The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of MT Identified by UPLC-Q/TOF-MS
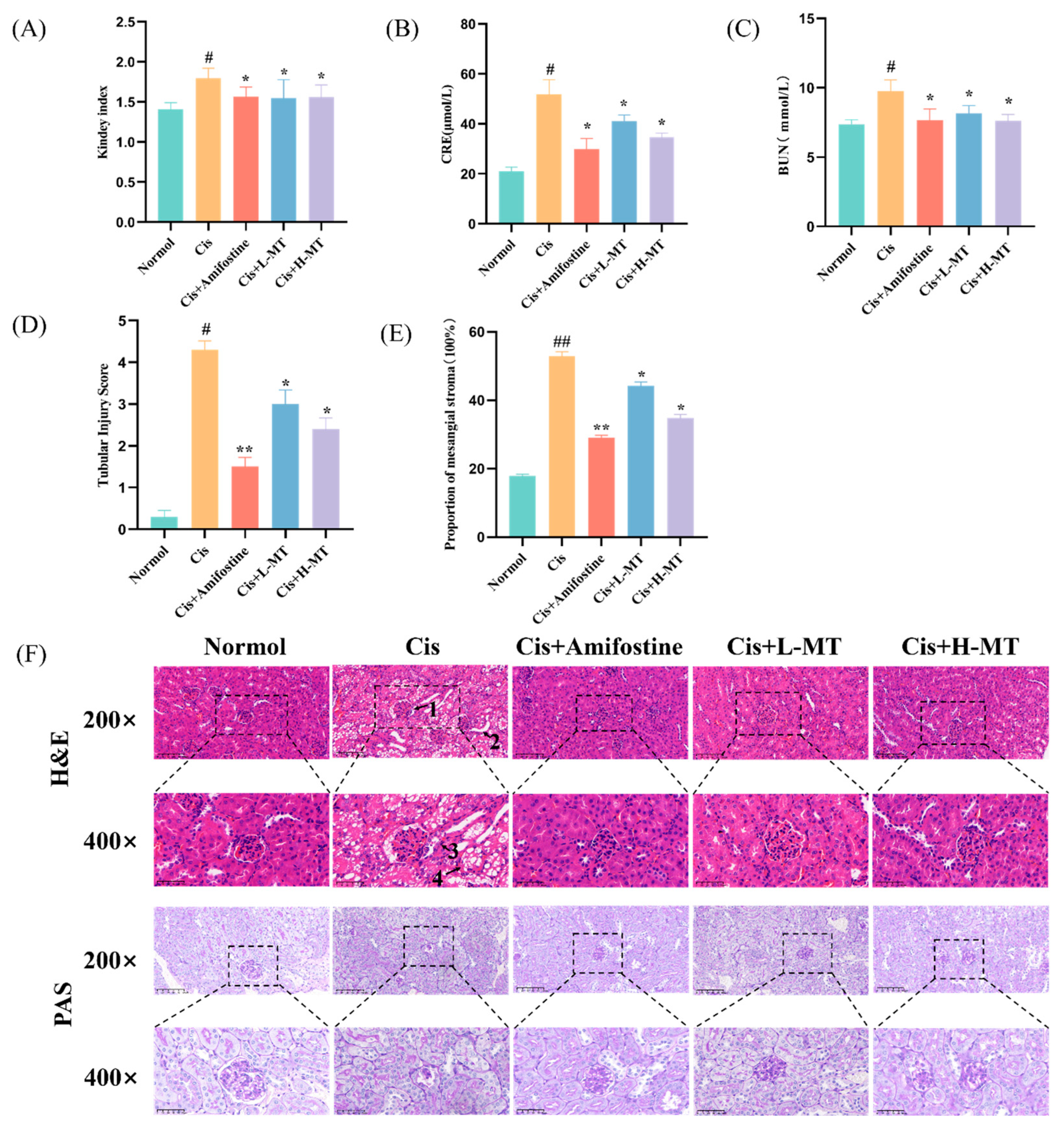
2.2. MT Ameliorates Cis-Induced Kidney Damage in Mice
2.3. Histopathological Study
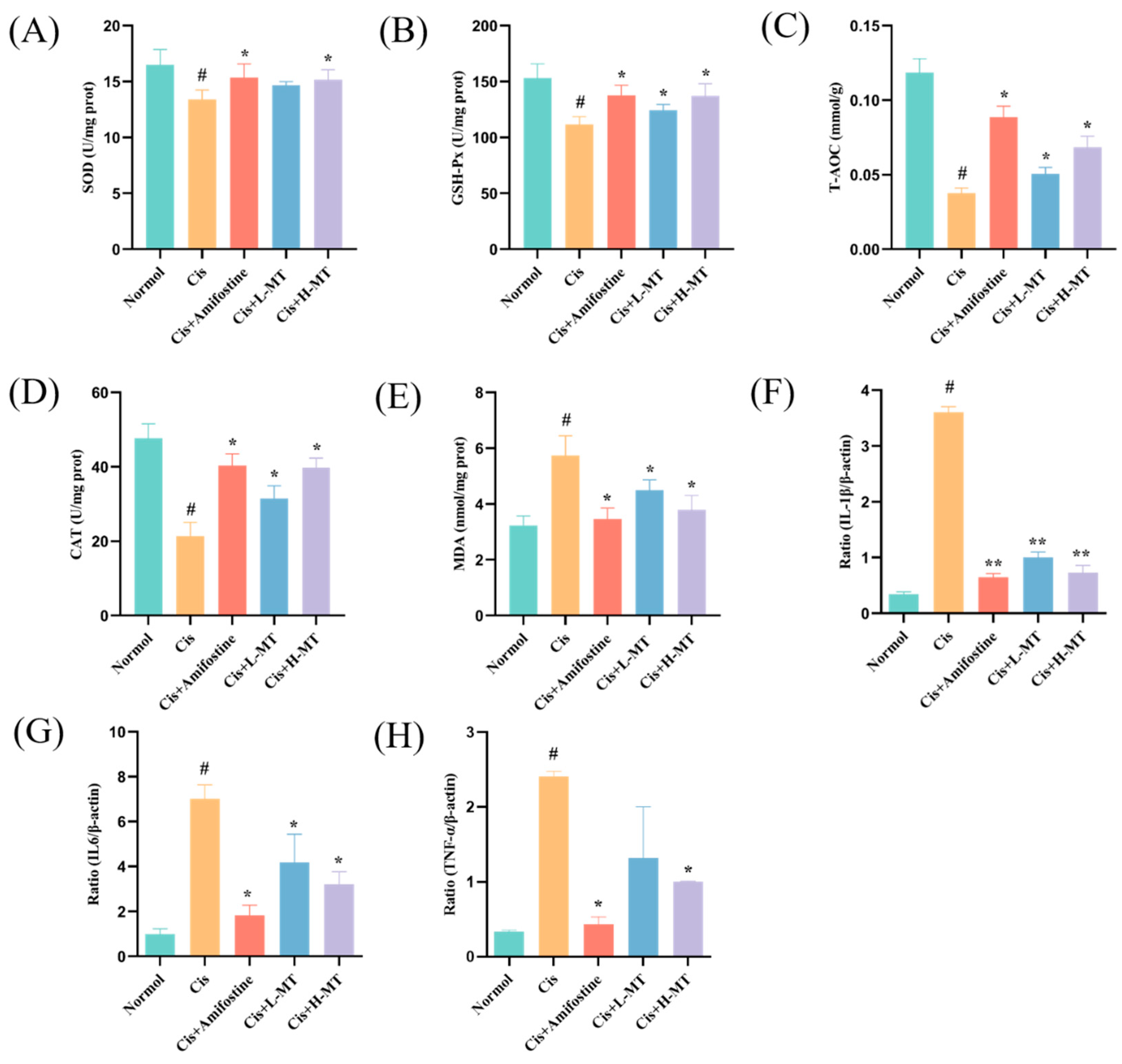
2.4. Effect of MT Treatment on Oxidative Stress Parameters
2.5. Effect of MT on Inflammation-Related Gene Expression in Renal Tissues
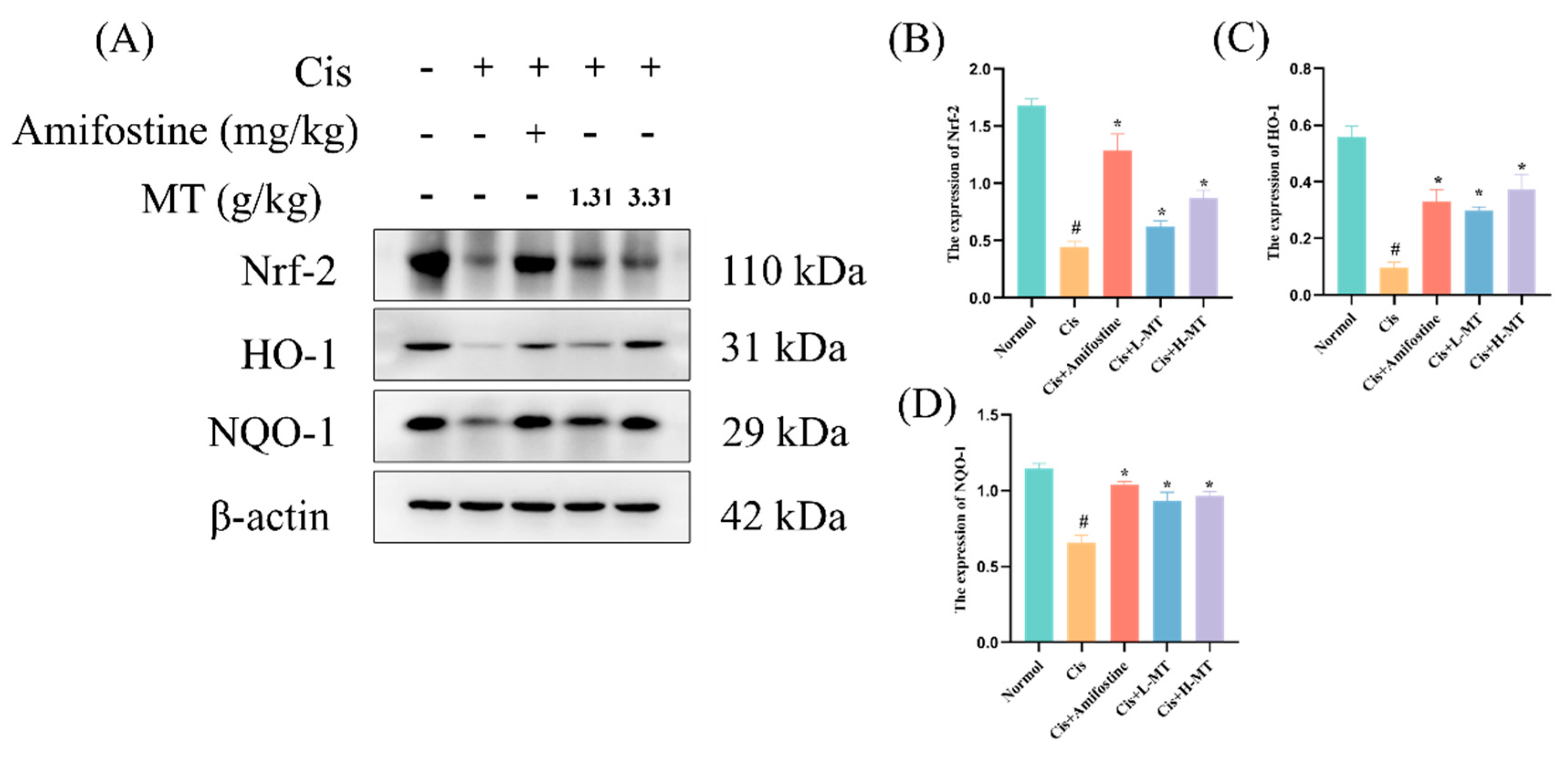
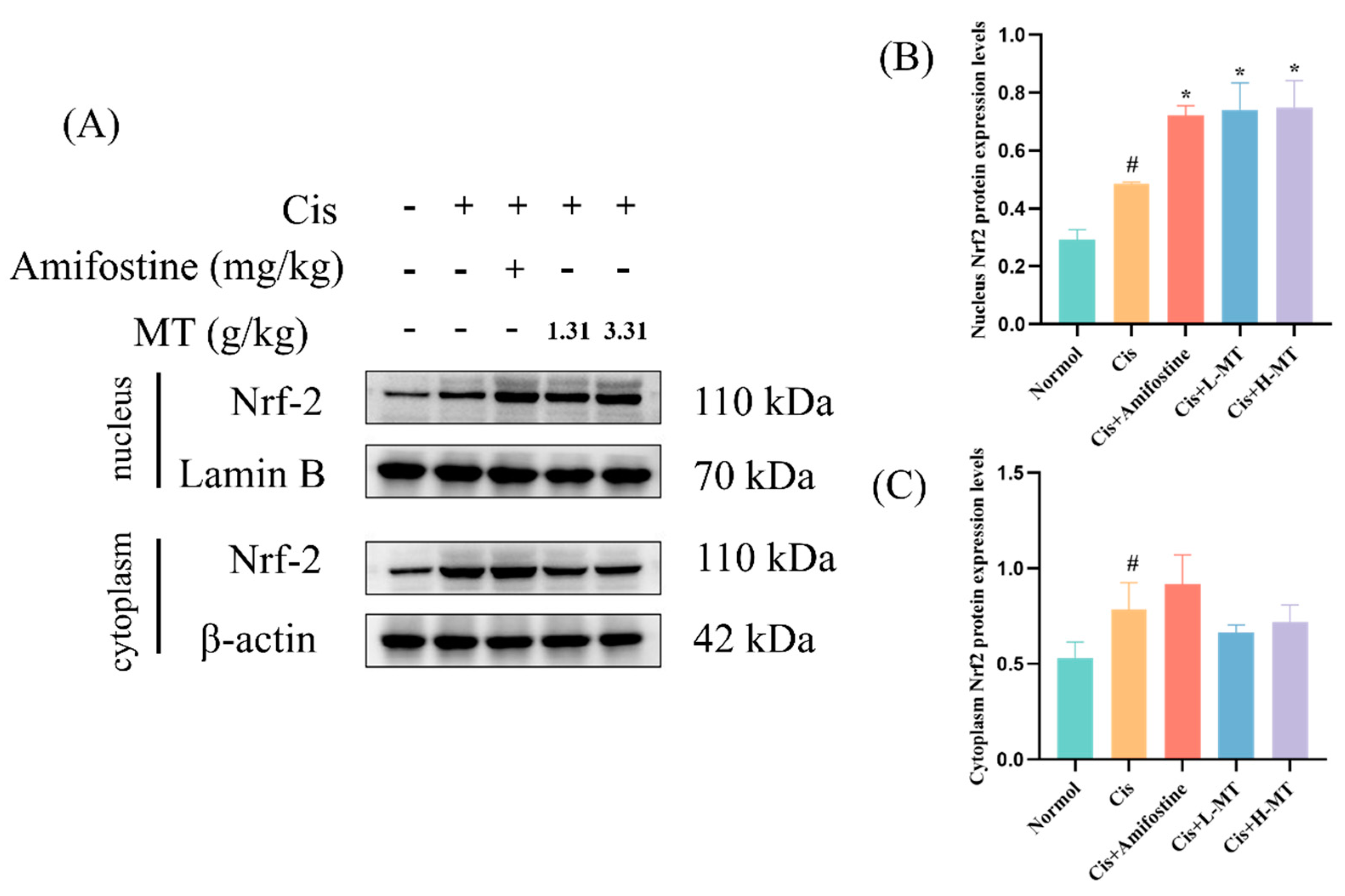
2.6. MT Activates the Nrf2-Mediated Antioxidant Response
2.7. MT Inhibits the Activation of NF-κB in Mice
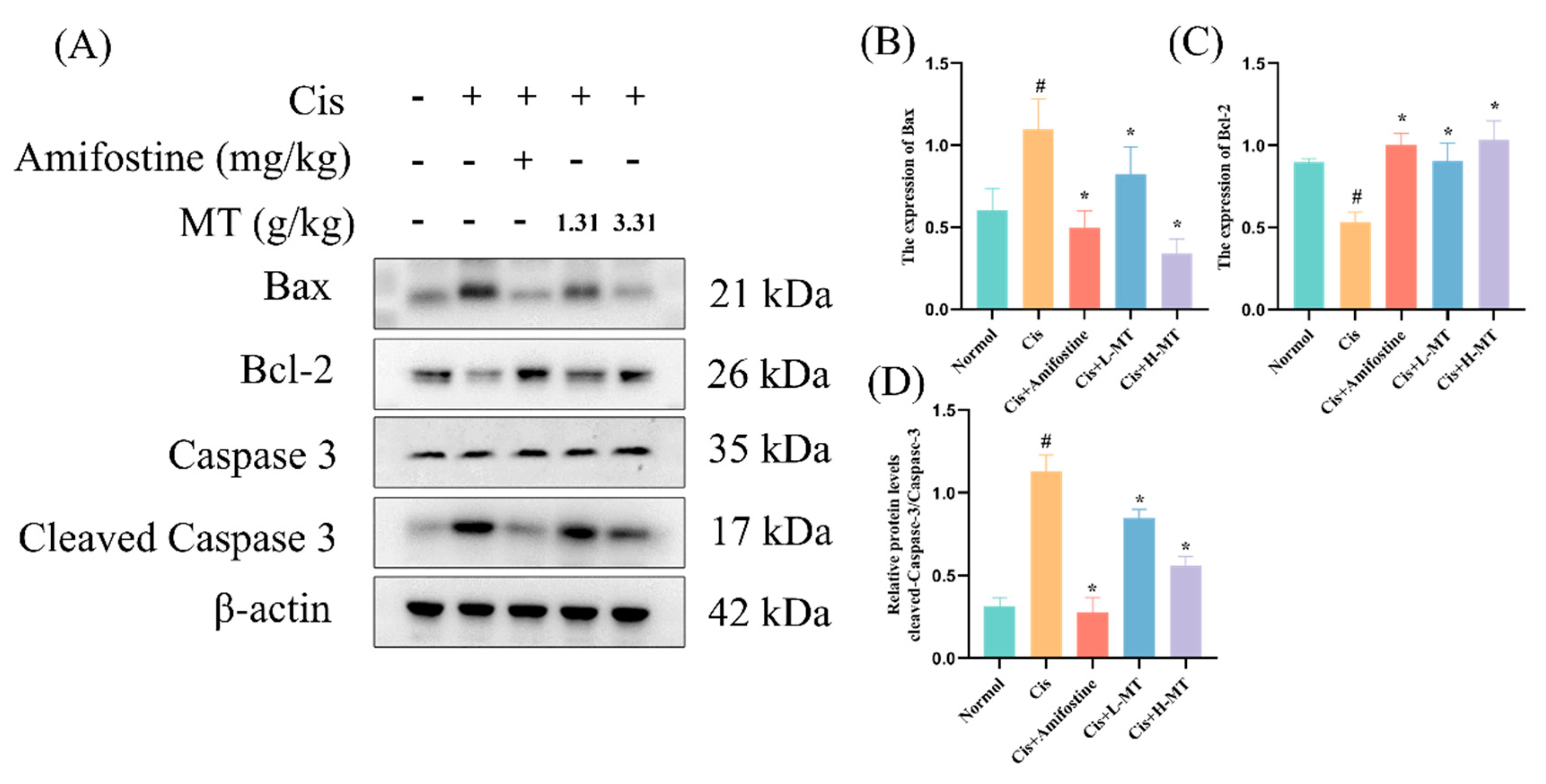
2.8. MT Attenuates Cis-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents and Antibodies
4.1.2. Plant Material
4.2. Methods
4.2.1. Preparation of MT Aqueous Extract
4.2.2. Sample Preparation
4.2.3. Standard Solutions
4.2.4. Mass Spectrum Condition
4.2.5. Animal Experiment Design and Drug Treatments
4.2.6. Serum Biochemical Analysis
4.2.7. Histopathological Examination
4.2.8. Assay of Antioxidant Enzyme Vitality and MDA Levels
4.2.9. Quantitative Real-Time PCR Analysis
4.2.10. Western Blot Analysis
4.2.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MT | Marsdenia tenacissima | Cis | cisplatin |
| XAP | Xiaoaiping | p-IKKβ | phospho-Inhibitor of nuclear factor kappa B kinase beta subunit |
| AKI | acute kidney injury | IKKβ | inhibitor of nuclear factor kappa B kinase beta subunit |
| CRE | creatinine | Bax | Bcl-2-associated X |
| BUN | blood urea nitrogen | HO-1 | heme oxygenase-1 |
| HE | hematoxylin–eosin staining | NQO1 | recombinant NADH dehydrogenase, quinone 1 |
| PAS | periodic acid–Schiff staining | Bcl-2 | B-cell lymphoma-2 |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 | ROS | reactive oxygen species |
| NF-κB | nuclear factor kappa-B | SOD | superoxide dismutase |
| IL-1β | interleukin-1β | GSH-Px | glutathione peroxidase |
| IL-6 | interleukin-6 | CAT | catalase |
| TNF α | tumor necrosis factor-α | TCM | traditional Chinese medicine |
| TH | tenacissoside H | T-AOC | total antioxidant capacity |
| p-p65 | phospho-p65 | MDA | malondialdehyde |
| CA | chlorogenic acid | SCR | serum creatinine |
| WB | Western blot | SPF | specific pathogen-free |
| RIPA | radioimmunoprecipitation assay | SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| PVDF | polyvinylidene fluoride | TIC | total ion chromatography |
| p65 | NFκB p65 protein | HP-β-CD | 2-hydroxypropyl-β-cyclodextrin |
