Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan
Abstract
:1. Introduction
2. Results and Discussion
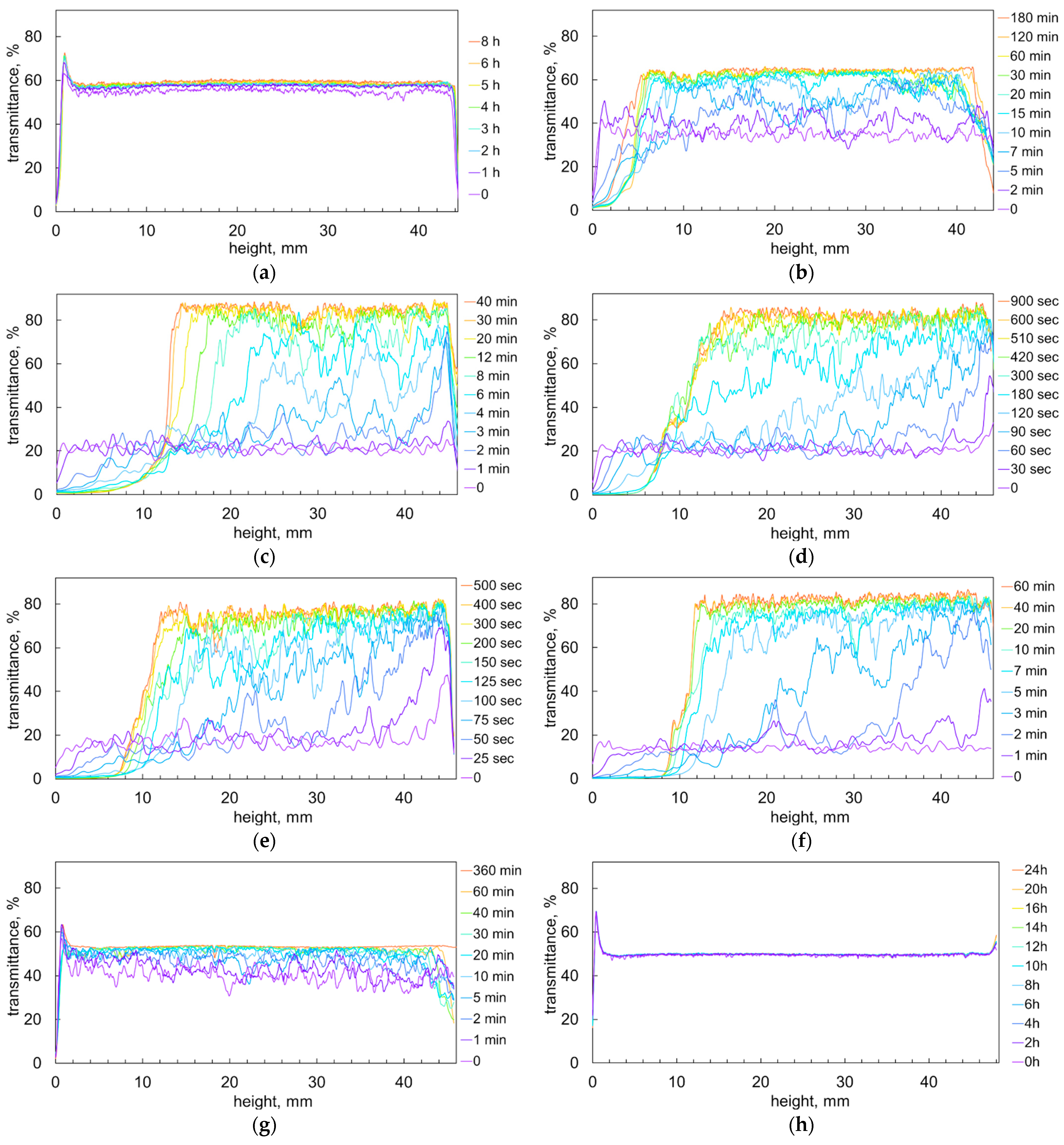
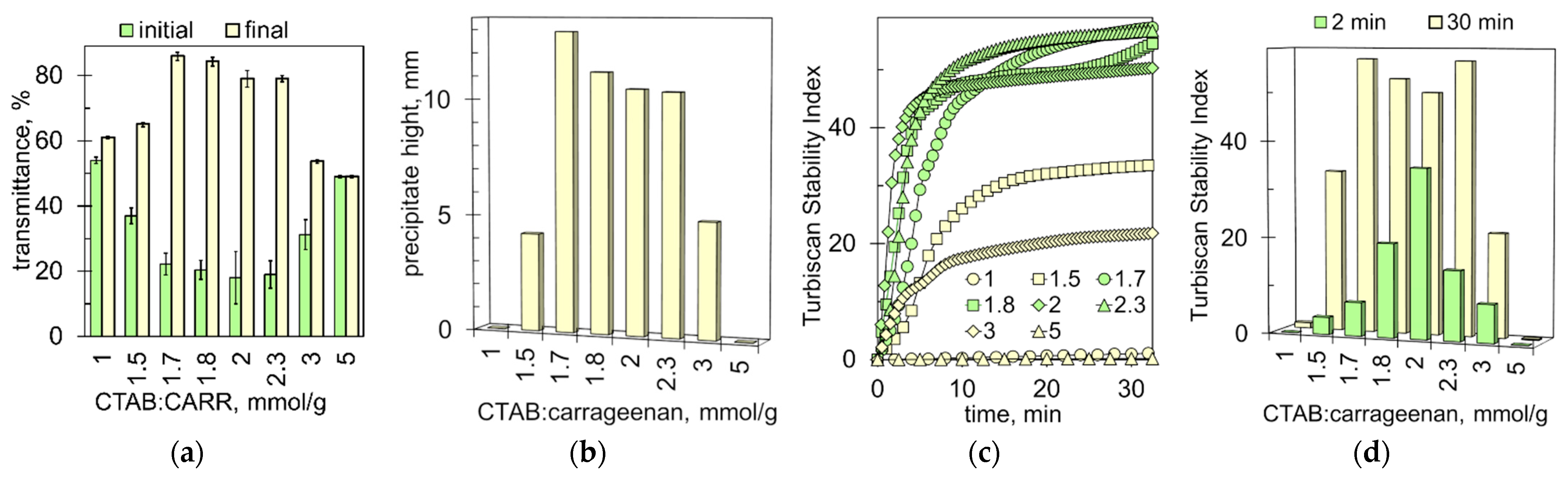
2.1. Sedimentation of the Ionic Associates
2.2. Shape and Size of Particles of Ionic Associates
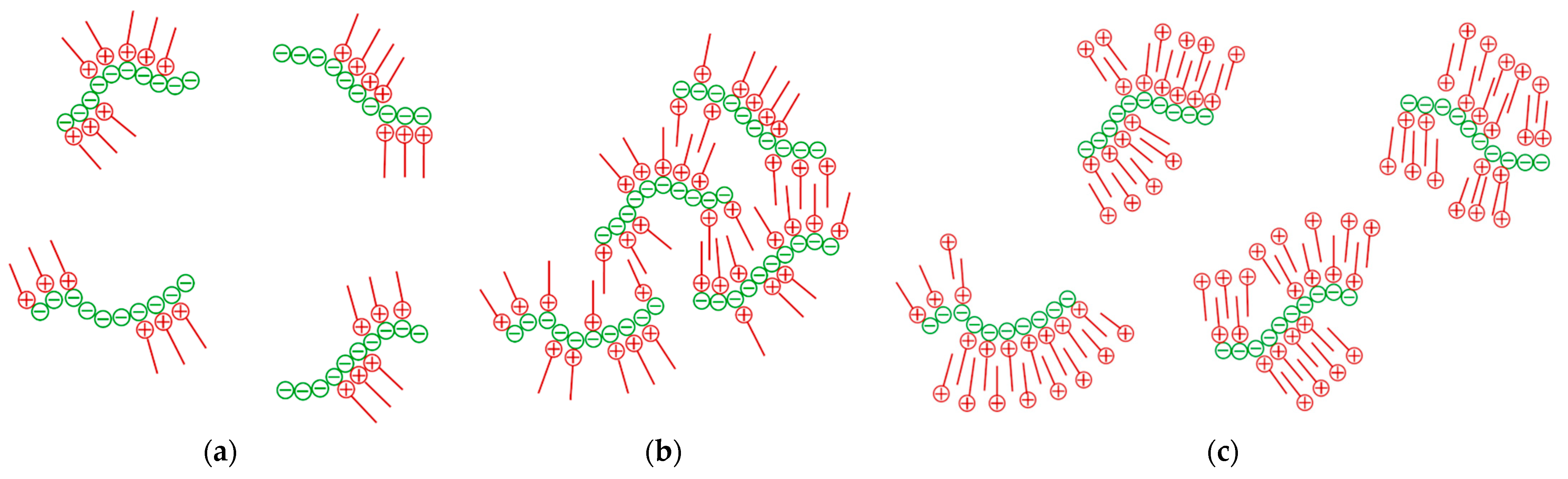
2.3. Probable Mechanism of Auto-Flocculation
2.4. The Ratio of CTAB to Carrageenan That Provides the Most Intense Flocculation
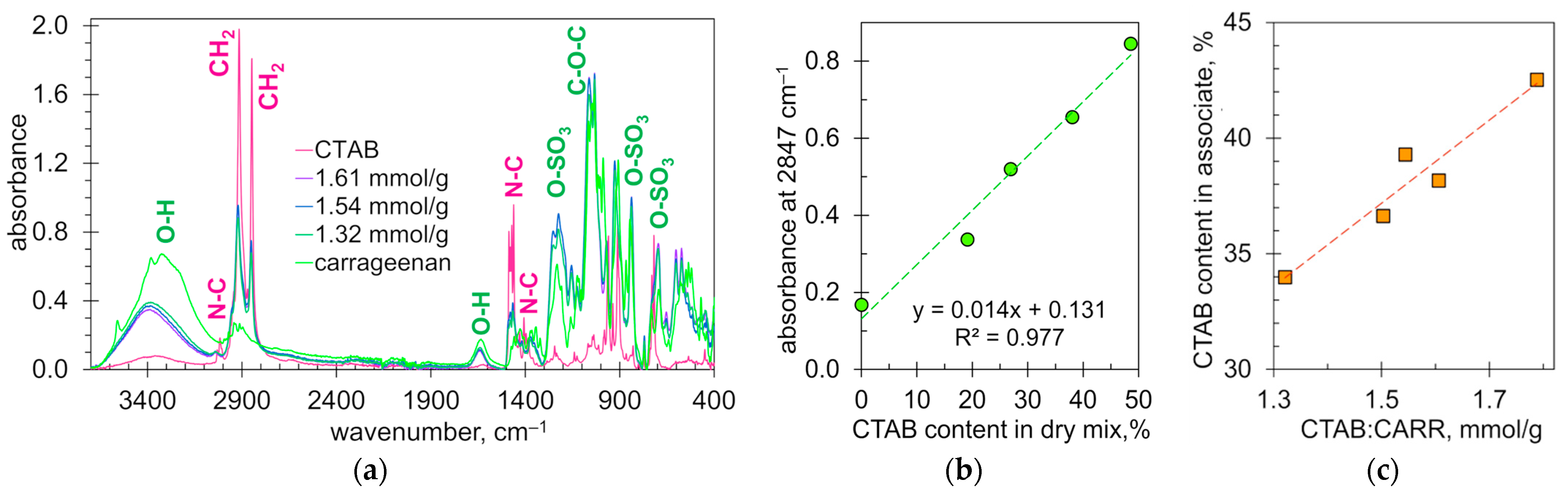
2.5. Structure and Composition of Ionic Associates Investigated by XRD and FTIR Methods
