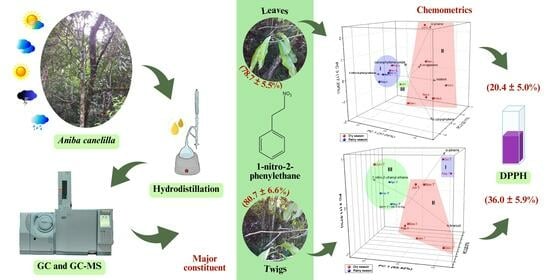
Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane
Abstract
:1. Introduction
2. Results and Discussion
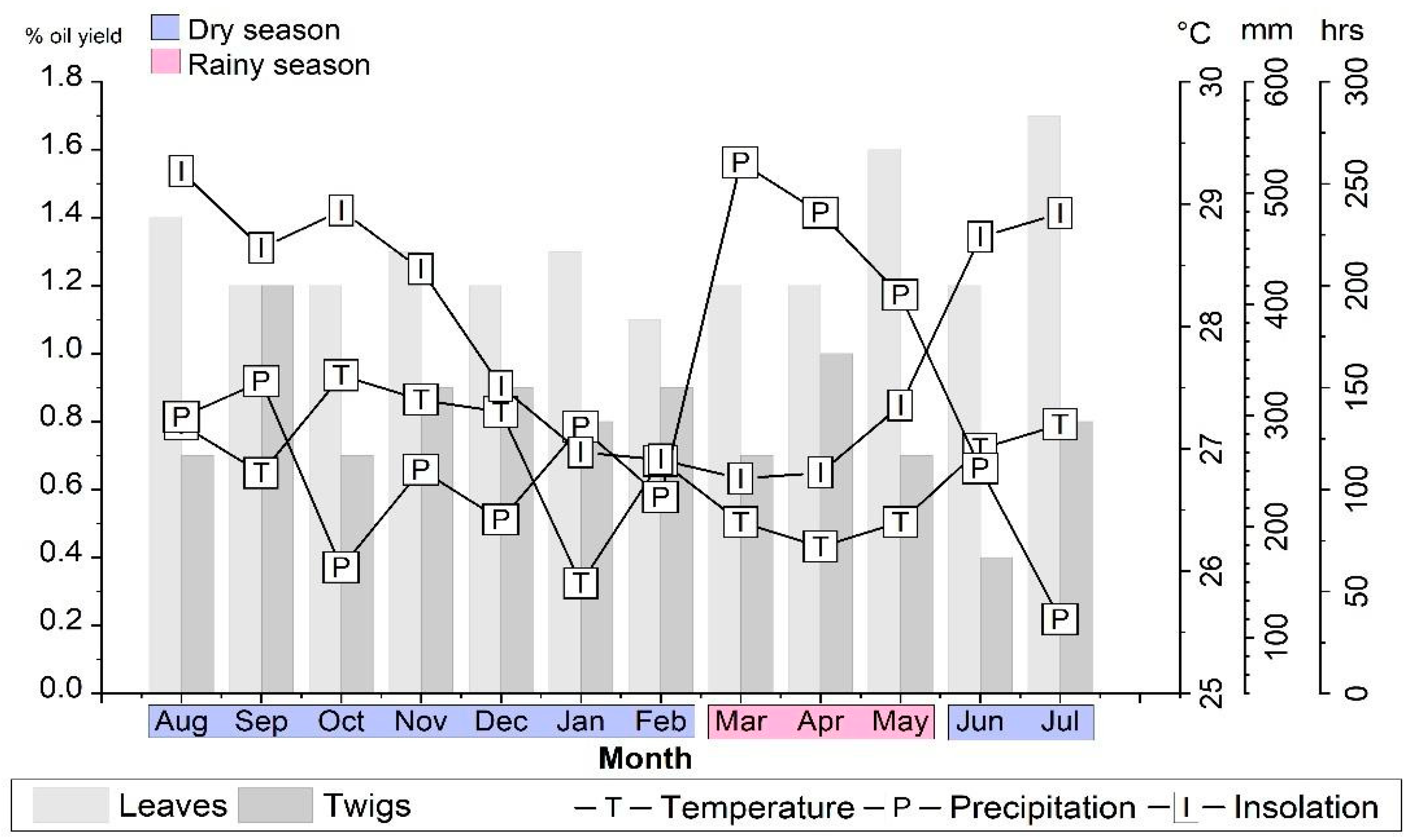
2.1. Essential Oil Yields vs. Environmental Conditions
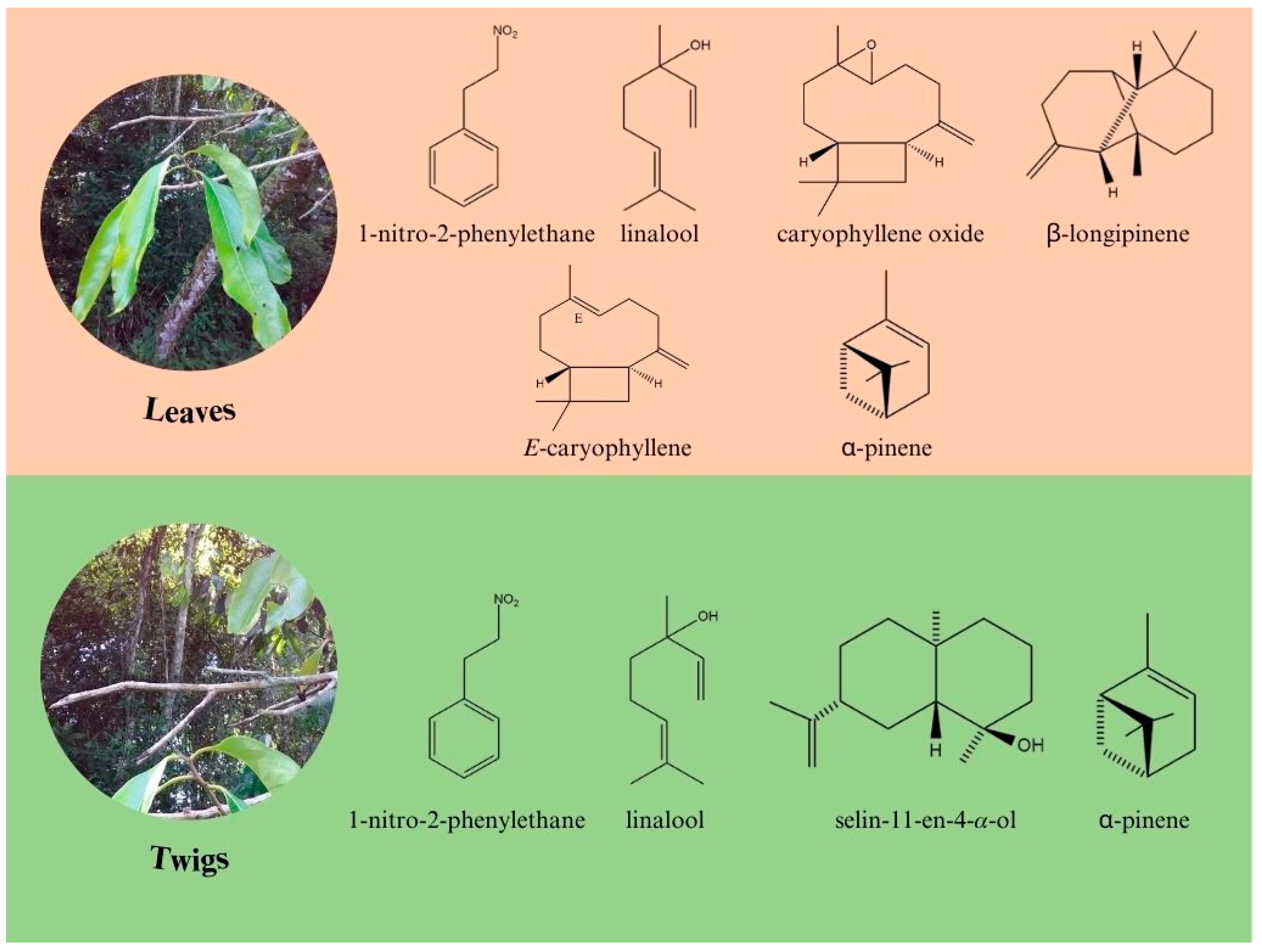
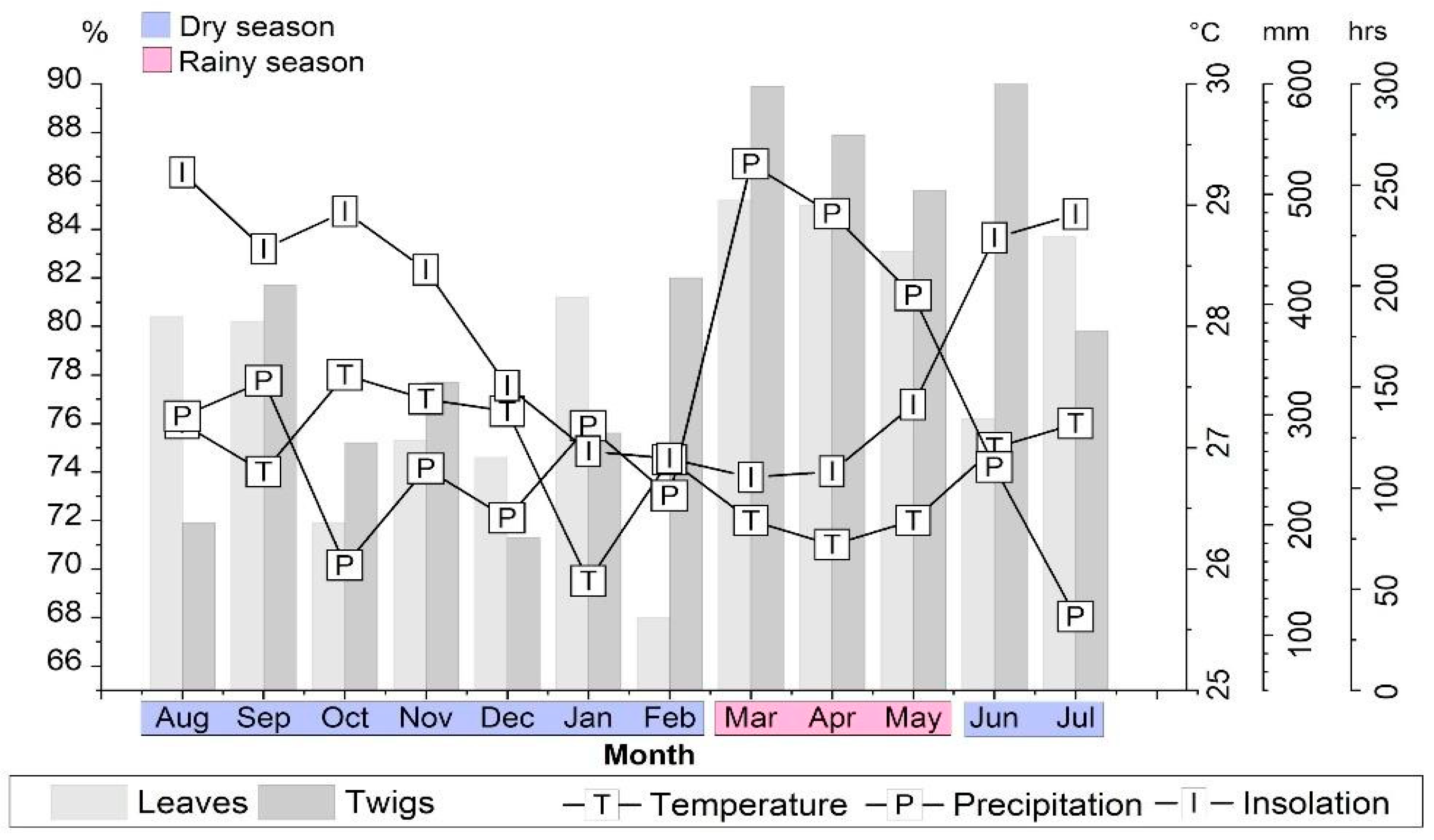
2.2. Chemical Composition vs. Environmental Conditions
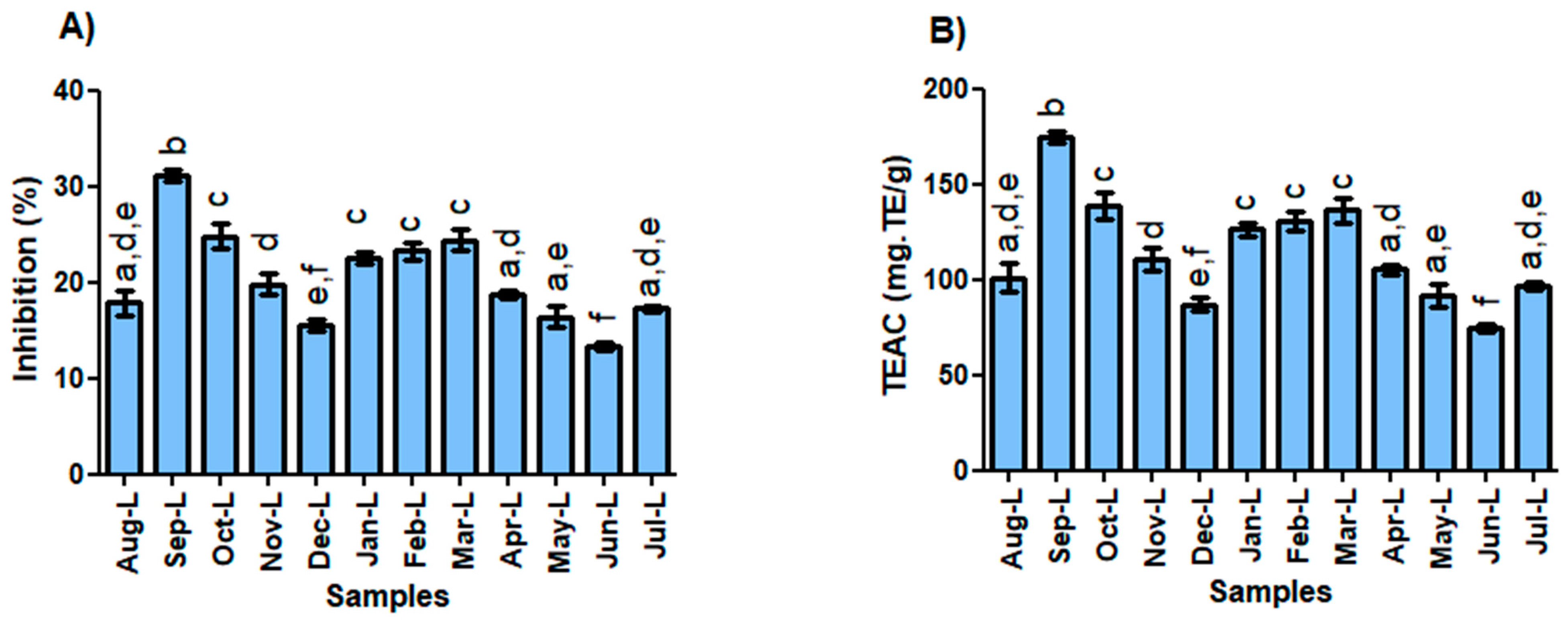
2.3. Antioxidant Capacity vs. Environmental Conditions
DPPH Radical Scavenging
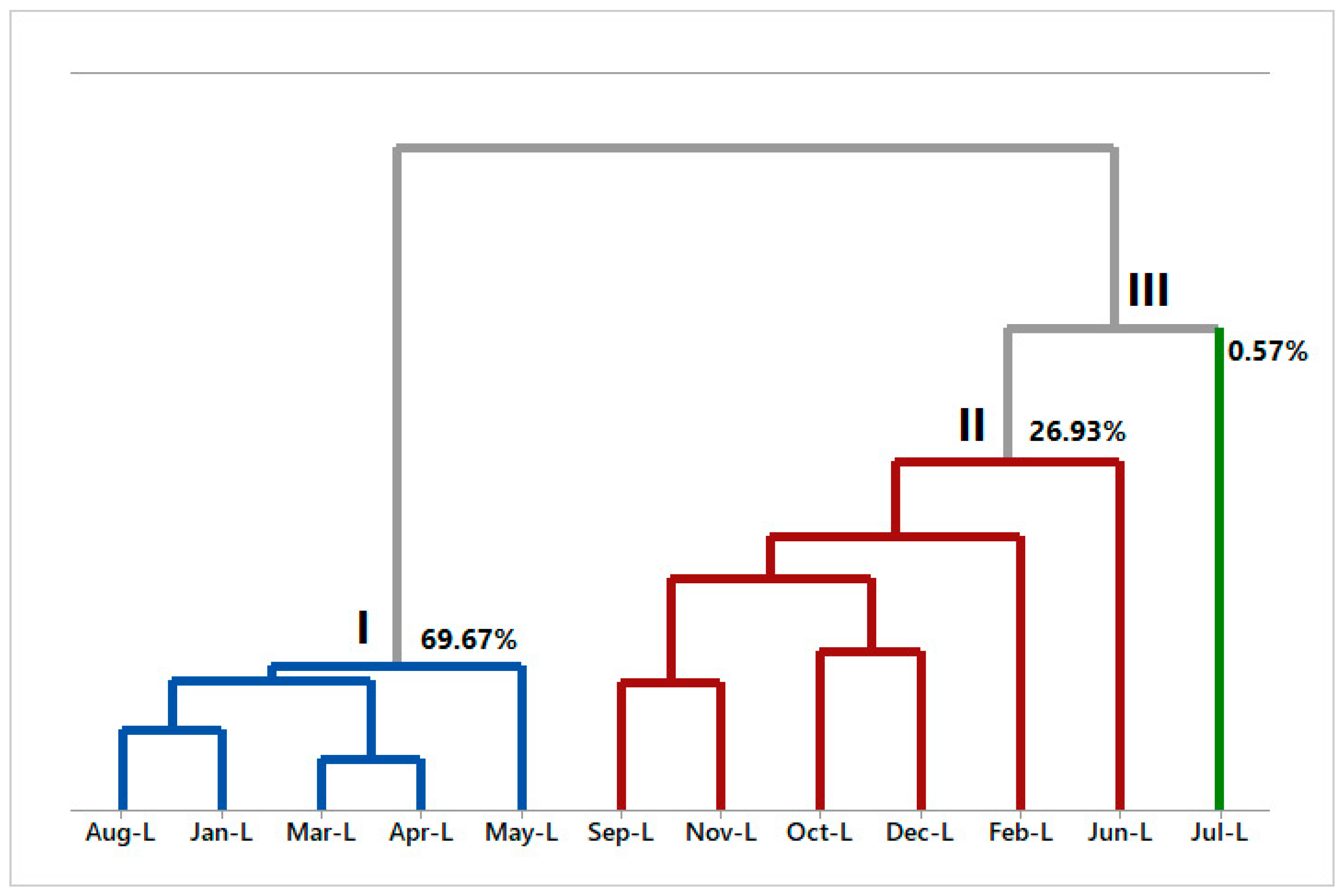
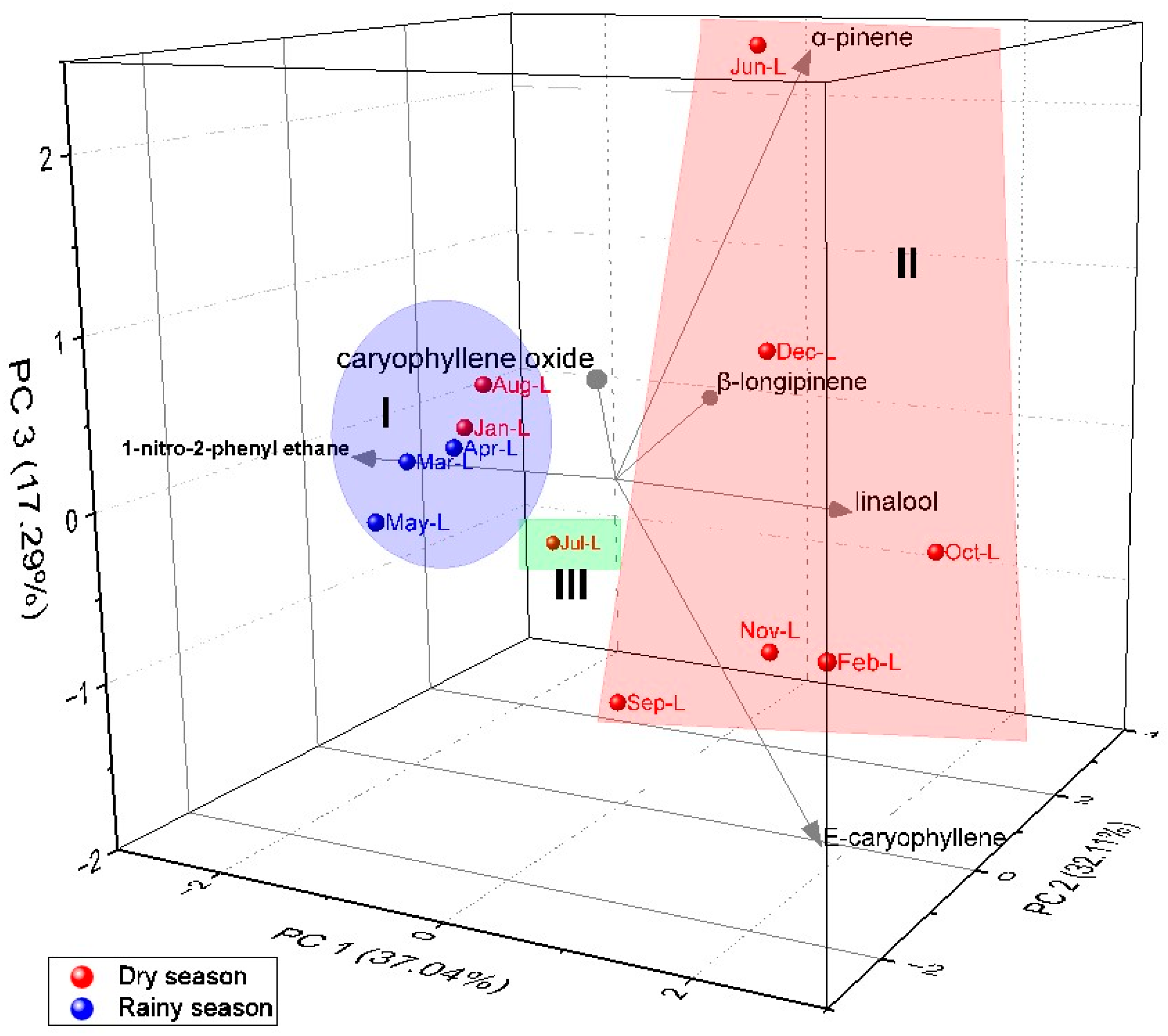
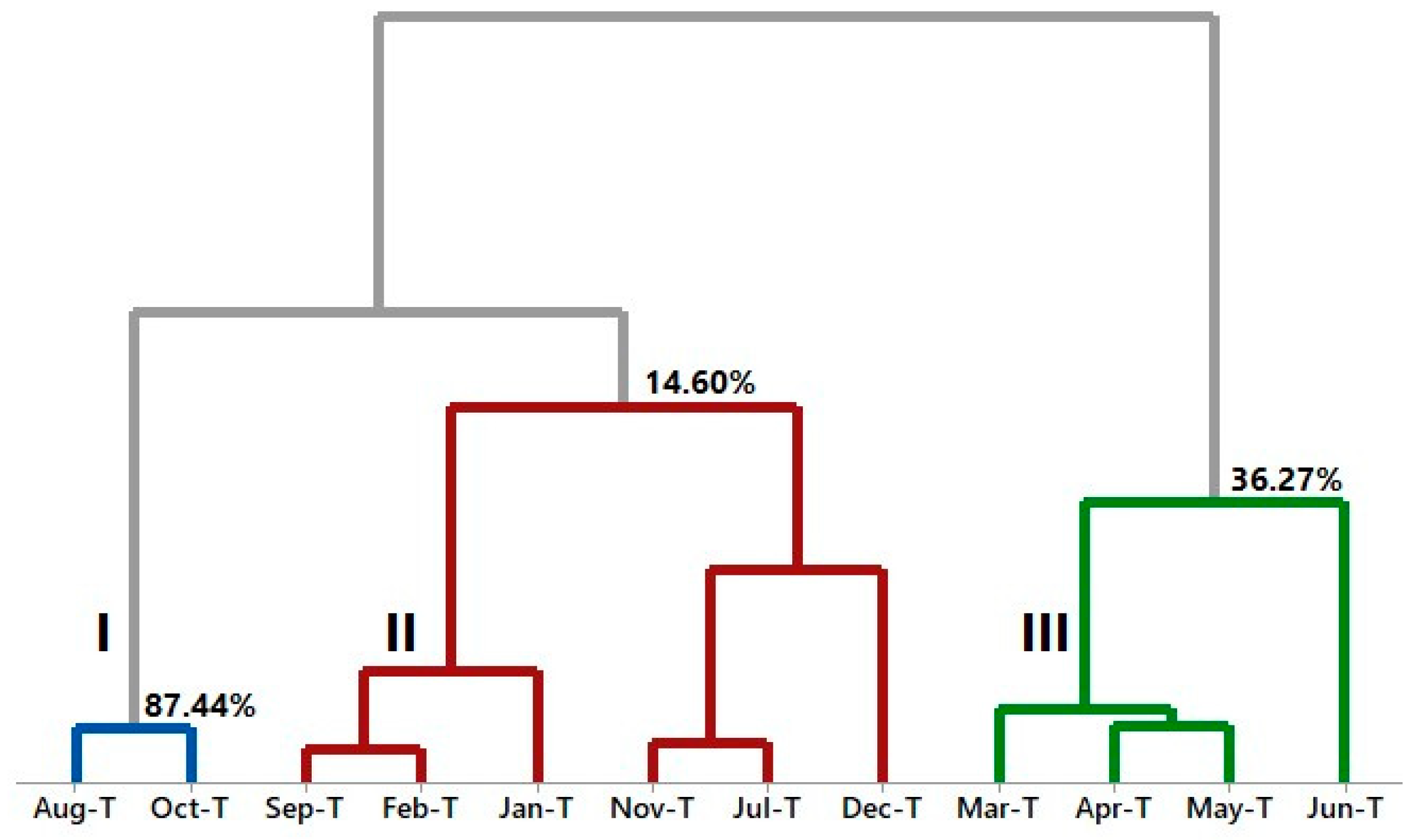
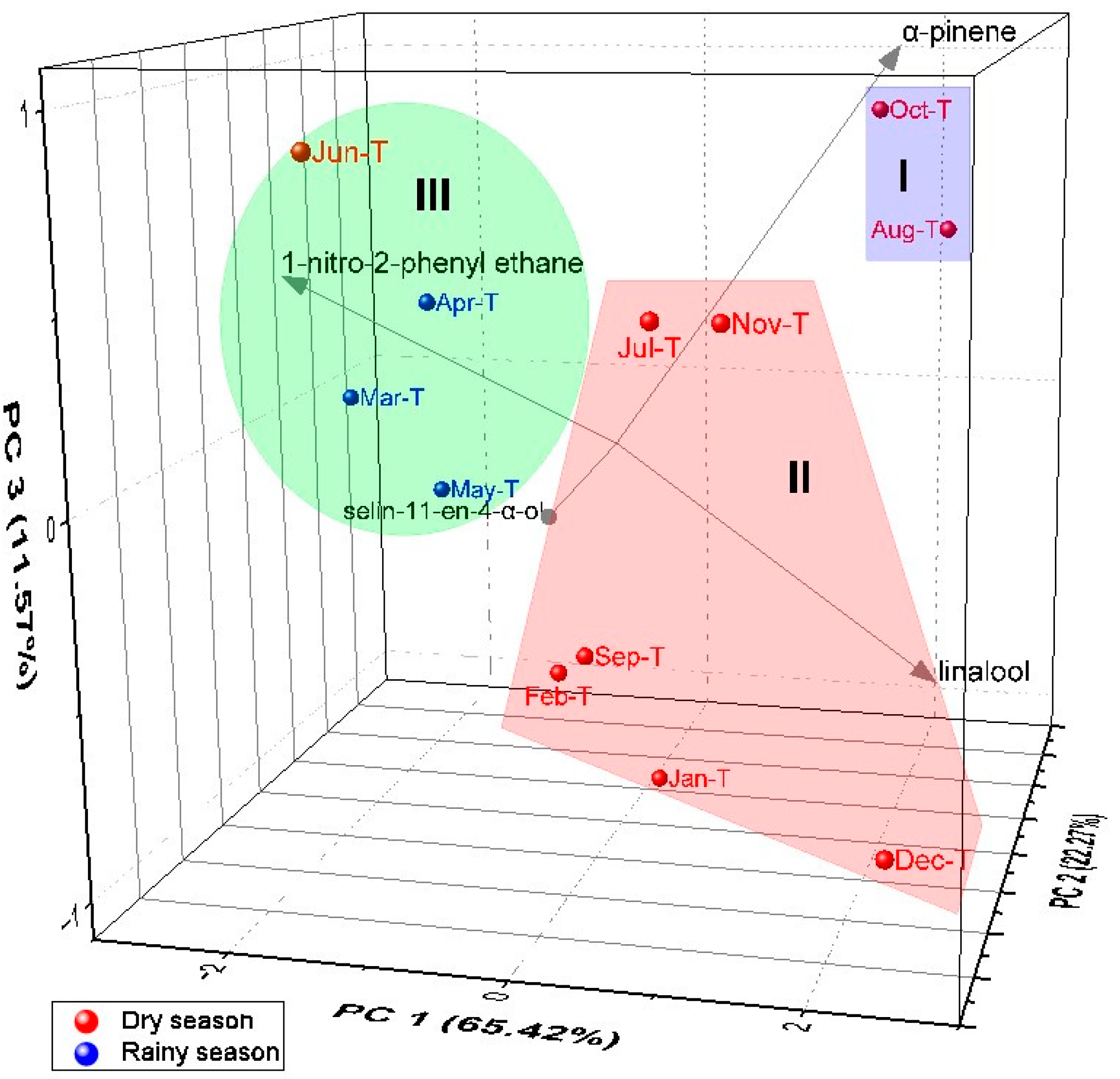
2.4. Multivariate Analysis of A. canelilla Leaf and Twig Essential Oils
3. Material and Methods
3.1. Plant Material and Climatic Data
3.2. Extraction and Oil Composition
3.3. Antioxidant Capacity
DPPH Radical Scavenging Method
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| RIC | RIL | Oil Constituents (%) | RT |
|---|---|---|---|
| 933 | 932 a | α-pinene | 5.820 |
| 948 | 946 a | camphene | 6.225 |
| 958 | 952 a | benzaldehyde | 6.485 |
| 973 | 974 a | sabinene | 6.900 |
| 977 | 974 a | β-pinene | 7.020 |
| 984 | 983 b | benzoic acid nitrile | 7.180 |
| 991 | 988 a | myrcene | 7.375 |
| 1006 | 1002 a | α-phellandrene | 7.848 |
| 1011 | 1008 a | δ-3-carene | 8.035 |
| 1024 | 1020 a | p-cymene | 8.495 |
| 1028 | 1024 a | limonene | 8.650 |
| 1029 | 1025 a | β-phellandrene | 8.669 |
| 1031 | 1026 a | 1.8-cineole | 8.740 |
| 1036 | 1032 a | Z-β-ocimene | 8.933 |
| 1041 | 1036 a | benzene acetaldehyde | 9.125 |
| 1046 | 1044 a | E-β-ocimene | 9.309 |
| 1058 | 1054 a | γ-terpinene | 9.723 |
| 1069 | 1059 a | acetophenone | 10.145 |
| 1071 | 1067 a | cis-linalool oxide | 10.210 |
| 1088 | 1084 a | trans-linalool oxide | 10.805 |
| 1089 | 1086 a | terpinolene | 10.827 |
| 1100 | 1095 a | linalool | 11.240 |
| 1137 | 1134 a | benzeneacetonitrile | 12.770 |
| 1139 | 1135 a | trans-pinocarveol | 12.820 |
| 1177 | 1174 a | terpinen-4-ol | 14.435 |
| 1190 | 1186 a | α-terpineol | 14.990 |
| 1195 | 1195 a | myrtenal | 15.235 |
| 1228 | 1227 a | nerol | 16.565 |