References
- Perse, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Shaili, E. Platinum anticancer drugs and photochemotherapeutic agents: Recent advances and future developments. Sci. Prog. 2014, 97, 20–40. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef]
- Pabla, N.; Murphy, R.F.; Liu, K.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef]
- Hu, S.; Leblanc, A.F.; Gibson, A.A.; Hong, K.W.; Kim, J.Y.; Janke, L.J.; Li, L.; Vasilyeva, A.; Finkelstein, D.B.; Sprowl, J.A.; et al. Identification of OAT1/OAT3 as Contributors to Cisplatin Toxicity. Clin. Transl. Sci. 2017, 10, 412–420. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Renal Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef]
- Jesse, C.R.; Bortolatto, C.F.; Wilhelm, E.A.; Roman, S.S.; Prigol, M.; Nogueira, C.W. The peroxisome proliferator-activated receptor-γ agonist pioglitazone protects against cisplatin-induced renal damage in mice. J. Appl. Toxicol. 2014, 34, 25–32. [Google Scholar] [CrossRef]
- Rubera, I.; Duranton, C.; Melis, N.; Cougnon, M.; Mograbi, B.; Tauc, M. Role of CFTR in oxidative stress and suicidal death of renal cells during cisplatin-induced nephrotoxicity. Cell Death Dis. 2013, 4, e817. [Google Scholar] [CrossRef]
- Dobyan, D.C.; Levi, J.; Jacobs, C.; Kosek, J.; Weiner, M.W. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther. 1980, 213, 6. [Google Scholar]
- Sancho-Martinez, S.M.; Piedrafita, F.J.; Cannata-Andia, J.B.; Lopez-Novoa, J.M.; Lopez-Hernandez, F.J. Necrotic concentrations of cisplatin activate the apoptotic machinery but inhibit effector caspases and interfere with the execution of apoptosis. Toxicol. Sci. 2011, 122, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Yang, J.; Zhu, Z.F.; Zhang, X.J. Marsdenia tenacissima: A Review of Traditional Uses, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2018, 46, 1449–1480. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Kang, L.P.; Guo, B.L.; Zhang, Z.L.; Guan, Y.H.; Pang, X.; Peng, C.Z.; Ma, B.P.; Zhang, L.X. Original plant identification of Dai nationality herb “Daibaijie”. China J. Chin. Mater. Med. 2014, 39, 4. [Google Scholar]