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Suspension Sedimentation Tests
3.3. Study of the Shape and Size of Suspended Particles
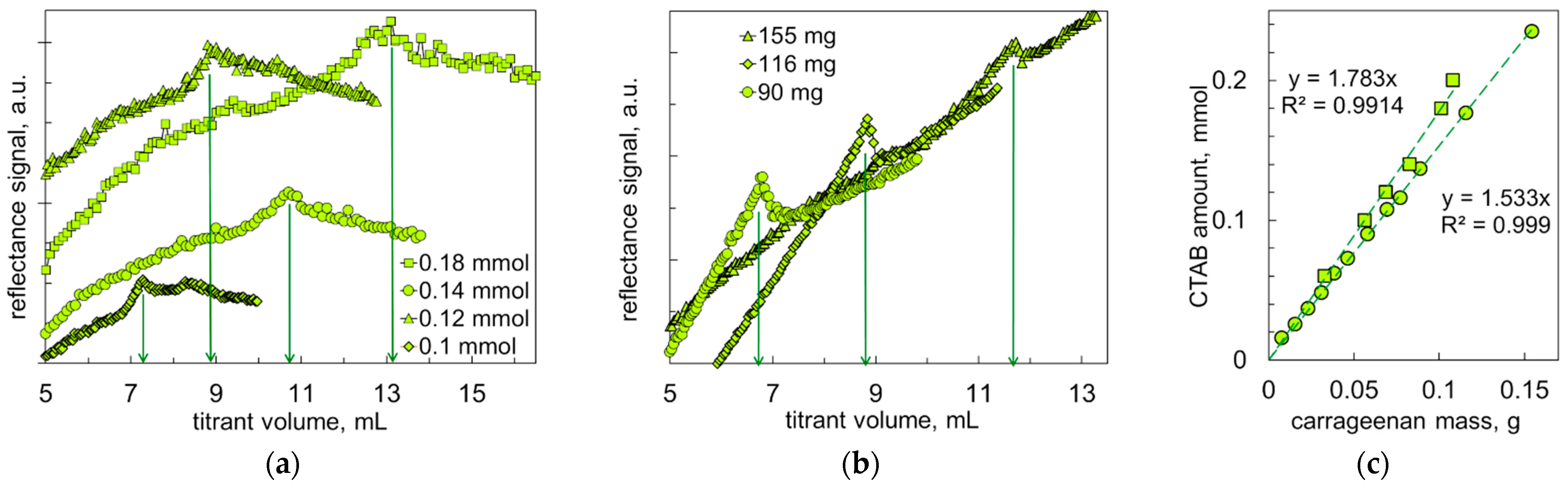
3.4. Turbidimetric Titrations
3.5. Study of Structure and Composition of Ion Pair Associates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurrey, R.; Deb, M.K.; Shrivas, K.; Khalkho, B.R.; Nirmalkar, J.; Sinha, D.; Jha, S. Citrate-capped gold nanoparticles as a sensing probe for determination of cetyltrimethylammonium surfactant using FTIR spectroscopy and colorimetry. Anal. Bioanal. Chem. 2019, 411, 6943–6957. [Google Scholar] [CrossRef]
- Azmat, M.A.; Khan, I.A.; Cheema, H.M.N.; Rajwana, I.A.; Khan, A.S.; Khan, A.A. Extraction of DNA suitable for PCR applications from mature leaves of Mangifera indica L. J. Zhejiang Univ. Sci. B 2012, 13, 239–243. [Google Scholar] [CrossRef]
- Clarke, J.D. Cetyltrimethyl Ammonium Bromide (CTAB) DNA Miniprep for Plant DNA Isolation. Cold Spring Harb. Protoc. 2009, 2009, pdb.prot5177. [Google Scholar] [CrossRef]
- Kumar, M.; Elahi, D.; Bhardwaj, A.; Sharma, S.; Khushi, K.; Singh, E.; Singh, N.; Srivastava, A. Physiochemical investigation of the excipients mixed micelles for improvement of encapsulation and controlled release of antihistamine drugs. J. Mol. Liq. 2022, 364, 119971. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kusunose, T.; Sekino, T. CTAB-Assisted Synthesis of Size- and Shape-Controlled Gold Nanoparticles in SDS Aqueous Solution. Mater. Lett. 2009, 63, 2038–2040. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). Off. J. Eur. Union 2009, L342/59–L342/209. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 7 October 2023).