| 1255 | 1249 a | geraniol | 17.690 |
| 1256 | 1254 a | 2-phenylethyl acetate | 17.795 |
| 1308 | 1294 a | 1-nitro-2-phenylethane | 19.803 |
| 1351 | 1345 a | α-cubebene | 21.810 |
| 1357 | 1356 a | eugenol | 22.110 |
| 1377 | 1374 a | α-copaene | 22.930 |
| 1393 | 1389 a | β-elemene | 23.605 |
| 1408 | 1400 a | β-longipinene | 24.230 |
| 1420 | 1417 a | E-caryophyllene | 24.728 |
| 1441 | 1439 a | 2-phenylethyl butanoate | 25.565 |
| 1454 | 1452 a | α-humulene | 26.096 |
| 1487 | 1490 a | 2-phenylethyl 3-methylbutanoate | 27.425 |
| 1496 | 1498 a | α-selinene | 27.815 |
| 1509 | 1505 a | β-bisabolene | 28.340 |
| 1524 | 1521 a | trans-calamenene | 28.920 |
| 1525 | 1522 a | δ-cadinene | 28.902 |
| 1564 | 1561 a | E-nerolidol | 30.455 |
| 1571 | 1565 a | 3Z-hexenyl benzoate | 30.710 |
| 1578 | 1577 a | spathulenol | 31.045 |
| 1584 | 1582 a | caryophyllene oxide | 31.203 |
| 1588 | 1590 a | β-copaen-4α-ol | 31.395 |
| 1599 | 1600 a | guaiol | 31.815 |
| 1610 | 1608 a | humulene epoxide II | 32.235 |
| 1630 | 1627 a | 1-epi-cubenol | 32.970 |
| 1634 | 1639 a | caryophylla-4(12),8(13)-dien-5α-ol | 33.090 |
| 1637 | 1639 a | caryophylla-4(12),8(13)-dien-5β-ol | 33.230 |
| 1656 | 1651 a | pogostol | 33.893 |
| 1656 | 1658 a | selin-11-en-4α-ol | 33.930 |
| 1659 | 1661 a | allo-himachalol | 34.025 |
| 1672 | 1668 a | 14-hydroxy-9-epi-E-caryophyllene | 34.540 |
| 1669 | 1670 a | bulnesol | 34.390 |
| 1678 | 1676 a | mustakone | 34.770 |
| 1759 | 1759 a | cyclocolorenone | 37.640 |
| Sample | Leaves | Twigs | ||
|---|---|---|---|---|
| Inhibition (%) * | TEAC (mg.TE/g) * | Inhibition (%) * | TEAC (mg.TE/g) * | |
| August | 17.9 ± 1.3 a,d,e | 11.8 ± 7.4 | 40.2 ± 1.3 a,e,g | 226.8 ± 7.2 |
| September | 31.3 ± 0.5 b | 174.3 ± 3.0 | 42.0 ± 3.9 a,b | 236.6 ± 22.2 |
| October | 24.7 ± 1.3 c | 138.6 ± 7.3 | 40.2 ± 1.3 a,b,e,g | 226.8 ± 7.2 |
| November | 19.8 ± 1.0 d | 110.8 ± 6.0 | 33.0 ± 0.9 c,h | 186.2 ± 5.2 |
| December | 15.6 ± 0.5 e,f | 87.0 ± 3.2 | 30.2 ± 1.2 c,d | 170.4 ± 6.8 |
| January | 22.5 ± 0.6 c | 126.2 ± 3.6 | 34.4 ± 1.1 c,d,h | 193.9 ± 6.1 |
| February | 23.3 ± 0.8 c | 130.3 ± 4.9 | 39.8 ± 1.5 a,b,e,g | 224.5 ± 8.4 |
