/z: [M+H]+ Calcd for C21H22NO4S+ 384.1265; Found 384.1262.
(2-Hydroxy-5-methylphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3c), a white solid (75%), Rf = 0.23 (n-Hexane/EtOAc, 8:2), m.p. = 134–135 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.10 (s, 1H), 10.54 (s, 1H), 7.86 (d, J = 8.1 Hz, 2H), 7.63 (s, 1H), 7.44 (d, J = 8.1 Hz, 2H), 7.31 (s, 1H), 7.24 (d, J = 8.2 Hz, 1H), 7.13 (s, 1H), 6.86 (d, J = 8.3 Hz, 1H), 2.38 (s, 3H), 2.24 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 191.36, 156.01, 144.75, 139.03, 134.62, 131.03, 130.86, 130.57, 128.21, 127.42, 125.25, 124.25, 117.35, 116.06, 21.50, 20.42. HRMS (ESI) m/z: [M+H]+ Calcd for C19H18NO4S+ 356.0952; Found 356.0953.
(5-Ethyl-2-hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3d), a white solid (77%), Rf = 0.25 (n-Hexane/EtOAc, 8:2), m.p. = 137–138 °C. 1H NMR (400 MHz, CDCl3) δ 11.59 (s, 1H), 9.86 (s, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.52 (d, J = 2.1 Hz, 1H), 7.46 (dd, J = 3.1, 1.6 Hz, 1H), 7.30 − 7.20 (m, 4H), 6.89 (d, J = 8.5 Hz, 1H), 2.53 (q, J = 7.6 Hz, 2H), 2.35 (s, 3H), 1.14 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 193.14, 160.61, 144.86, 138.30, 135.89, 134.62, 130.20, 127.52, 127.23, 125.63, 119.52, 118.26, 116.17, 27.96, 21.65, 15.73. HRMS (ESI) m/z: [M+H]+ Calcd for C20H20NO4S+ 370.1108; Found 370.1109.
4-Hydroxy-3-(5-tosyl-1H-pyrrole-3-carbonyl)phenyl acetate (3e), a white solid (75%), Rf = 0.21 (n-Hexane/EtOAc, 8:2), m.p. = 133–134 °C. 1H NMR (400 MHz, CDCl3) δ 12.05 (s, 1H), 9.92 (s, 1H), 7.77 (d, J = 8.2 Hz, 3H), 7.46 (s, 1H), 7.26 (d, J = 8.1 Hz, 2H), 6.71 (d, J = 2.1 Hz, 1H), 6.62 (dd, J = 8.7, 2.1 Hz, 1H), 2.35 (s, 3H), 2.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 192.26, 168.68, 164.19, 156.23, 144.92, 138.24, 132.88, 131.11, 130.19, 127.69, 127.24, 125.31, 116.04, 112.91, 111.27, 21.65, 21.22. HRMS (ESI) m/z: [M+H]+ Calcd for C20H18NO6S+ 400.0850; Found 400.0851.
(4-Chloro-2-hydroxy-5-methylphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3f), a white solid (72%), Rf = 0.23 (n-Hexane/EtOAc, 8:2), m.p. = 131–132 °C. 1H NMR (400 MHz, CDCl3) δ 11.69 (s, 1H), 10.17 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.66 (s, 1H), 7.46 (dd, J = 3.0, 1.6 Hz, 1H), 7.26 (d, J = 8.2 Hz, 2H), 7.20 (d, J = 2.2 Hz, 1H), 6.84 (s, 1H), 2.34 (s, 3H), 2.31 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 191.65, 161.02, 145.13, 144.95, 138.20, 131.20, 130.95, 130.19, 127.68, 127.22, 125.06, 124.30, 120.49, 118.67, 116.00, 21.61, 20.71. HRMS (ESI) m/z: [M+H]+ Calcd for C19H17ClNO4S+ 390.0562; Found 390.0560.