- Srivastava, A.; Sharma, S.; Kumar, M.; Raghav, S.; Alfakeer, M.; Rub, M.A.; Asiri, A.M. Mixed micellization between sunset yellow dye and hexadecyltrimethylammonium chloride/sodium tetradecyl sulphate surfactants in an aqueous medium. Chem. Pap. 2023, 1–13. [Google Scholar] [CrossRef]
- Seres, L.; Csapó, E.; Varga, N.; Juhász, Á. The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels. Gels 2023, 9, 529. [Google Scholar] [CrossRef]
- Arab, M.p.; Yousefi, M.; Khanniri, E.; Azari, M.; Ghasemzadeh-Mohammadi, V.; Mollakhalili-Meybodi, N. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. J. Food Sci. Technol. 2023, 60, 1656–1665. [Google Scholar] [CrossRef]
- Dille, M.J.; Knutsen, S.H.; Draget, K.I. Gels and gelled emulsions prepared by acid-induced gelation of mixtures of faba bean (Vicia faba) protein concentrate and λ-carrageenan. Appl. Food Res. 2022, 2, 100174. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Zhang, P.; Gao, Y.; Zhao, Y.; Liu, M.; Gu, J.; Wang, Z.; Han, J. A novel multifunctional Salecan/κ-carrageenan composite hydrogel with anti-freezing properties: Advanced rheology, thermal analysis and model fitting. Int. J. Biol. Macromol. 2022, 208, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Meng, R.; Xu, B.-C.; Zhang, B.; Cui, B.; Wu, Z.-Z. Function emulsion gels prepared with carrageenan and zein/carboxymethyl dextrin stabilized emulsion as a new fat replacer in sausages. Food Chem. 2022, 389, 133005. [Google Scholar] [CrossRef] [PubMed]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ghosh, S.; Nickerson, M.T. Effect of biopolymer mixing ratios and aqueous phase conditions on the interfacial and emulsifying properties of lentil protein isolate–κ-carrageenan and lentil protein isolate–ι-carrageenan complexes. Cereal Chem. 2022, 99, 169–183. [Google Scholar] [CrossRef]
- Wasinnitiwong, N.; Benjakul, S.; Hong, H. Effects of κ-carrageenan on gel quality of threadfin bream (Nemipterus spp.) surimi containing salted duck egg white powder. Int. J. Biol. Macromol. 2022, 221, 61–70. [Google Scholar] [CrossRef]
- Westermeier, R.; González, C.; Murúa, P.; Morales, J.; Patiño, D.J.; Fabres, N.; Zamorano, J.; Müller, D.G. Seasonal variation of carrageenan yield, gel strength and viscosity in Sarcopeltis (ex Gigartina) skottsbergii from Southern Chile. Phycol. Res. 2022, 70, 42–49. [Google Scholar] [CrossRef]
- Elnahtawy, A.I.; Elshafei, N.S.; Elzoghby, A.O. Marine Polymer-Based Nano-carriers for Drug Delivery Applications. In Marine Biomaterials; Jana, S., Jana, S., Eds.; Springer: Singapore, 2022; pp. 15–59. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Qamar, S.A.; Junaid, M.; Riasat, A.; Jahangeer, M.; Bilal, M.; Mu, B. Carrageenan-Based Hybrids with Biopolymers and Nano-Structured Materials for Biomimetic Applications. Starch Stärke 2022, 2200018. [Google Scholar] [CrossRef]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyup, N.H.; Abdullah, S.; Mohamed Hussin, N.A.; Tan, Y.C.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Huc-Mathis, D.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Compositional and rheological analysis of carrageenan from the gametophyte phase of the red seaweed Chondrus crispus neutrally extracted at varying temperatures and time. Food Hydrocoll. 2022, 133, 107995. [Google Scholar] [CrossRef]
- Narvarte, B.C.v.; Hinaloc, L.A.R.; Genovia, T.G.T.; Gonzaga, S.M.C.; Tabonda-Nabor, A.M.; Roleda, M.Y. Physiological and biochemical characterization of new wild strains of Kappaphycus alvarezii (Gigartinales, Rhodophyta) cultivated under land-based hatchery conditions. Aquat. Bot. 2022, 183, 103567. [Google Scholar] [CrossRef]
- Solorzano-Chavez, E.G.; Paz-Cedeno, F.R.; Ezequiel de Oliveira, L.; Gelli, V.C.; Monti, R.; Conceição de Oliveira, S.; Masarin, F. Evaluation of the Kappaphycus alvarezii growth under different environmental conditions and efficiency of the enzymatic hydrolysis of the residue generated in the carrageenan processing. Biomass Bioenergy 2019, 127, 105254. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Nicolai, T.; Renou, F. Mixed iota and kappa carrageenan gels in the presence of both calcium and potassium ions. Carbohydr. Polym. 2019, 223, 115107. [Google Scholar] [CrossRef] [PubMed]