| March | 24.3 ± 1.1 c | 136.3 ± 6.2 | 40.6 ± 1.0 a,b,e,g | 229.2 ± 6.0 |
| April | 18.8 ± 0.4 a,d | 105.3 ± 2.3 | 37.2 ± 0.4 a,b,h | 210.1 ± 2.3 |
| May | 16.4 ± 1.0 a,e | 91.9 ± 5.8 | 36.7 ± 2.2 e,h | 206.8 ± 12.3 |
| June | 13.4 ± 0.3 f | 74.9 ± 2.0 | 20.8 ± 1.0 f | 117.6 ± 5.4 |
| July | 17.2 ± 0.3 a,d,e | 96.6 ± 1.6 | 36.8 ± 0.9 g,h | 207.7 ± 4.9 |
References
- da Trindade, R.C.S.; Xavier, J.K.A.M.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Chemical Diversity and Therapeutic Effects of Essential Oils of Aniba Species from the Amazon: A Review. Plants 2021, 10, 1854. [Google Scholar] [CrossRef]
- Chanderbali, P.A.S.; Van Der Werff, H.; Renner, S.S. Phylogeny and Historical Biogeography of Lauraceae: Evidence from the Chloroplast and Nuclear Genomes. Ann. Mo. Bot. Gard. 2001, 88, 104–134. [Google Scholar] [CrossRef]
- Lauraceae in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB143 (accessed on 7 November 2023).
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. A Review on Chemical Constituents and Biological Activities of the Genus Beilschmiedia (Lauraceae). Trop. J. Pharm. Res. 2015, 14, 2139–2150. [Google Scholar] [CrossRef]
- Damasceno, C.S.B.; Fabri Higaki, N.T.; Dias, J.D.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition and Biological Activities of Essential Oils in the Family Lauraceae: A Systematic Review of the Literature. Planta Med. 2019, 85, 1054–1072. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.K.A.M.; Maia, L.; Figueiredo, P.L.B.; Folador, A.; Ramos, A.R.; Andrade, E.H.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.R. Essential Oil Composition and DNA Barcode and Identification of Aniba Species (Lauraceae) Growing in the Amazon Region. Molecules 2021, 26, 1914. [Google Scholar] [CrossRef]
- de Oliveira, F.P.; Ana Carolina, A.C.B.; de Lima, E.J.S.P.; Silva, V.R.; de S. Santos, L.; da Anunciação, T.A.; Nogueira, M.L.; Soares, M.B.P.; Dias, R.B.; Gurgel Rocha, C.A.; et al. Essential Oil from Bark of Aniba parviflora (Meisn.) Mez (Lauraceae) Reduces HepG2 Cell Proliferation and Inhibits Tumor Development in a Xenograft Model. Chem. Biodivers. 2021, 18, e2000938. [Google Scholar] [CrossRef]