(5-Chloro-2-hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3g), a white solid (74%), Rf = 0.23 (n-Hexane/EtOAc, 8:2), m.p. = 133–134 °C. 1H NMR (400 MHz, CDCl3) δ 11.66 (s, 1H), 10.28 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 2.6 Hz, 1H), 7.47 (dd, J = 3.2, 1.6 Hz, 1H), 7.35 (dd, J = 8.9, 2.6 Hz, 1H), 7.27 (d, J = 8.2 Hz, 2H), 7.22 − 7.19 (m, 1H), 6.92 (d, J = 8.9 Hz, 1H), 2.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 192.00, 160.98, 145.01, 138.12, 135.73, 131.40, 130.55, 130.22, 127.96, 127.24, 124.86, 123.69, 120.43, 120.08, 116.04, 21.65. HRMS (ESI) m/z: [M+H]+ Calcd for C18H15ClNO4S+ 376.0405; Found 376.0404.
(5-Bromo-2-hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3h), A white solid (76%), Rf = 0.25 (n-Hexane/EtOAc, 8:2), m.p. = 130–131 °C. 1H NMR (400 MHz, CDCl3) δ 11.67 (s, 1H), 10.31 (s, 1H), 7.79 (d, J = 7.8 Hz, 3H), 7.50 − 7.44 (m, 2H), 7.26 (d, J = 8.1 Hz, 2H), 7.22 − 7.19 (m, 1H), 6.86 (d, J = 8.9 Hz, 1H), 2.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 191.90, 161.42, 145.04, 138.50, 138.08, 133.54, 131.37, 130.24, 128.02, 127.25, 124.84, 121.07, 120.48, 116.08, 110.56, 21.66. HRMS (ESI) m/z: [M+H]+ Calcd for C18H15BrNO4S+ 419.9900; Found 419.9900.
(5-Fluoro-2-hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3i), a white solid (71%), Rf = 0.23 (n-Hexane/EtOAc, 8:2), m.p. = 138–139 °C. 1H NMR (400 MHz, CDCl3) δ 11.49 (s, 1H), 9.85 (s, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.51 − 7.45 (m, 1H), 7.41 (dd, J = 8.8, 3.1 Hz, 1H), 7.27 (d, J = 8.2 Hz, 2H), 7.19 − 7.13 (m, 2H), 6.93 (dd, J = 9.1, 4.6 Hz, 1H), 2.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 192.11, 158.70, 156.17, 153.68, 144.98, 138.17, 131.45, 130.20, 129.93, 127.55, 125.13, 123.50, 119.80, 116.62, 115.84, 21.65. HRMS (ESI) m/z: [M+H]+ Calcd for C18H15NO4S+ 360.0701; Found 360.0700.
(4,5-Dichloro-2-hydroxyphenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3j), a white solid (69%), Rf = 0.24 (n-Hexane/EtOAc, 8:2), m.p. = 140–141 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.20 (s, 1H), 10.72 (s, 1H), 7.87 (d, J = 8.3 Hz, 2H), 7.73 (d, J = 2.6 Hz, 1H), 7.67 (dd, J = 3.3, 1.7 Hz, 1H), 7.50 − 7.38 (m, 3H), 7.20 − 7.09 (m, 1H), 2.38 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 188.60, 151.69, 144.83, 138.91, 132.36, 131.70, 131.67, 130.57, 128.68, 128.31, 127.46, 124.82, 123.64, 123.34, 115.77, 21.51. HRMS (ESI) m/z: [M+H]+ Calcd for C18H14Cl2NO4S+ 410.0016; Found 410.0017.
(2-Hydroxy-5-nitrophenyl)(5-tosyl-1H-pyrrol-3-yl)methanone (3k), a white solid (60%), Rf = 0.17 (n-Hexane/EtOAc, 8:2), m.p. = 145–146 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.14 (s, 1H), 11.57 (s, 1H), 8.25 (dd, J = 9.1, 2.8 Hz, 1H), 8.15 (d, J = 2.8 Hz, 1H), 7.86 (d, J = 8.2 Hz, 2H), 7.65 (s, 1H), 7.44 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 9.0 Hz, 2H), 2.38 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 187.87, 161.93, 144.80, 139.78, 138.95, 131.54, 131.48, 130.57, 128.02, 127.41, 125.82, 125.52, 117.71, 115.61, 21.50. HRMS (ESI) m/z: [M+H]+ Calcd for C18H15N2O6S+ 387.0646; Found 387.0645.