- Elmarhoum, S.; Mathieu, S.; Ako, K.; Helbert, W. Sulfate groups position determines the ionic selectivity and syneresis properties of carrageenan systems. Carbohydr. Polym. 2023, 299, 120166. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Nogami, S.; Uchiyama, H.; Tozuka, Y. Controlled release behavior of curcumin from kappa-carrageenan gels with flexible texture by the addition of metal chlorides. Food Hydrocoll. 2020, 101, 105564. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Cui, B.; Liu, Y. Influence of cations on texture, compressive elastic modulus, sol-gel transition and freeze-thaw properties of kappa-carrageenan gel. Carbohydr. Polym. 2018, 202, 530–535. [Google Scholar] [CrossRef]
- Wen, C.; Wang, N.; Dong, Y.; Tian, J.; Song, S.; Qi, H. Calcium-induced-gel properties for ιcarrageenan in the presence of different charged amino acids. LWT 2021, 146, 111418. [Google Scholar] [CrossRef]
- Bartlová, M.; Ziółkowska, D.; Pospiech, M.; Shyichuk, A.; Tremlová, B. Determination of carrageenan in jellies with new methylene blue dye using spectrophotometry, smartphone-based colorimetry and spectrophotometric titration. Food Sci. Technol. 2021, 41, 81–90. [Google Scholar] [CrossRef]
- Tian, J.; Li, T.; Janaswamy, S.; Wang, N.; Song, S.; Qi, H.; Wen, C. The aggregation behavior and structure of blends of κ-carrageenan and ε-polylysine hydrochloride. Polym. Int. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Structure and Flocculation of Ion Associates of Carrageenan and Poly(diallyldimethylammonium chloride) Depending on the Component Ratio. Molecules 2022, 27, 8075. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Abelenda-Nuñez, I.; Hernández-Rivas, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Impact of the bulk aggregation on the adsorption of oppositely charged polyelectrolyte-surfactant mixtures onto solid surfaces. Adv. Colloid Interface Sci. 2020, 282, 102203. [Google Scholar] [CrossRef] [PubMed]
- Grządka, E.; Godek, E.; Słowik, G.; Kowalczuk, A.; Matusiak, J.; Maciołek, U. Interactions between Nanoclay, CTAB and Linear/Star Shaped Polymers. Int. J. Mol. Sci. 2022, 23, 3051. [Google Scholar] [CrossRef] [PubMed]
- Grządka, E. Interactions between kappa-carrageenan and some surfactants in the bulk solution and at the surface of alumina. Carbohydr. Polym. 2015, 123, 1–7. [Google Scholar] [CrossRef]
- Das, A.K.; Sequeira, R.A.; Maity, T.K.; Prasad, K. Bio-ionic liquid promoted selective coagulation of κ-carrageenan from Kappaphycus alvarezii extract. Food Hydrocoll. 2020, 111, 106382. [Google Scholar] [CrossRef]
- Godek, E.; Bastrzyk, A.; Maciołek, U.; Orzeł, J.; Grządka, E. How does the type of background electrolyte change the flocculation effectiveness of κ-carrageenan towards nanoclay? Research on the mechanisms involved. J. Mol. Liq. 2023, 375, 121382. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Szulc, J.; Runka, T. Manufacturing homogenous PVC/graphene nanocomposites using a novel dispersion agent. Polym. Test. 2020, 91, 106868. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, S.; Zhao, G.; Li, Y.; Liu, X.; Yang, L.; Zhu, L.; Liu, H. Fabrication and emulsifying properties of non-covalent complexes between soy protein isolate fibrils and soy soluble polysaccharides. Food Funct. 2022, 13, 386–397. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Flocculation of silica nanoparticles by natural, wood-based polyelectrolytes. Sep. Purif. Technol. 2020, 231, 115888. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Li, Y.; Yang, L.; Song, H. Properties and microstructure of pickering emulsion synergistically stabilized by silica particles and soy hull polysaccharides. Food Hydrocoll. 2023, 134, 108084. [Google Scholar] [CrossRef]