- Souza-Junior, F.J.C.; Luz-Moraes, D.; Pereira, F.S.; Barros, M.A.; Fernandes, L.M.P.; Queiroz, L.Y.; Maia, C.F.; Maia, J.G.S.; Fontes-Junior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae): A Review of Ethnobotany, Phytochemical, Antioxidant, Anti-Inflammatory, Cardiovascular, and Neurological Properties. Front. Pharmacol. 2020, 11, 699. [Google Scholar] [CrossRef]
- de Melo Alves Silva, L.C.; de Oliveira Mendes, F.d.C.; de Castro Teixeira, F.; de Lima Fernandes, T.E.; Barros Ribeiro, K.R.; da Silva Leal, K.C.; Dantas, D.V.; Neves Dantas, R.A. Use of Lavandula Angustifolia Essential Oil as a Complementary Therapy in Adult Health Care: A Scoping Review. Heliyon 2023, 9, e15446. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Ramezani, L. Evaluation of Five Essential Oils as Botanical Acaricides against the Strawberry Spider Mite Tetranychus Turkestani Ugarov and Nikolskii. Int. Biodeterior. Biodegrad. 2015, 98, 101–106. [Google Scholar] [CrossRef]
- de Oliveira Carvalho, I.; Purgato, G.A.; Píccolo, M.S.; Pizziolo, V.R.; Coelho, R.R.; Diaz-Muñoz, G.; Alves Nogueira Diaz, M. In Vitro Anticariogenic and Antibiofilm Activities of Toothpastes Formulated with Essential Oils. Arch. Oral Biol. 2020, 117, 104834. [Google Scholar] [CrossRef]
- Brito, T.S.; Lima, F.J.B.; Aragão, K.S.; De Siqueira, R.J.B.; Sousa, P.J.C.; Maia, J.G.S.; Filho, J.D.; Lahlou, S.; Magalhães, P.J.C. The Vasorelaxant Effects of 1-Nitro-2-Phenylethane Involve Stimulation of the Soluble Guanylate Cyclase-CGMP Pathway. Biochem. Pharmacol. 2013, 85, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.D.F.L.; Dos Ramos-Alves, F.E.; De Siqueira, R.J.B.; Xavier, F.E.; Duarte, G.P.; Magalhães, P.J.C.; Maia, J.G.S.; Sousa, P.J.D.C.; Lahlou, S. Vasorelaxant Effects of 1-Nitro-2-Phenylethane, the Main Constituent of the Essential Oil of Aniba canelilla, in Superior Mesenteric Arteries from Spontaneously Hypertensive Rats. Eur. J. Pharm. Sci. 2013, 48, 709–716. [Google Scholar] [CrossRef] [PubMed]