Methyl 4-(2-hydroxybenzoyl)-1H-pyrrole-2-carboxylate (3l), a white solid (61%), Rf = 0.30 (n-Hexane/EtOAc, 8:2), m.p. = 124–125 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.72 (s, 1H), 10.94 (s, 1H), 7.60 (dd, J = 7.7, 1.6 Hz, 1H), 7.56 (dd, J = 3.4, 1.6 Hz, 1H), 7.44 (ddd, J = 8.7, 7.4, 1.7 Hz, 1H), 7.13 (dd, J = 2.4, 1.7 Hz, 1H), 7.01 − 6.88 (m, 2H), 3.81 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 191.83, 160.97, 158.55, 134.01, 130.81, 129.91, 125.31, 124.33, 123.90, 119.51, 117.52, 116.36, 52.07. HRMS (ESI) m/z: [M+H]+ Calcd for C13H12NO4+ 246.0761; Found 246.0757.
Methyl 4-(2-hydroxy-5-isopropylbenzoyl)-1H-pyrrole-2-carboxylate (3m), a white solid (64%), Rf = 0.32 (n-Hexane/EtOAc, 8:2), m.p. = 128–129 °C. 1H NMR (400 MHz, CDCl3) δ 11.83 (s, 1H), 9.63 (s, 1H), 7.73 (d, J = 2.3 Hz, 1H), 7.59 (dd, J = 3.2, 1.6 Hz, 1H), 7.39 (ddd, J = 5.4, 4.0, 2.0 Hz, 2H), 7.01 (d, J = 8.5 Hz, 1H), 3.94 (s, 3H), 2.92 (dt, J = 13.8, 6.9 Hz, 1H), 1.27 (d, J = 6.9 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 193.69, 161.18, 160.64, 139.13, 134.15, 129.04, 127.46, 125.35, 123.92, 119.72, 118.15, 116.45, 52.05, 33.29, 24.11. HRMS (ESI) m/z: [M+H]+ Calcd for C16H18NO4+ 288.1231; Found 288.1229.
Methyl 4-(5-fluoro-2-hydroxybenzoyl)-1H-pyrrole-2-carboxylate (3n), a white solid (63%), Rf = 0.30 (n-Hexane/EtOAc, 8:2), m.p. = 126–127 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.71 (s, 1H), 10.33 (s, 1H), 7.53 (s, 1H), 7.25 (t, J = 8.2 Hz, 2H), 7.09 (s, 1H), 6.96 (dd, J = 8.2, 4.3 Hz, 1H), 3.80 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 194.54, 161.17, 158.83, 158.03, 134.93, 131.38, 130.24, 128.79, 124.57, 123.35, 120.89, 120.47, 56.83. HRMS (ESI) m/z: [M+H]+ Calcd for C13H11FNO4+ 264.0667; Found 264.0664.
Methyl 4-(5-chloro-2-hydroxybenzoyl)-1H-pyrrole-2-carboxylate (3o), a white solid (63%), Rf = 0.33 (n-Hexane/EtOAc, 8:2), m.p. = 123–124 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.70 (s, 1H), 10.48 (s, 1H), 7.50 (dd, J = 3.3, 1.6 Hz, 1H), 7.41 (dd, J = 8.7, 2.7 Hz, 1H), 7.37 (d, J = 2.6 Hz, 1H), 7.08 − 7.04 (m, 1H), 6.98 (d, J = 8.7 Hz, 1H), 3.80 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 194.14, 165.71, 160.16, 137.05, 134.92, 133.78, 132.83, 130.39, 128.79, 127.68, 123.80, 120.80, 56.83. HRMS (ESI) m/z: [M+H]+ Calcd for C13H11ClNO4+ 280.0372; Found 280.0376.
Methyl 4-(5-bromo-2-hydroxybenzoyl)-1H-pyrrole-2-carboxylate (3p), a white solid (60%), Rf = 0.32 (n-Hexane/EtOAc, 8:2), m.p. = 125–126 °C. 1H NMR (400 MHz, CDCl3) δ 11.84 (s, 1H), 9.74 (s, 1H), 7.91 (d, J = 2.4 Hz, 1H), 7.53 (dd, J = 3.1, 1.5 Hz, 1H), 7.49 (dd, J = 8.9, 2.4 Hz, 1H), 7.31 − 7.26 (m, 1H), 6.89 (d, J = 8.9 Hz, 1H), 3.86 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 192.42, 161.52, 161.11, 138.25, 133.72, 127.78, 124.67, 124.31, 121.37, 120.43, 116.31, 110.43, 52.11. HRMS (ESI) m/z: [M+H]+ Calcd for C13H11BrNO4+ 323.9866; Found 323.9863.
