Detrimental Impacts of Pharmaceutical Excipient PEG400 on Gut Microbiota and Metabolome in Healthy Mice
Abstract
:1. Introduction
2. Results
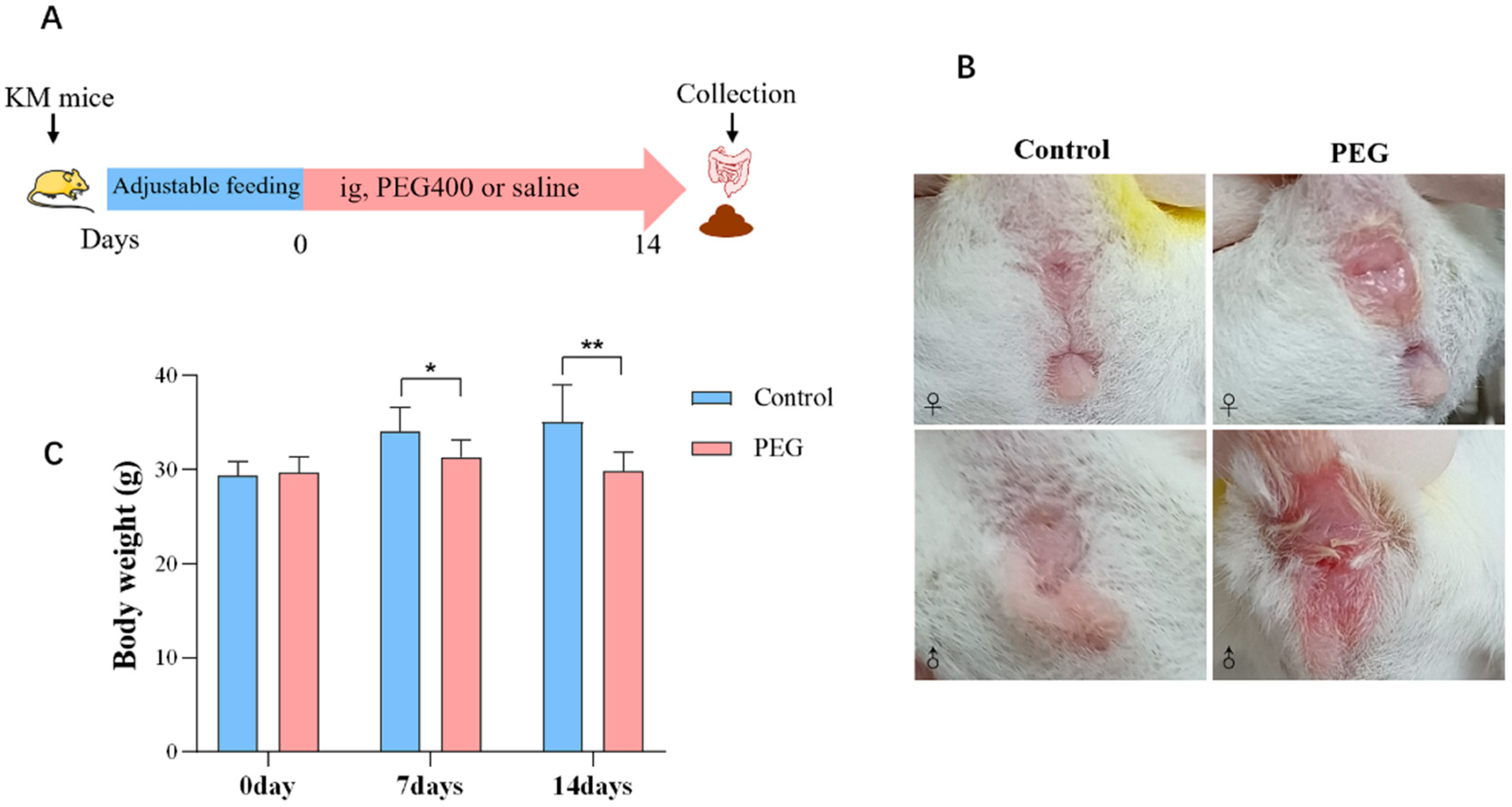
2.1. Impact of PEG400 on General Conditions in Mice
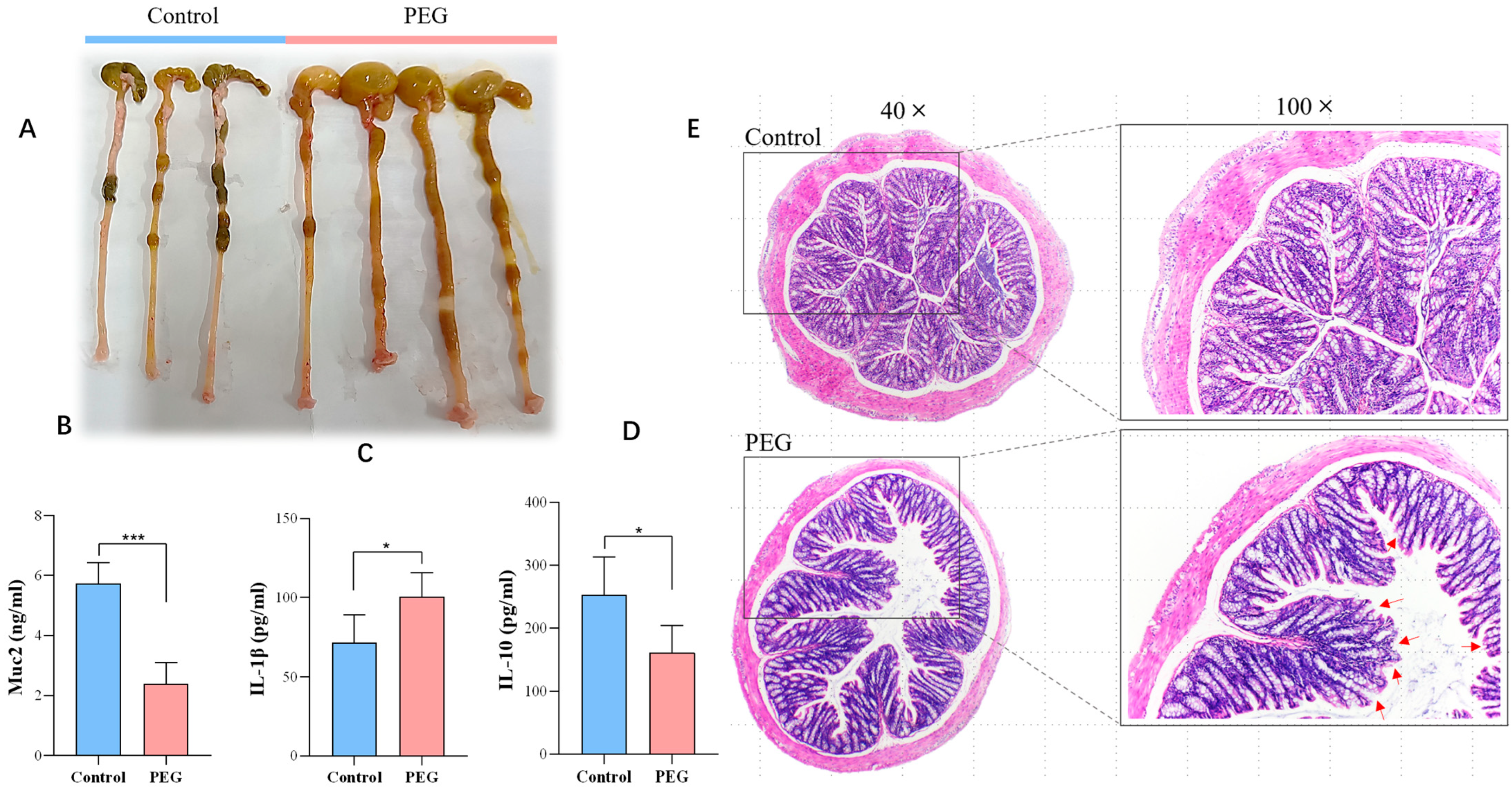
2.2. Effect of PEG400 on Colon Tissue
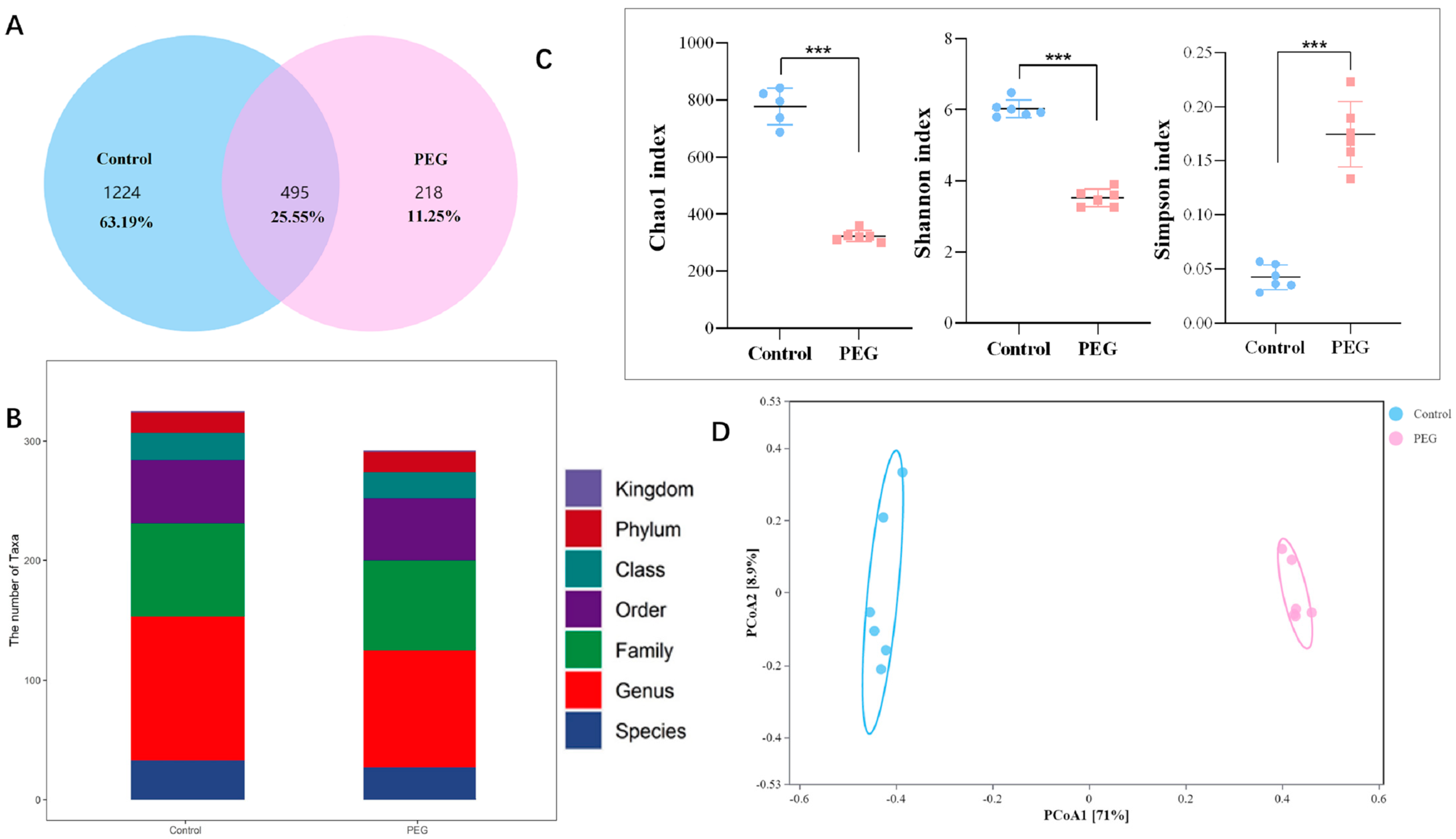
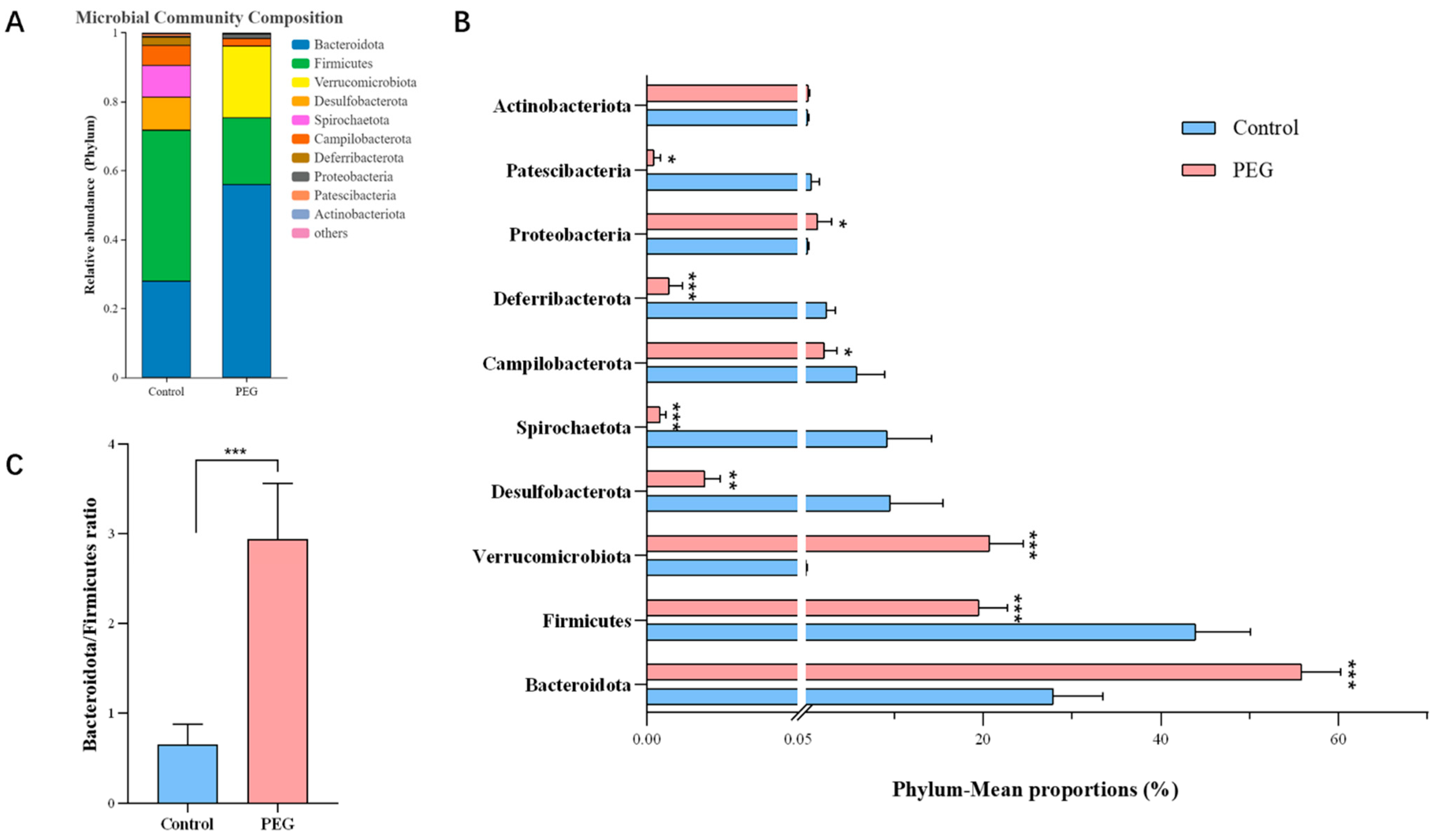
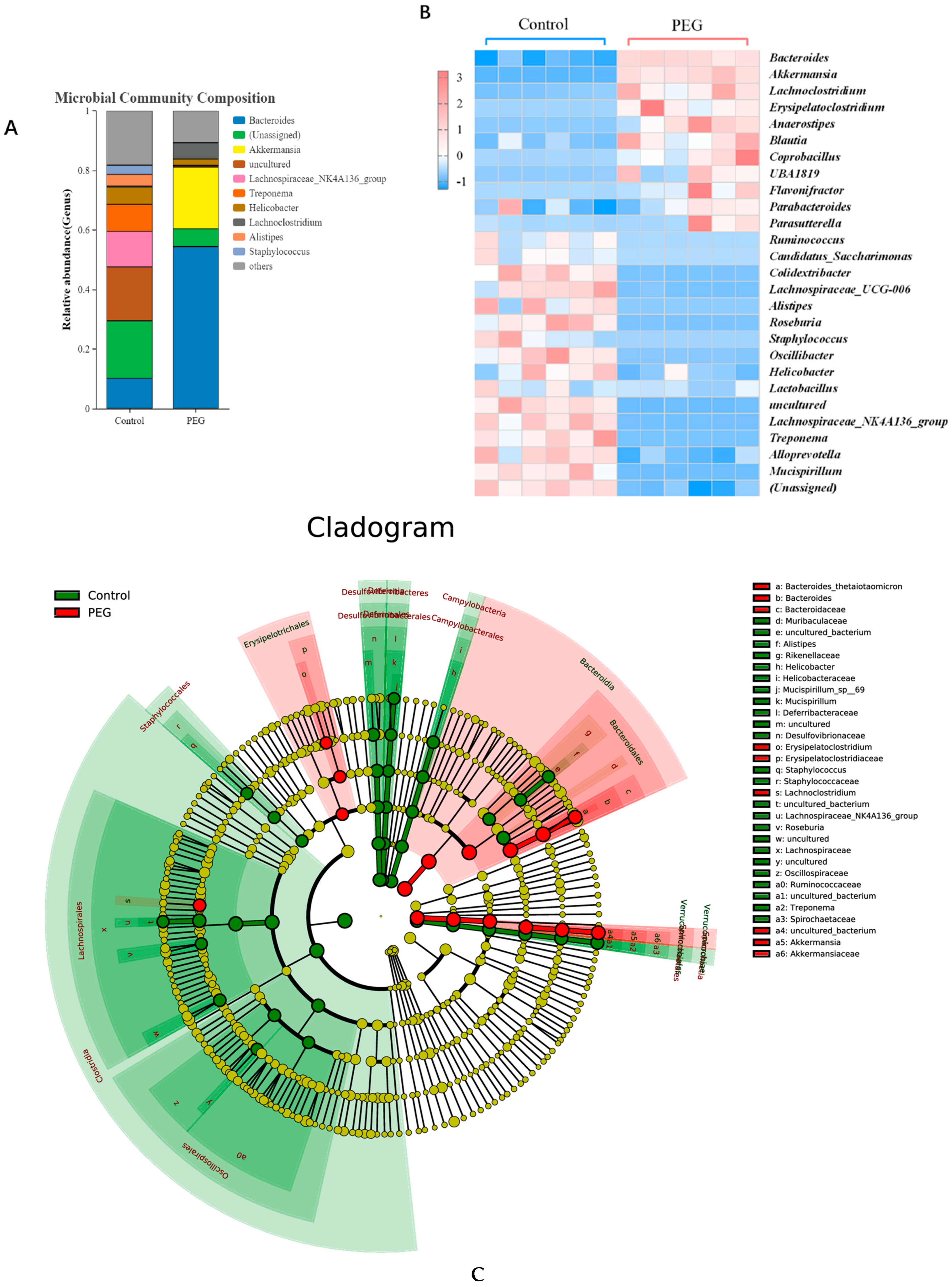
2.3. Effect of PEG400 on Gut Microbiota
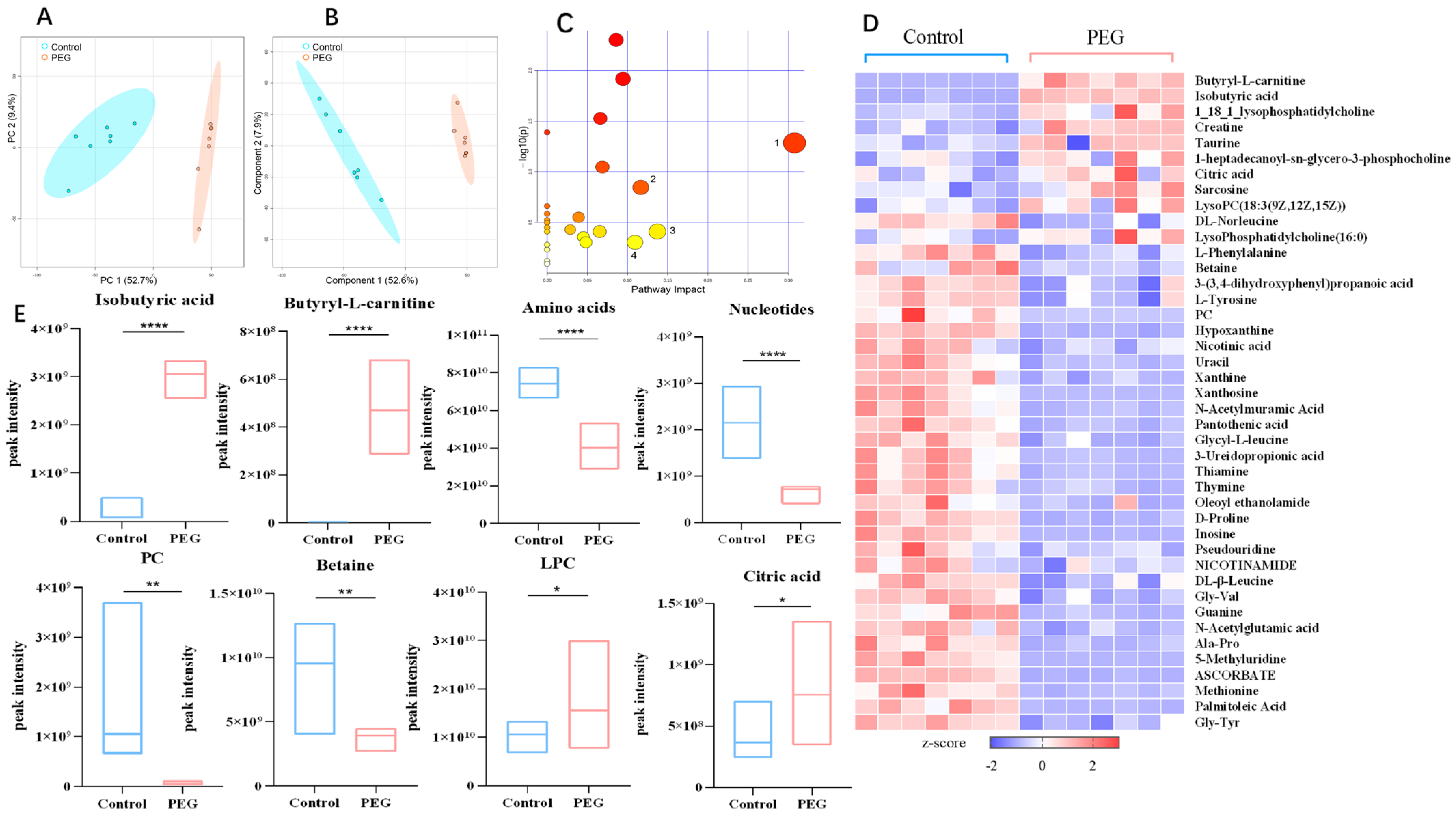
2.4. Metabolomics Analysis
2.5. Relationship between Gut Microbiota and Metabolites
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Animals
4.3. Biochemical and Histological Analysis
4.4. Gut Microbiota Composition Analysis
4.5. Untargeted Metabolomic Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Tian, Y.; Zheng, A.; Cui, C. Design Strategies for and Stability of mRNA–Lipid Nanoparticle COVID-19 Vaccines. Polymers 2022, 14, 4195. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Zhang, M.; Zhang, S.; Wang, P.J.; Meng, X.; Cao, S.Y.; Yang, Q.M.; Gao, X.L. Effect of molecular weight of polyethylene glycol on pharmacokinetics of baicalin. Acta Pharm. Sin. 2021, 56, 1416–1423. [Google Scholar] [CrossRef]
- Iyer, K.; Shah, T.; Kambl, S.; Patil, P.G. Effect of water-miscible organic solvents on CYP450-mediated metoprolol and imipramine metabolism in rat liver microsomes. Indian J. Pharm. Sci. 2015, 77, 382–390. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; He, Y.; Ren, T.; Yin, L.; Fawcett, J.P.; Gu, J.; Sun, H. Impact of molecular weight on the mechanism of cellular uptake of polyethylene glycols (PEGs) with particular reference to P-glycoprotein. Acta Pharm. Sin. B 2020, 10, 2002–2009. [Google Scholar] [CrossRef]
- Mai, Y.; Dou, L.; Murdan, S.; Basit, A.W. An animal’s sex influences the effects of the excipient PEG 400 on the intestinal P-gp protein and mRNA levels, which has implications for oral drug absorption. Eur. J. Pharm. Sci. 2018, 120, 53–60. [Google Scholar] [CrossRef]
- Houle, R.; Gu, Y.Z.; Sistare, F.D.; Koeplinger, K.; Cox, K.; Chu, X. Effects of PEG200 and PEG400 on human renal uptake/efflux transporters. Drug Metab. Pharmacokinet. 2019, 34, S72. [Google Scholar] [CrossRef]
- Ma, B.L.; Yang, Y.; Dai, Y.; Li, Q.; Lin, G.; Ma, Y.M. Polyethylene glycol 400 (PEG400) affects the systemic exposure of oral drugs based on multiple mechanisms: Taking berberine as an example. RSC Adv. 2017, 7, 2435–2442. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhang, S.; Zhang, M.; Meng, X.X.; Wang, P.J.; Zhang, R.P.; Gao, X.L. Study of Pharmaceutical Excipient PEG400 Alteration of Pharmacokinetics and Tissue Distribution of Main Flavonoids Metabolites of Baicalin. Curr. Pharm. Anal. 2021, 17, 609–623. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, M.; Yuan, M.; Yang, D.; Zhao, M.; Zhang, S.; Wang, P.; Zhang, R.; Gao, X. The pharmaceutical excipient PEG400 affect the absorption of baicalein in Caco-2 monolayer model by interacting with UDP-glucuronosyltransferases and efflux transport proteins. Pharmacol. Res. Perspect. 2022, 10, e00928. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.Y.; Zhou, M.H.; Cao, S.Y.; Zhang, M.; Wang, P.J.; Zhang, S.; Meng, X.X.; Yang, Q.M.; Gao, X.L. Effect of polyethylene glycol 400 on the pharmacokinetics and tissue distribution of baicalin by intravenous injection based on the enzyme activity of UGT1A8/1A9. Eur. J. Pharm. Sci. 2023, 180, 106328. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wagele, B.; Altmaier, E.; Gram, C.; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, A.; Zhang, H.; Sun, H.; Wang, X. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother. Res. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- Fung, C.; Van den Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell. Mol. Life Sci. 2020, 77, 4505–4522. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2021, 162, 743–756. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M.; Pihlsgård, M.; et al. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Romero, E.S.; Cotoner, C.A.; Camacho, C.P.; Bedmar, M.C.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 108, 686–696. [Google Scholar] [CrossRef]
- Feng, Y.; Weng, H.; Ling, L.; Zeng, T.; Zhang, Y.; Chen, D.; Li, H. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 2019, 132, 1001–1011. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L.-Q. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Experiment 2020, 26, e926789. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Tang, L.; Wang, F.; Huang, S.; Liu, S.; Lei, Y.; Wang, S.; Xie, Z.; Wang, W.; et al. TLR4 regulates RORγt+ regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.