- Yang, L.; Peng, L.Q.; Tai, H.C.; Zhang, X.F. Research Progress of Dai-Bai-jie. J. Med. Pharm. Chin. Minorities 2021, 27, 3. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Chang, Y.Z.; Shen, C.Y.; Han, J.; Chang, R.A. Xiaoaiping injection combined with chemotherapy for advanced gastric cancer: An updated systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1023314. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Huang, J.; Wang, Z.; Zhang, J.; Han, D.; Wu, Q.; He, H.; Zhou, X. Xiao-ai-ping injection adjunct with platinum-based chemotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 3. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, J.K.; Yu, S.H.; Wang, J.; Hu, H.Y. Biological activities and detoxification mechanisms of Clerodendrum chinense var. simplex, Marsdenia tenacissima and Arundina graminifolia: The Dai antidotes. Acta Sci. Nat. Univ. Sunyatseni 2023, 62, 11. [Google Scholar] [CrossRef]
- Badr, A.M.; Al-Kharashi, L.A.; Attia, H.; Alshehri, S.; Alajami, H.N.; Ali, R.A.; Mahran, Y.F. TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity. Pharmaceuticals 2023, 16, 337. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Moridnia, A.; Sabbagh, S.; Ghaffaripour, R.; Jafaripour, L.; Behzadifard, M. Ameliorative Effects of Gallic Acid on Cisplatin-Induced Nephrotoxicity in Rat Variations of Biochemistry, Histopathology, and Gene Expression. Biomed. Res. Int. 2021, 2021, 2195238. [Google Scholar] [CrossRef]
- Lee, J.; Nguyen, Q.N.; Park, J.Y.; Lee, S.; Hwang, G.S.; Yamabe, N.; Choi, S.; Kang, K.S. Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies. Plants 2020, 9, 1681. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, Y.; Han, L.W.; Tian, Q.P.; He, Q.X.; Wang, X.M.; Sun, C.; Han, J.; Liu, K.C. Tenacissoside H exerts an anti-inflammatory effect by regulating the nf-κb and p38 pathways in zebrafish. Fish. Shellfish. Immun. 2018, 83, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.L.; Zhang, L.J. Protective effects of Marsdeniatenacissima extract on CCl4-induced chronic liver injury in rats. Chin. New Drugs J. 2017, 26, 6. [Google Scholar]
- Chang, W.; Li, P.P.; Wei, W. Protective effect of saponins of Marsdenia Tenacissima on N-acetyl-p-aminophenol-induced hepatic injury in mice. Chin. New Drugs J. 2015, 24, 5. [Google Scholar]
- Mathew, A.; Asirvatham, R.; Tomy, D. Cardioprotective effect of Marsdenia tenacissima and Sansevieria roxburghiana in Doxorubicin induced cardiotoxicity in rats in vivo: The role of Dresgenin and Lupeol. Turk. J. Pharm. Sci. 2021, 18, 11. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A. Acute kidney injury. Crit. Care Med. 2008, 36, S141–S145. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Acute kidney injury. Lancet 2008, 372, 2. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Hu, Y.L.; Liu, P.; Kang, L.W.; Li, J.Y.; Li, R.T.; Liu, T.X. Mechanism of Marsdenia tenacissima extract promoting apoptosis of lung cancer by regulating Ca2+/CaM/CaMK signaling. J. Ethnopharmacol. 2020, 251, 112535. [Google Scholar] [CrossRef]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-kappaB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef]
- Meng, X.M.; Li, H.D.; Wu, W.F.; Ming-Kuen Tang, P.; Ren, G.L.; Gao, L.; Li, X.F.; Yang, Y.; Xu, T.; Ma, T.T.; et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab. Investig. 2018, 98, 79–94. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, C.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Shi, M.; Yang, Z.; Fu, Y. Protective role of apigenin in cisplatin-induced renal injury. Eur. J. Pharmacol. 2016, 789, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, K.; Lee, D.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Protective effect and mechanism of action of lupane triterpenes from Cornus walteri in cisplatin-induced nephrotoxicity. Bioorg. Med. Chem. Lett. 2015, 25, 5613–5618. [Google Scholar] [CrossRef]
- Potocnjak, I.; Simic, L.; Vukelic, I.; Domitrovic, R. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemosensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem. Toxicol. 2019, 132, 110676. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, M.; Koubaa, M.; Soudani, N.; Elwej, A.; Grati, M.; Jamoussi, K.; Boudawara, T.; Ellouze Chaabouni, S.; Zeghal, N. Nitraria retusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes. Pharm. Biol. 2017, 55, 1061–1073. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Shino, Y.; Itoh, Y.; Kubota, T.; Yano, T.; Sendo, T.; Oishi, R. Role of poly(ADP-ribose)polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radic. Biol. Med. 2003, 35, 966–977. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Ma, Q.; Wang, D.; Zhou, T. Cisplatin-based chemoradiotherapy with 5-fluorouracil or pemetrexed in patients with locally advanced, unresectable esophageal squamous cell carcinoma: A retrospective analysis. Mol. Clin. Oncol. 2017, 6, 743–747. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.; Liu, S.; Yu, Q.; Chen, Y.; Xie, J. Ameliorative effect of Cyclocarya paliurus polysaccharides against carbon tetrachloride induced oxidative stress in liver and kidney of mice. Food Chem. Toxicol. 2020, 135, 111014. [Google Scholar] [CrossRef] [PubMed]