The results of the X-ray diffraction analysis for compound 3g were deposited with the Cambridge Crystallographic Data Centre (CCDC 2282072) (Supplementary Material).
4. Conclusions
In summary, we have developed a novel protocol for the synthesis of substituted pyrroles using DBU-driven sequences. This transformation involved the cleavage of one old C-C bond and the formation of two new C-C bonds in the process of aldehyde oxidation, Michael/retro-Michael decarboxylation, [3+2] cycloaddition, and aromatization. After investigating the generalizability of this cascade reaction, we found that the reaction performed well under optimized conditions, yielding a series of pyrroles in moderate to good yields. All synthesized compounds were tested using the MTT assay, and 3l exhibited potent anti-colon cancer activity in vitro. Currently, we are making further efforts to develop efficient decarboxylation cycloaddition methods for the synthesis of privileged scaffolds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28227602/s1, including 1H and 13C NMR. In addition, crystallographic data on compound 3g (CCDC 2282072) (CIF) are provided in the supporting information.
Author Contributions
Conceptualization, Z.-Z.C. and Z.-G.X.; methodology, X.-Y.C., B.-Y.F. and Q.Y.; formal analysis, X.L. and J.L.; investigation, X.-Y.C., B.-Y.F. and Q.Y.; writing—original draft preparation, X.L., J.L. and Z.-G.X.; supervision, Z.-Z.C. and Z.-G.X. All authors approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202301349, KJQN202201302, KJQN202101340, and KJZD-M201801301), the Natural Science Foundation Project of CQ CSTSC (cstc2021ycjh-bgzxm0163, cstc2020jcyj-msxmX0849, and cstc2021jcyj-bshX0242), and the Chongqing University of Arts and Sciences: Program for Talents Introduction (R2022YX07, R2021FYX05, and P2022YX10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to thank H.-Z.L. for obtaining the LC/MS, HRMS, and NMR data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matsui, K.; Shibuya, M.; Yamamoto, Y. Synthesis of pyrroles via ruthenium-catalyzed nitrogen-transfer [2 + 2 + 1] cycloaddition of α,ω-diynes using sulfoximines as nitrene surrogates. Commun. Chem. 2018, 1, 21–28. [Google Scholar] [CrossRef]
- Shi, T.; Yin, G.F.; Wang, X.D.; Xiong, Y.X.; Peng, Y.; Li, S.; Zeng, Y.F.; Wang, Z. Recent advances in the syntheses of pyrroles. Green Synth. Catal. 2023, 4, 20–34. [Google Scholar] [CrossRef]
- Michlik, S.; Kempe, R. A sustainable catalytic pyrrole synthesis. Nat. Chem. 2013, 5, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiou, M.-F.; Li, Y.; Ye, C.; Su, M.; Xue, M.; Yuan, X.; Wang, C.; Wan, W.-M.; Li, D.; et al. Synthesis of unsymmetrically tetrasubstituted pyrroles and studies of AIEE in pyrrolo[1,2-a]pyrimidine derivatives. Chem. Sci. 2022, 13, 5667–5673. [Google Scholar] [CrossRef]
- La Regina, G.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New pyrrole derivatives with potent tubulin polymerization inhibiting activity as anticancer agents including hedgehog-dependent cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef]
- Bailey, D.M.; Johnson, R.E.; Salvador, U.J. Pyrrole antibacterial agents. 1. Compounds related to pyoluteorin. J. Med. Chem. 1973, 16, 1298–1300. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Zhang, J.; Hu, X.; Liu, Y.; Yin, H.; Lv, T.; Zhang, H.; Liu, L.; An, H.; et al. Sunitinib induces cellular senescence via p53/Dec1 activation in renal cell carcinoma cells. Cancer Sci. 2013, 104, 1052–1061. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef]