- de Lima, A.B.; Santana, M.B.; Cardoso, A.S.; da Silva, J.K.R.; Maia, J.G.S.; Carvalho, J.C.T.; Sousa, P.J.C. Antinociceptive Activity of 1-Nitro-2-Phenylethane, the Main Component of Aniba canelilla Essential Oil. Phytomedicine 2009, 16, 555–559. [Google Scholar] [CrossRef]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional Efficacy of Biologically Active Nitro Compounds Included in Medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Analgesic-like Activity of Essential Oils Constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef]
- da Fonsêca, D.V.; da Silva Maia Bezerra Filho, C.; Lima, T.C.; de Almeida, R.N.; de Sousa, D.P. Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomolecules 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- De Vincenzi, M.; Silano, M.; Stacchini, P.; Scazzocchio, B. Constituents of Aromatic Plants: I. Methyleugenol. Fitoterapia 2000, 71, 216–221. [Google Scholar] [CrossRef]
- Taveira, F.S.N.; De Lima, W.N.; Andrade, E.H.A.; Maia, J.G.S. Seasonal Essential Oil Variation of Aniba canelilla. Biochem. Syst. Ecol. 2003, 31, 69–75. [Google Scholar] [CrossRef]
- Barros, L.d.S.P.; Santos da Cruz, E.d.N.; de Araújo Guimarães, B.; Setzer, W.N.; Veras Mourão, R.H.; do Rosário da Silva, J.K.; Silva da Costa, J.; Baia Figueiredo, P.L. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition and Antioxidant Activity of a New Geraniol Chemotype of Lippia alba (Mill.) N.E.Br. Ex Britton & P. Wilson from the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104503. [Google Scholar] [CrossRef]
- de Loureiro, R.S.; Saraiva, J.M.; Saraiva, I.; Senna, R.C.; Fredó, A.S. Estudo Dos Eventos Extremos de Precipitação Ocorridos Em 2009 No Estado Do Pará. Rev. Bras. Meteorol. 2014, 29, 83–94. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant Capacity and Cytotoxicity of Essential Oil and Methanol Extract of Aniba canelilla (H.B.K.) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.V.L.; da Cruz, E.d.N.S.; Barroso, A.d.S.; Mourão, R.H.V.; Setzer, W.N.; da Silva, J.K.; do Nascimento, W.M.O.; da Costa, J.S.; Figueiredo, P.L.B. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition of Psidium Acutangulum Growing in the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104528. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential Oil of Wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) Leaves from Amazonian Ecuador. Flavour Fragr. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Steam, IL, USA, 2017; ISBN 9781932633214. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds,Mass Spectral Database; John Wiley & Sons Inc: New York, NY, USA, 2011. [Google Scholar]
- Cardoso, E.K.S.; Kubota, K.; Luz, D.A.; Mendes, P.F.S.; Figueiredo, P.L.B.; Lima, R.R.; Maia, C.S.F.; Fontes-Júnior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants 2022, 11, 1903. [Google Scholar] [CrossRef]
- da Silva, D.T.; Bianchini, N.H.; Amaral, L.d.P.; Longhi, S.J.; Heinzmann, B.M. Análise Do Efeito Da Sazonalidade Sobre O Rendimento Do Óleo Essencial Das Folhas De Nectandra grandiflora Nees1. Rev. Árvore 2015, 39, 1065–1072. [Google Scholar] [CrossRef]
- Silva, I.G.R.; Sousa, E.M.; Moraes, A.A.B.; Sarges, M.d.S.R.; Cascaes, M.M.; Nascimento, L.D.; Andrade, E.H.d.A. Avaliação Sazonal Do Rendimento e Composição Química Do Óleo Essencial Das Folhas de Aniba parviflora (Meisn) Mez. (Lauraceae). Braz. J. Dev. 2020, 6, 41334–41345. [Google Scholar] [CrossRef]
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.F.; Barbosa, W.; Raposo, N.R.B. Antioxidant Activity and Potential Photoprotective from Amazon Native Flora Extracts. J. Photochem. Photobiol. B Biol. 2016, 161, 34–39. [Google Scholar] [CrossRef]
- Santos, P.V.L.; da Cruz, E.d.N.S.; Nunes, J.d.A.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal Influence on Volatile Composition of Psidium friedrichsthalianum Leaves, Sampled in the Brazilian Amazon. Horticulturae 2023, 9, 768. [Google Scholar] [CrossRef]
- da Cruz, E.d.N.S.; Peixoto, L.d.S.; da Costa, J.S.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal Variability of a Caryophyllane Chemotype Essential Oil of Eugenia Patrisii Vahl Occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef]
- Instituto Nacional de Metereologia (INMET), Brazilian Government. Available online: http://www.inmet.gov.br/portal (accessed on 23 September 2023).