- Sha, F.; Chang, Y.; Ding, J. Effects of two cooling modes of low temperature stress on antioxidant enzyme activities and malondiadehyde level in sea cucumber Apostichopu japonicus. J. Dalian Ocean. Univ. 2015, 30, 4. [Google Scholar]
- Fang, C.Y.; Lou, D.Y.; Zhou, L.Q.; Wang, J.C.; Yang, B.; He, Q.J.; Wang, J.J.; Weng, Q.J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- Ben-Yehuda Greenwald, M.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. Skin Redox Balance Maintenance: The Need for an Nrf2-Activator Delivery System. Cosmetics 2016, 3, 1. [Google Scholar] [CrossRef]
- Sasaki, A.; Koike, N.; Murakami, T.; Suzuki, K. Dimethyl fumarate ameliorates cisplatin-induced renal tubulointerstitial lesions. J. Toxicol. Pathol. 2019, 32, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004, 65, 8. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef]
- Yu, X.; Meng, X.; Xu, M.; Zhang, X.; Zhang, Y.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-kappaB and improving mitochondrial function. EBioMedicine 2018, 36, 266–280. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-kappaB and Nrf2/HO1 Pathways. Biomolecules 2020, 10, 1488. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Zou, X.; Yang, Y.; Qiu, Y.; Wen, Y. Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 10. [Google Scholar]
- Tsuruya, K.; Ninomiya, T.; Tokumoto, M.; Hiraata, H.; Iida, M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003, 63, 10. [Google Scholar] [CrossRef] [PubMed]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Renal cell apoptosis induced by nephrotoxic drugs: Cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, X.T.; Zhao, L.C.; Ren, S.; He, Y.F.; Liu, W.C.; Wang, Z.; Li, X.D.; Jiang, S.; Li, W. alpha-Mangostin, a Dietary Xanthone, Exerts Protective Effects on Cisplatin-Induced Renal Injury via PI3K/Akt and JNK Signaling Pathways in HEK293 Cells. ACS Omega 2020, 5, 19960–19967. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zheng, J.; Tian, X.; Yuan, F.; Liu, Z.; Zhou, Y.; Yang, Z.; Ding, X. Protective mechanism of traditional Chinese medicine guizhi fuling pills against carbon tetrachloride-induced kidney damage is through inhibiting oxidative stress, inflammation and regulating the intestinal flora. Phytomedicine 2022, 101, 154129. [Google Scholar] [CrossRef]