- Liu, J.; Ming, B.; Gong, G.H.; Wang, D.; Bao, G.L.; Yu, L.J. Current research on anti-breast cancer synthetic compounds. RSC Adv. 2018, 8, 4386–4416. [Google Scholar] [CrossRef]
- Abdel-Aziz, Y.; Metz, D.C.; Howden, C.W. Review article: Potassium-competitive acid blockers for the treatment of acid-related disorders. Aliment. Pharmacol. Ther. 2021, 53, 794–809. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, M.; Kidarsa, T.; Johnson, C.P.; Loper, J.E. Two pathway-specific transcriptional regulators, PltR and PltZ, coordinate autoinduction of pyoluteorin in pseudomonas protegens Pf-5. Microorganisms 2021, 9, 1489. [Google Scholar] [CrossRef]
- Menichincheri, M.; Albanese, C.; Alli, C.; Ballinari, D.; Bargiotti, A.; Caldarelli, M.; Ciavolella, A.; Cirla, A.; Colombo, M.; Colotta, F.; et al. Cdc7 kinase inhibitors: 5-heteroaryl-3-carboxamido-2-aryl pyrroles as potential antitumor agents. 1. Lead finding. J. Med. Chem. 2010, 53, 7296–7315. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Miranda-Cervantes, A.; Licea-Navarro, A.; Mansour, C.; Beltrán-Partida, E.; Donis-Maturano, L.; Delgado De la Herrán, H.C.; Villarreal, F.; Álvarez-Delgado, C. (-)-Epicatechin stimulates mitochondrial biogenesis and cell growth in C2C12 myotubes via the G-protein coupled estrogen receptor. Eur. J. Pharmacol. 2018, 822, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R. The first approved agent in the Glitazar’s Class: Saroglitazar. Curr. Drug Targets 2014, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Boonlarppradab, C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Marineosins A and B, cytotoxic spiroaminals from a marine-derived actinomycete. Org. Lett. 2008, 10, 5505–5508. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiu, G.; Li, C.; Sun, K.; Wang, Z.C.; Wang, X. Heterocycle synthesis via decarboxylative cyclization methods. Adv. Synth. Catal. 2022, 364, 3756–3781. [Google Scholar] [CrossRef]
- Xu, X.J.; Van der Eycken, E.V.; Feng, H.D. Metal-free decarboxylation of α,β-unsaturated carboxylic acids for carbon-carbon and carbon-heteroatom coupling reactions. Chin. J. Chem. 2020, 38, 1780–1792. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, Q.; Zhu, J.P. Palladium-catalyzed three-component reaction of propargyl carbonates, isocyanides, and alcohols or water: Switchable synthesis of pyrroles and its bicyclic analogues. Org. Lett. 2017, 19, 270–273. [Google Scholar] [CrossRef]
- Hu, H.; Wang, C.; Lai, H.; Wang, S.; Ni, H.; Yu, W.; Cao, P. Fe(III)-catalyzed decarboxylative cycloaddition of β-Ketoacids and 2H-azirines for the synthesis of pyrrole derivatives. Org. Chem. Front. 2020, 7, 3686–3691. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, H.; Yang, Y.; Yang, C. Synthesis of substituted pyrroles using a silver-catalysed reaction between isocyanoacetates/benzyl isocyanides and chromones. RSC Adv. 2015, 5, 98549–98552. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, H.; Yang, C. Synthesis of functionalized chromeno[2,3-b]pyrrol-4(1H)-ones by silver-catalyzed cascade reactions of chromones/thiochromones and isocyanoacetates. Org. Lett. 2015, 17, 5590–5593. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Yap, W.J.; Wu, J.E.; Wong, M.W.; Zhao, Y. Three-component reactions of isocyanoacetates, amines and 3-formylchromones initiated by an unexpected aza-Michael addition. Chem. Commun. 2017, 53, 9067–9070. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.G.P.R.; Tome, A.C.; Silva, A.M.S.; Cavaleiro, J.A.S. Reaction of chromone-3-carbaldehyde with a-amino acids-syntheses of 3- and 4-(2-hydroxybenzoyl)pyrroles. Tetrahedron 2007, 63, 910–917. [Google Scholar] [CrossRef]