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, Antioxidant Capacity and Cytotoxic Activity of Eugenia Uniflora L. Chemotype-Oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-Icrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.; da Silva, N.; Ribeiro, A.; Montenegro, R.; Maia, J. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]

| Yield/ Components | Temperature | Insolation | Precipitation | |||
|---|---|---|---|---|---|---|
| L | T | L | T | L | T | |
| Oil yield | 0.01 | −0.11 | 0.30 | −0.20 | −0.17 | 0.10 |
| 1-nitro-2-phenylethane | −0.59 * | −0.47 | −0.16 | −0.37 | 0.61 * | 0.60 * |
| Linalool | 0.49 | 0.56 | 0.12 | 0.38 | −0.18 | −0.65 * |
| β-longipinene | 0.55 | 0.26 | 0.68 * | −0.14 | −0.64 * | −0.23 |
| E-caryophyllene | 0.43 | 0.06 | 0.16 | −0.10 | 0.29 | −0.33 |
| Selin-11-en-α-ol | −0.24 | −0.68 * | −0.13 | −0.55 * | −0.37 | 0.72 * |
| Caryophyllene oxide | −0.11 | −0.01 | −0.37 | −0.14 | 0.29 | −0.26 |
| α-pinene | 0.29 | 0.65 * | 0.24 | 0.67 * | −0.33 | −0.42 |
| Monoterpene hydrocarbons | 0.35 | 0.69 * | 0.28 | 0.71 * | −0.40 | −0.51 |
| Oxygenated monoterpenes | 0.78 * | 0.54 | 0.41 | 0.33 | −0.60 * | −0.63 * |
| Sesquiterpene hydrocarbons | 0.70 * | −0.08 | 0.49 | −0.13 | −0.70 * | −0.22 |
| Oxygenated sesquiterpenes | −0.13 | −0.70 * | −0.36 | −0.43 | 0.41 | 0.48 |
| Benzenoids | −0.58 * | −0.48 | −0.14 | −0.38 | 0.58 * | 0.61 * |
| RIC | RIL | August | September | October | November | December | January | February | March | April | May | June | July | Class | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aniba canelilla | L | T | L | T | L | T | L | T | L | T | L | T | L | T | L | T | L | T | L | T | L | T | L | T | |||
| Oil Yields (%) | 1.4 | 0.7 | 1.2 | 1.2 | 1.2 | 0.7 | 1.3 | 0.9 | 1.2 | 0.9 | 1.3 | 0.8 | 1.1 | 0.9 | 1.2 | 0.7 | 1.2 | 1.0 | 1.6 | 0.7 | 1.2 | 0.4 | 1.7 | 0.8 | |||
| Oil Constituents (%) | (%) | ||||||||||||||||||||||||||
| 933 | 932 a | α-pinene | 0.3 | 2.2 | 0.1 | 0.2 | 0.8 | 2.2 | 0.3 | 1.0 | 0.8 | 0.6 | 0.3 | 0.2 | 0.6 | 0.1 | tr | 0.2 | 0.4 | tr | 0.1 | 2.2 | 0.1 | tr | 0.8 | MH | |
| 948 | 946 a | camphene | tr | 0.1 | tr | 0.1 | tr | tr | tr | tr | tr | 0.1 | tr | MH | |||||||||||||
| 958 | 952 a | benzaldehyde | 1.1 | 0.1 | 0.4 | 0.1 | 0.5 | 0.1 | 0.5 | tr | 1.2 | 0.1 | 0.7 | 0.8 | 1.0 | 0.7 | 0.5 | tr | 0.5 | 0.4 | 0.1 | BZ | |||||
| 973 | 974 a | sabinene | tr | 0.1 | 0.1 | 0.1 | tr | 0.6 | tr | 0.4 | 0.6 | 0.7 | 0.4 | MH | |||||||||||||
| 977 | 974 a | β-pinene | 0.3 | 1.2 | 0.1 | 0.2 | 0.8 | 1.2 | 0.2 | 0.7 | 0.6 | 0.5 | 0.3 | 0.2 | 0.6 | 0.1 | 0.1 | tr | 0.2 | 0.3 | tr | 0.1 | 1.3 | 0.2 | 0.2 | 0.7 | MH |
| 984 | 983 b | benzoic acid nitrile | tr | 0.2 | 0.2 | 0.2 | tr | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.2 | BZ | |||||||||||||
| 991 | 988 a | myrcene | 0.3 | tr | 0.3 | 0.1 | 0.1 | 0.1 | tr | tr | 0.1 | tr | 0.1 | MH | |||||||||||||
| 1006 | 1002 a | α-phellandrene | 0.1 | 0.1 | 0.1 | tr | tr | tr | MH | ||||||||||||||||||
| 1011 | 1008 a | δ-3-carene | 0.2 | 0.2 | 0.1 | 0.1 | tr | tr | tr | tr | 0.1 | MH | |||||||||||||||
| 1024 | 1020 a | p-cymene | 0.1 | 0.3 | tr | tr | 0.1 | 0.3 | tr | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | tr | 0.2 | tr | 0.1 | MH | ||||||
| 1028 | 1024 a | limonene | 0.1 | tr | 0.4 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | tr | 0.5 | tr | MH | |||||||||||||
| 1029 | 1025 a | β-phellandrene | 1.1 | 0.2 | 1.1 | 0.6 | 0.4 | 0.3 | 0.1 | 0.6 | MH | ||||||||||||||||
| 1031 | 1026 a | 1.8-cineole | 0.2 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | tr | 0.1 | tr | 0.3 | tr | 0.1 | OM | ||||||||
| 1036 | 1032 a | Z-β-ocimene | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | MH | ||||||||||||||||
| 1041 | 1036 a | benzene acetaldehyde | 1.0 | 0.1 | 1.1 | 1.3 | 0.1 | 1.4 | 0.1 | 0.3 | 0.2 | 1.2 | 0.1 | 1.1 | 0.1 | 0.6 | 0.6 | 1.7 | tr | 0.7 | 1.8 | 0.1 | BZ | ||||
| 1046 | 1044 a | E-β-ocimene | 0.3 | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | 0.1 | tr | tr | 0.2 | MH | ||||||||||||||
| 1058 | 1054 a | γ-terpinene | tr | tr | tr | tr | tr | tr | MH | ||||||||||||||||||
| 1069 | 1059 a | acetophenone | 0.1 | 0.1 | 0.1 | tr | 0.1 | BZ | |||||||||||||||||||
| 1071 | 1067 a | cis-linalool oxide | 0.1 | 0.1 | tr | tr | tr | tr | tr | 0.1 | tr | tr | 0.1 | 0.1 | tr | tr | OM | ||||||||||
| 1088 | 1084 a | trans-linalool oxide | 0.1 | 0.1 | tr | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | OM | ||||||||||
| 1089 | 1086 a | terpinolene | 0.1 | MH | |||||||||||||||||||||||
| 1100 | 1095 a | linalool | 2.2 | 16.1 | 2.7 | 13.0 | 3.5 | 14.2 | 3.1 | 13.2 | 3.4 | 20.1 | 2.3 | 12.6 | 2.3 | 12.0 | 2.5 | 4.5 | 2.6 | 6.1 | 1.9 | 6.5 | 2.6 | 5.5 | 2.7 | 12.1 | OM |
| 1137 | 1134 a | benzeneacetonitrile | 0.3 | 0.2 | 0.3 | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.1 | BZ | |
| 1139 | 1135 a | trans-pinocarveol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | OM | |||||||||||||||||||
| 1177 | 1174 a | terpinen-4-ol | tr | 0.1 | tr | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | tr | 0.1 | 0.1 | tr | tr | MH | ||||||||
| 1190 | 1186 a | α-terpineol | 0.3 | 0.8 | 0.4 | 0.8 | 0.5 | 0.6 | 0.4 | 0.7 | 0.5 | 1.0 | 0.3 | 0.8 | 0.3 | 0.8 | 0.3 | 0.6 | 0.3 | 0.5 | 0.3 | 0.7 | 0.4 | 0.4 | 0.4 | 0.5 | OM |
| 1195 | 1195 a | myrtenal | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | OM | ||||||||||||||||
| 1228 | 1227 a | nerol | tr | tr | 0.1 | 0.1 | tr | 0.1 | 0.1 | OM | |||||||||||||||||
| 1255 | 1249 a | geraniol | 0.2 | tr | 0.1 | 0.1 | 0.1 | tr | 0.2 | tr | 0.3 | tr | 0.3 | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 | tr | OM | |||||||
| 1256 | 1254 a | 2-phenylethyl acetate | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | BZ | |||||||||
| 1308 | 1294 a | 1-nitro-2-phenylethane | 80.4 | 71.9 | 80.2 | 81.7 | 71.9 | 75.2 | 75.3 | 77.7 | 74.6 | 71.3 | 81.2 | 75.6 | 68.0 | 82.0 | 85.2 | 89.9 | 85.0 | 87.9 | 83.1 | 85.6 | 76.2 | 89.8 | 83.7 | 79.8 | BZ |