| No. | RT (min) | Name | Formula | Ion. | Cal. m/z | Mea. m/z | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.70 | Gallic acid | C7H6O5 | M − H | 169.01425 | 169.01445 | 1.2 | 169.0156, 125.0241, 97.0292, 69.0349 |
| 2 | 3.05 | Protocatechuic acid | C7H6O4 | M − H | 153.01933 | 153.01959 | 1.7 | 153.0198, 109.0291, 108.0215, 91.0187 |
| 3 | 3.22 | Chlorogenic acid | C16H18O9 | M − H | 353.08781 | 353.08801 | 0.6 | 191.0559, 179.0349, 161.0249, 135.0444 |
| 4 | 3.22 | Shikimic acid | C7H10O5 | M − H | 173.04555 | 173.04599 | 2.6 | 173.0469, 109.0280, 93.0341 |
| 5 | 3.56 | Esculetin | C9H6O4 | M − H | 177.01933 | 177.01944 | 0.6 | 177.0189, 149.0241, 133.0294, 77.0399, 105.0501, 89.0388 |
| 6 | 3.58 | Caffeic acid | C9H8O4 | M − H | 179.03498 | 179.0351 | 0.6 | 135.0448, 134.0374, 89.0389 |
| 7 | 3.66 | Vanillic acid | C8H8O4 | M − H | 167.03498 | 167.03526 | 1.7 | 167.0392, 152.0129, 123.0469, 108.0231, 91.0183 |
| 8 | 3.71 | 3-O-Feruloyl-quinic acid | C17H20O9 | M − H | 367.10346 | 367.10322 | −0.7 | 191.0563, 173.0456, 155.0358, 134.0373, 93.0346, 67.0193 |
| 9 | 4.25 | Rutin | C27H30O16 | M − H | 609.14611 | 609.14622 | 0.2 | 609.1476, 301.0367, 300.0284, 271.0267, 151.0033 |
| 10 | 4.25 | Scopoletin | C10H8O4 | M + H | 193.04954 | 193.04957 | 0.2 | 178.0255, 149.0585, 133.0282, 122.0359 |
| 11 | 4.30 | 3′,5′-Dimethoxy-4′-hydroxyacetophenone | C10H12O4 | M − H | 195.06628 | 195.06683 | 195.0670, 136.0530, 119.0506 | |
| 12 | 4.47 | Isoferulic acid | C10H10O4 | M − H | 193.05063 | 193.05066 | 0.2 | 193.0504, 178.0255, 149.0248, 134.0372, 133.0289 |
| 13 | 4.82 | Isochlorogenic acid A | C25H24O12 | M − H | 515.1195 | 515.11967 | 0.3 | 353.0870, 191.0555, 179.0343, 135.0443 |
| 14 | 5.51 | 4-Hydroxybenzoic acid | C7H6O3 | M − H | 137.02442 | 137.02438 | −0.3 | 93.0347, 65.0397 |
| 15 | 5.95 | Tenacigenin B | C21H32O5 | M − H | 363.2177 | 363.21777 | 0.2 | 327.1965, 311.1659, 276.1371, |
| 16 | 6.20 | Xanthyletin | C14H12O3 | M − H | 227.07137 | 227.07162 | 1.1 | 212.0469, 199.0771, 183.0448, 155.0496 |
| 17 | 6.55 | Quercetin | C15H10O7 | M − H | 301.03538 | 301.03508 | −1 | 301.0353, 178.9999, 151.0041 |
| 18 | 6.60 | Wogonin | C16H12O5 | M + H | 285.07575 | 285.07628 | 1.9 | 285.0753, 270.0522, 253.0493, 213.0542, 197.0600 |
| 19 | 6.67 | Kaempferol | C15H10O6 | M − H | 285.04046 | 285.04078 | 1.1 | 285.0412, 175.0402, 151.0045, 133.0229 |