- Maltauro, R.; Stone, M.; Collins, A.L.; Krishnappan, B.G.; Silins, U. The effect of sheardependent flocculation on the multimodality of effective particle size distributions in a gravel-bed river during high flows. J. Soils Sediments 2023, 23, 3589–3601. [Google Scholar] [CrossRef]
- Spencer, K.L.; Wheatland, J.A.T.; Bushby, A.J.; Carr, S.J.; Droppo, I.G.; Manning, A.J. A structure–function based approach to floc hierarchy and evidence for the non-fractal nature of natural sediment flocs. Sci. Rep. 2021, 11, 14012. [Google Scholar] [CrossRef]
- Zarate-Vilet, N.; Wisniewski, C.; Gué, E.; Delalonde, M. Towards a better identification of naringin and narirutin dispersion state in grapefruit peel press liquor. Chem. Eng. Res. Des. 2020, 159, 205–214. [Google Scholar] [CrossRef]
- Gradzielski, M. Polyelectrolyte–Surfactant Complexes As a Formulation Tool for Drug Delivery. Langmuir 2022, 38, 13330–13343. [Google Scholar] [CrossRef]
- Gradzielski, M. Polymer–surfactant interaction for controlling the rheological properties of aqueous surfactant solutions. Curr. Opin. Colloid Interface Sci. 2023, 63, 101662. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, M.; Kumar Deb, D.; Muzaffar, F.; Singh, S. Utilization of amphiphilic antihistamines drugs to enhance micellization of anionic surfactant and improve the binding and solubility of Itraconazole drug. J. Mol. Liq. 2022, 348, 118018. [Google Scholar] [CrossRef]
- Bai, G.; Wu, H.; Lou, P.; Wang, Y.; Nichifor, M.; Zhuo, K.; Wang, J.; Bastos, M. Cationic gemini surfactant as a dual linker for a cholic acid-modified polysaccharide in aqueous solution: Thermodynamics of interaction and phase behavior. Phys. Chem. Chem. Phys. 2017, 19, 1590–1600. [Google Scholar] [CrossRef]
- Gómez-Hernández, M.; Rodríguez-García, C.M.; Peraza-Echeverría, L.; Peraza-Sánchez, S.R.; TorresTapia, L.W.; Pérez-Brito, D.; Vargas-Coronado, R.F.; Cauich-Rodríguez, J.V. In vitro antifungal activity screening of beach-cast seaweeds collected in Yucatan, Mexico. J. Appl. Phycol. 2021, 33, 1229–1237. [Google Scholar] [CrossRef]
- Hirota, N.; Nagai, K. Helical structures and water vapor sorption properties of carrageenan membranes derived from red algae. Carbohydr. Polym. Technol. Appl. 2022, 3, 100200. [Google Scholar] [CrossRef]
- Rezaie, M.; Dinari, M.; Chermahini, A.N.; Saraji, M.; Shahvar, A. Preparation of kapa carrageenan-based acidic heterogeneous catalyst for conversion of sugars to high-value added materials. Int. J. Biol. Macromol. 2020, 165, 1129–1138. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. Reinforcement of Refined and Semi-Refined Carrageenan Film with Nanocellulose. Polymers 2020, 12, 1145. [Google Scholar] [CrossRef]
- Vinceković, M.; Pustak, A.; Tušek-Božić, L.J.; Liu, F.; Ungar, G.; Bujan, M.; Šmit, I.; FilipovićVinceković, N. Structural and thermal study of mesomorphic dodecylammonium carrageenates. J. Colloid Interface Sci. 2010, 341, 117–123. [Google Scholar] [CrossRef]
- Chandekar, K.v.; Kant, K.M. Relaxation phenomenon and relaxivity of cetrimonium bromide (CTAB) coated CoFe2O4 nanoplatelets. Phys. B Condens. Matter 2018, 545, 536–548. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyichuk, A.; Ziółkowska, D.; Szulc, J. Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan. Molecules 2023, 28, 7584. https://doi.org/10.3390/molecules28227584
Shyichuk A, Ziółkowska D, Szulc J. Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan. Molecules. 2023; 28(22):7584. https://doi.org/10.3390/molecules28227584
Chicago/Turabian StyleShyichuk, Alexander, Dorota Ziółkowska, and Joanna Szulc. 2023. "Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan" Molecules 28, no. 22: 7584. https://doi.org/10.3390/molecules28227584
APA StyleShyichuk, A., Ziółkowska, D., & Szulc, J. (2023). Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan. Molecules, 28(22), 7584. https://doi.org/10.3390/molecules28227584