| 1351 | 1345 a | α-cubebene | 0.1 | 0.1 | tr | 0.1 | SH | ||||||||||||||||||||
| 1357 | 1356 a | eugenol | 0.2 | 0.6 | 0.5 | 0.7 | 0.3 | 0.7 | 0.3 | 0.6 | 0.3 | 0.6 | 0.3 | 0.6 | 0.4 | 0.8 | 0.2 | 0.9 | 0.2 | 0.9 | 0.3 | 1.0 | 0.1 | 0.8 | 0.2 | 0.5 | BZ |
| 1377 | 1374 a | α-copaene | 0.5 | 0.7 | 1.2 | 1.0 | 0.4 | 0.2 | 1.6 | 0.2 | 0.3 | 0.4 | 0.6 | 0.8 | SH | ||||||||||||
| 1393 | 1389 a | β-elemene | tr | tr | 0.1 | 0.1 | tr | 0.1 | tr | tr | SM | ||||||||||||||||
| 1408 | 1400 a | β-longipinene | 1.9 | 0.6 | 2.5 | 1.0 | 1.5 | 0.1 | 0.6 | 0.7 | 0.6 | 2.4 | 4.8 | SM | |||||||||||||
| 1420 | 1417 a | E-caryophyllene | 0.5 | 0.2 | 4.9 | 0.2 | 5.4 | 0.2 | 5.1 | 0.3 | 1.0 | 0.3 | 0.6 | 0.3 | 6.6 | 0.2 | 0.2 | 0.2 | 1.0 | 0.2 | 1.9 | 0.2 | 0.8 | 0.1 | 1.4 | 0.3 | SM |
| 1441 | 1439 a | 2-phenylethyl butanoate | 0.1 | 0.1 | 0.1 | 0.1 | BZ | ||||||||||||||||||||
| 1454 | 1452 a | α-humulene | 0.1 | tr | 0.5 | 0.7 | tr | 0.6 | tr | 0.3 | tr | 0.1 | tr | 0.6 | tr | 0.1 | 0.2 | tr | 0.3 | 0.5 | tr | SH | |||||
| 1487 | 1490 a | 2-phenylethyl 3-methylbutanoate | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.2 | 0.4 | 0.1 | 0.2 | 0.2 | BZ | ||||||||||||||
| 1496 | 1498 a | α-selinene | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | SH | |||||||
| 1509 | 1505 a | β-bisabolene | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | tr | 0.1 | 0.1 | SH | ||||||||||||||
| 1524 | 1521 a | trans-calamenene | 0.1 | 0.1 | 0.2 | tr | 0.1 | SH | |||||||||||||||||||
| 1525 | 1522 a | δ-cadinene | tr | 0.1 | 0.2 | 0.2 | tr | 0.1 | 0.1 | 0.1 | tr | SH | |||||||||||||||
| 1564 | 1561 a | E-nerolidol | 0.1 | 0.1 | 0.1 | tr | 0.1 | tr | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | OS | ||||||||||
| 1571 | 1565 a | 3Z-hexenyl benzoate | 0.1 | 0.1 | 0.1 | 0.1 | BZ | ||||||||||||||||||||
| 1578 | 1577 a | spathulenol | 0.1 | tr | 0.1 | tr | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | OS | ||||||||
| 1584 | 1582 a | caryophyllene oxide | 5.1 | 0.2 | 3.4 | 4.9 | 0.2 | 4.9 | 0.3 | 5.2 | 0.3 | 4.5 | 0.3 | 5.6 | 0.2 | 4.8 | 0.1 | 5.4 | 0.2 | 5.5 | 0.4 | 4.2 | 0.2 | 0.7 | 0.3 | OS | |
| 1588 | 1590 a | β-copaen-4α-ol | tr | tr | tr | tr | tr | 0.1 | 0.1 | OS | |||||||||||||||||
| 1599 | 1600 a | guaiol | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | tr | 0.1 | OS | |||||||||||||
| 1610 | 1608 a | humulene epoxide II | 0.4 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | tr | 0.2 | tr | OS | |||||||||||
| 1630 | 1627 a | 1-epi-cubenol | 0.1 | tr | tr | 0.1 | 0.1 | tr | tr | 0.1 | 0.1 | tr | 0.1 | 0.1 | OS | ||||||||||||
| 1634 | 1639 a | caryophylla-4(12),8(13)-dien-5α-ol | 0.3 | 0.2 | 0.3 | 0.3 | 0.8 | 0.2 | tr | OS | |||||||||||||||||
| 1637 | 1639 a | caryophylla-4(12),8(13)-dien-5β-ol | 0.8 | 0.6 | 0.8 | 1.0 | 1.3 | 1.0 | 1.7 | 0.2 | 0.9 | 1.4 | 0.9 | OS | |||||||||||||
| 1656 | 1651 a | pogostol | 1.0 | 1.0 | 1.0 | 1.9 | 0.6 | 1.8 | OS | ||||||||||||||||||
| 1656 | 1658 a | selin-11-en-4α-ol | 0.9 | 1.5 | 0.8 | 1.3 | 0.9 | 1.5 | 0.6 | 1.9 | 1.1 | 1.6 | 2.5 | 0.9 | 2.0 | 1.0 | 2.4 | OS | |||||||||
| 1659 | 1661 a | allo-himachalol | 0.2 | 0.7 | OS | ||||||||||||||||||||||
| 1672 | 1668 a | 14-hydroxy-9-epi-E-caryophyllene | 0.5 | 0.3 | tr | 0.4 | 0.4 | OS | |||||||||||||||||||
| 1669 | 1670 a | bulnesol | tr | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | OS | ||||||||||||||||
| 1678 | 1676 a | mustakone | 0.1 | tr | tr | 0.1 | OS | ||||||||||||||||||||
| 1759 | 1759 a | cyclocolorenone | 0.4 | 0.2 | 0.4 | 0.1 | 0.8 | 0.5 | 0.2 | 0.5 | 0.1 | OS | |||||||||||||||
| Monoterpene hydrocarbons | 0.7 | 5.9 | 0.3 | 0.6 | 2.1 | 5.9 | 0.6 | 3.0 | 1.7 | 2.1 | 0.7 | 0.9 | 1.6 | 0.6 | 0.7 | 0.1 | 0.5 | 1.4 | 0.1 | 0.2 | 4.8 | 1.0 | 1.0 | 3.2 | |||
| Oxygenated monoterpenes | 2.9 | 17.6 | 3.3 | 14.0 | 4.5 | 15.2 | 3.9 | 14.4 | 4.0 | 21.8 | 2.8 | 14.0 | 3.3 | 13.5 | 3.0 | 5.3 | 2.9 | 6.8 | 2.3 | 7.7 | 3.5 | 5.8 | 3.2 | 12.8 | |||
| Sesquiterpene hydrocarbons | 3.2 | 0.3 | 7.2 | 0.2 | 10.5 | 0.2 | 8.2 | 0.4 | 3.4 | 0.5 | 1.7 | 0.5 | 9.5 | 0.2 | 1.1 | 0.3 | 1.9 | 0.2 | 2.6 | 0.4 | 4.2 | 0.1 | 7.8 | 0.5 | |||
| Oxygenated sesquiterpenes | 8.2 | 2.3 | 5.5 | 1.3 | 7.1 | 2.1 | 7.6 | 1.0 | 6.9 | 0.9 | 6.4 | 3.4 | 9.1 | 2.2 | 7.4 | 3.3 | 7.6 | 2.7 | 8.3 | 3.9 | 7.5 | 2.2 | 1.4 | 2.6 | |||
| Benzenoids | 83.1 | 73.1 | 82.5 | 82.4 | 74.4 | 76.3 | 77.9 | 78.8 | 77.0 | 72.5 | 84.1 | 76.7 | 70.8 | 83.1 | 87.5 | 91.0 | 86.6 | 88.9 | 85.8 | 87.0 | 78.8 | 90.8 | 86.8 | 80.8 | |||
| Total | 98.0 | 99.1 | 98.8 | 98.4 | 98.5 | 99.7 | 98.2 | 97.6 | 93.0 | 97.8 | 95.8 | 95.5 | 94.3 | 99.6 | 99.7 | 99.8 | 99.4 | 99.9 | 99.0 | 99.2 | 98.8 | 99.9 | 100.0 | 99.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, E.d.N.S.d.; Barros, L.d.S.P.; Guimarães, B.d.A.; Mourão, R.H.V.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.d.R.; Figueiredo, P.L.B. Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane. Molecules 2023, 28, 7573. https://doi.org/10.3390/molecules28227573
Cruz EdNSd, Barros LdSP, Guimarães BdA, Mourão RHV, Maia JGS, Setzer WN, da Silva JKdR, Figueiredo PLB. Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane. Molecules. 2023; 28(22):7573. https://doi.org/10.3390/molecules28227573
Chicago/Turabian StyleCruz, Ellen de Nazaré S. da, Luana de Sousa P. Barros, Bruna de A. Guimarães, Rosa Helena V. Mourão, José Guilherme S. Maia, William N. Setzer, Joyce Kelly do R. da Silva, and Pablo Luis B. Figueiredo. 2023. "Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane" Molecules 28, no. 22: 7573. https://doi.org/10.3390/molecules28227573
APA StyleCruz, E. d. N. S. d., Barros, L. d. S. P., Guimarães, B. d. A., Mourão, R. H. V., Maia, J. G. S., Setzer, W. N., da Silva, J. K. d. R., & Figueiredo, P. L. B. (2023). Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-phenylethane. Molecules, 28(22), 7573. https://doi.org/10.3390/molecules28227573