| 20 | 6.74 | Acacetin | C16H12O5 | M − H | 283.0612 | 283.06135 | 0.5 | 283.0605, 268.0367, 224.0468, 195.0460, 167.0495, 132.0206 |
| 21 | 7.7 | Apigenin | C15H10O5 | M − H | 269.04555 | 269.04566 | 0.4 | 269.0473, 151.0033, 117.0347 |
| 22 | 8.34 | Isoliquiritigenin | C15H12O4 | M + H | 257.08084 | 257.08059 | −0.9 | 257.0806, 147.0422, 137.0232, 119.0493 |
| 23 | 8.36 | Liquiritigenin | C15H12O4 | M − H | 255.06628 | 255.06634 | 0.2 | 255.0661, 135.0081, 119.0495 |
| 24 | 8.61 | 3-O-β-d-glucopyranosyl-(1→4)-6-deoxy3-O-methyl-β-d-allopyranosyl-(1→4)-β-d-oleandro-pyranosyl-11α-O-acetyltenacigenin B | C43H68O18 | M − H | 871.43329 | 871.43215 | −1.3 | 811.4124, 829.4221, 667.3720, 631.3589 |
| 25 | 8.97 | 12-O-tigloyltenacigenin A | C26H38O6 | M + H | 447.27412 | 447.27306 | −2.4 | 347.2206, 329.2100, 311.2002, 293.1893 |
| 26 | 9.61 | 3-O-β-d-glucopyranosyl-(1→4)-6-deoxy3-O-methyl-β-d-allopyranosyl-(1→4)-β-d-oleandro-pyranosyl-11α-O-Tigloyl-12β-O-acetyltenacigenin C | C48H76O20 | M − H | 971.48572 | 971.48517 | −0.6 | 811.4116, 775.3915, 613.3334 |
| 27 | 10.94 | Glycyrrhizic acid | C42H62O16 | M + H | 823.41106 | 823.40643 | −5.6 | 647.3764, 471.3443 |
| 28 | 12.32 | marsdenoside D | C40H64O13 | M − H | 751.42742 | 751.42489 | −3.4 | 751.4214, 667.3692 |
| 29 | 13.05 | Tenacissoside G | C42H64O14 | M + H | 793.43688 | 793.43266 | −5.3 | 639.3783, 651.3702, 633.3592, 347.2195, 311.1994 |
| 30 | 13.55 | 11α,12β-Di-O-tigloyltenacigeninB | C31H44O7 | M − H | 529.31598 | 529.3138 | −4.1 | 347.2208, 329.2102, 311.1993, 293.1898, 203.1068 |
| 31 | 13.56 | Isokobusone | C14H22O2 | M − H | 221.1547 | 221.15386 | −3.8 | 205.1239, 141.8689 |
| 32 | 13.65 | Tenacissoside H | C42H66O14 | M + H | 795.45253 | 795.44815 | −5.5 | 633.3608, 431.2743, 329.2097, 311.2020 |
| 33 | 13.82 | 11α-O-Tigloyl-12β-O-Benzoyltenacigenin B | C33H42O7 | M + H | 551.30033 | 551.29757 | −5 | 433.2358, 329.2119, 311.2010, 293.1906 |
| 34 | 14.00 | marstenacisside B5 | C57H90O24 | M − H | 1157.57493 | 1157.57149 | −3 | 1055.5049, 995.5205 |
| 35 | 14.25 | 11α-O-2-Methylbutyryl-12β-O-2-tigloyl tenacigeninB | C31H46O7 | M + H | 531.33163 | 531.32961 | −3.8 | 329.2114, 311.2002, 293.1908, 203.1077 |
| 36 | 14.47 | 11α-O-2-Methylbutyryl-12β-O-2-benzoyl tenacigeninB | C33H44O7 | M + H | 553.31598 | 553.31245 | −6.4 | 329.2104, 311.1989, 293.1882 |
| 37 | 15.99 | Glycyrrhetinic acid | C30H46O4 | M − H | 469.33233 | 469.33067 | −3.6 | 469.3301, 425.3414 |