- Plaskon, A.; Ryabukhin, S.V.; Volochnyuk, D.M.; Shivanyuk, A.N.; Tolmachev, A.A. A synthesis of 5-hetaryl-3-(2-hydroxybenzoyl)pyrroles. Tetrahedron 2008, 64, 5933–5943. [Google Scholar] [CrossRef]
- Jadala, C.; Prasad, B.; Prasanthi, A.V.G.; Shankaraiah, N.; Kamal, A. Transition metal-free one-pot synthesis of substituted pyrroles by employing aza-Wittig reaction. RSC Adv. 2019, 9, 30659–30665. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.D.; Lv, Y.; Lu, Z.H.; Yan, S.J. Cu-Catalyzed decarboxylative annulation of N-substituted glycines with 3-formylchromones: Synthesis of functionalized chromeno[2,3-b]pyrrol-4(1H)-ones. Chem. Commun. 2022, 58, 10194–10197. [Google Scholar] [CrossRef]
- An, Y.N.; Huang, J.H.; Xu, S.F.; Wang, X.L.; Zhou, C.H.; Xu, Z.G.; Lei, J.; Chen, Z.Z. Unexpected cascade sequence forming the C(sp3)–N/C(sp2)–C(sp2) bond: Direct access to γ-lactam-fused pyridones with anticancer activity. J. Org. Chem. 2023, 88, 7998–8009. [Google Scholar] [CrossRef]
- Lei, J.; Ding, Y.; Zhou, H.-Y.; Gao, X.-Y.; Cao, Y.-H.; Tang, D.-Y.; Li, H.Y.; Xu, Z.-G.; Chen, Z.-Z. Practical synthesis of quinolone drugs via a novel TsCl-mediated domino reaction sequence. Green Chem. 2022, 24, 5755–5759. [Google Scholar] [CrossRef]
- Lei, J.; Li, Y.; Xu, J.; Tang, D.-Y.; Shao, J.-W.; Li, H.; Chen, Z.-Z.; Xu, Z.-G. An acid-catalyzed 1,4-addition isocyanide-based multicomponent reaction in neat water. Green Chem. 2020, 22, 3716–3720. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Pinto, D.C.G.A.; Silva, A.M.S. Microwave irradiation: Alternative heating process for the synthesis of biologically applicable chromones, quinolones, and their precursors. Molecules 2021, 26, 6293. [Google Scholar] [CrossRef]
- Goossen, L.J.; Manjolinho, F.; Khan, B.A.; Rodríguez, N. Microwave-assisted Cu-catalyzed protodecarboxylation of aromatic carboxylic acids. J. Org. Chem. 2009, 74, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Åberg, V.; Norman, F.; Chorell, E.; Westermark, A.; Olofsson, A.; Sauer-Erikssonb, A.E.; Almqvist, F. Microwave-assisted decarboxylation of bicyclic 2-pyridone scaffolds and identification of A β-peptide aggregation inhibitors. Org. Biomol. Chem. 2005, 3, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Neo, A.G.; Díaz, J.; Marcaccinib, S.; Marcos, C.F. Conjugate addition of isocyanides to chromone 3-carboxylic acid: An efficient one-pot synthesis of chroman-4-one 2-carboxamides. Org. Biomol. Chem. 2012, 10, 3406–3416. [Google Scholar] [CrossRef]
- Lei, J.; Xu, J.; Tang, D.-Y.; Shao, J.-W.; Li, H.; Chen, Z.-Z.; Xu, Z.-G. A concise and unexpected one-pot methodology for the synthesis of pyrazinone-fused pyridines. Org. Chem. Front. 2020, 7, 2657–2663. [Google Scholar] [CrossRef]
- Qu, C.-H.; Song, G.-T.; Huang, J.-H.; Huang, R.; Chen, Y.; Liu, T.; Tang, D.-Y.; Xu, Z.-G.; Chen, Z.-Z. Tandem isonitrile insertion/azacyclopropylideneannulated cyclohexenone–tropone rearrangement of p-QMs and TosMIC: De novo synthesis of pyrrolotropones with anti-cancer activity. Org. Chem. Front. 2021, 8, 6515–6521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).