| 38 | 16.41 | Betulinic acid | C30H48O3 | M + H | 457.36762 | 457.36687 | −1.6 | 457.3652, 161.1799 |
| 39 | 16.93 | Oleanolic acid | C30H48O3 | M − H | 455.35307 | 455.3526 | −1 | 455.3518 |
| Name | Company | Lot Number | Dilution Ratio |
|---|---|---|---|
| Anti-p65 | Bioss, Beijing, China | Lot: BB10125523 | 1:1000 |
| Anti-Bax | Bioss, Beijing, China | Lot: BA12063356 | 1:1000 |
| Anti-Nrf2 | Bioss, Beijing, China | Lot: BB01286971 | 1:1000 |
| Anti-Bcl-2 | Bioss, Beijing, China | Lot: BB09268767 | 1:1000 |
| Anti-HO-1 | Bioss, Beijing, China | Lot: BB07252595 | 1:1000 |
| Anti-NQO1 | Bioss, Beijing, China | Lot: BB10121621 | 1:1000 |
| Anti-caspase 3 | Bioss, Beijing, China | Lot: BA08247137 | 1:1000 |
| Anti-cleaved caspase 3 | Abcam, Cambridge, UK | Lot: ab2302 | 1:1000 |
| Anti-p-p65 | Abcam, Cambridge, UK | Lot: ab86299 | 1:1000 |
| Anti--IKKβ | Abcam, Cambridge, UK | Lot: ab124957 | 1:1000 |
| Anti-p-IKKB | Abcam, Cambridge, UK | Lot: ab59195 | 1:1000 |
| Anti-β-actin | Servicebio, Wuhan, China | Lot: AC220730001 | 1:1000 |
| Lamin B | Abcam, Cambridge, UK | Lot: ab0054 | 1:1000 |
| Gene | Primer Sequence (5′ to 3′) | Length | Accession Number |
|---|---|---|---|
| TNF-α | F: CAGGCGGTGCCTATGTCTC | 19 | NM_013693.3 |
| R: CGATCACCCCGAAGTTCAGTAG | 22 | ||
| IL-1β | F: GCAACTGTTCCTGAACTCAACT | 22 | NM_008361.4 |
| R: ATCTTTTGGGGTCCGTCAACT | 21 | ||
| IL-6 | F: TAGTCCTTCCTACCCCAATTTCC | 23 | NM_031168.2 |
| R: TTGGTCCTTAGCCACTCCTTC | 21 | ||
| β-actin | F: GGCTGTATTCCCCTCCATCG | 20 | NM_007393.1 |
| R: CCAGTTGGTAACAATGCCATGT | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liang, B.; Jike, W.; Li, R.; Su, X.; Yu, J.; Liu, T. The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis. Molecules 2023, 28, 7582. https://doi.org/10.3390/molecules28227582
Zhang Z, Liang B, Jike W, Li R, Su X, Yu J, Liu T. The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis. Molecules. 2023; 28(22):7582. https://doi.org/10.3390/molecules28227582
Chicago/Turabian StyleZhang, Zhiguang, Boya Liang, Wugemo Jike, Runtian Li, Xinxin Su, Jie Yu, and Tongxiang Liu. 2023. "The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis" Molecules 28, no. 22: 7582. https://doi.org/10.3390/molecules28227582
APA StyleZhang, Z., Liang, B., Jike, W., Li, R., Su, X., Yu, J., & Liu, T. (2023). The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis. Molecules, 28(22), 7582. https://doi.org/10.3390/molecules28227582