-M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Ghotaslou, R.; Nabizadeh, E.; Memar, M.Y.; Law, W.M.H.; Ozma, M.A.; Abdi, M.; Yekani, M.; Kadkhoda, H.; Hosseinpour, R.; Bafadam, S.; et al. The metabolic, protective, and immune functions of Akkermansia muciniphila. Microbiol. Res. 2022, 266, 127245. [Google Scholar] [CrossRef]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Strous, G.J.; Dekker, J. Mucin-Type Glycoproteins. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 57–92. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, H.J.; Ko, J.; Lee, J.H. Metabolically unhealthy overweight individuals have high lysophosphatide levels, phospholipase activity, and oxidative stress. Clin. Nutr. 2020, 39, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Stremmel, W.; Merle, U.; Zahn, A.; Autschbach, F.; Hinz, U.; Ehehalt, R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut 2005, 54, 966–971. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. Lysophospholipid-Related Diseases and PPARγ Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 2730. [Google Scholar] [CrossRef]
- Ma, N.; Yang, Y.; Liu, X.; Kong, X.; Li, S.; Qin, Z.; Jiao, Z.; Li, J. UPLC-Q-TOF/MS-based metabonomic studies on the intervention effects of aspirin eugenol ester in atherosclerosis hamsters. Sci. Rep. 2017, 7, 10544. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef]
- Cui, L.; Feng, L.; Zhang, Z.H.; Bin Jia, X. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Lei, L.; Zheng, Y.; Ai, J.; Chen, L.; Xiong, H.; Mei, Z.; Cheng, Y.-C.; Ren, Y. The Comparative Study of the Therapeutic Effects and Mechanism of Baicalin, Baicalein, and Their Combination on Ulcerative Colitis Rat. Front. Pharmacol. 2019, 10, 1466. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, S.; He, W.X.; Lu, J.L.; Xu, Y.J.; Yang, J.Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef]
- Shi, Y.; Study on the Effect of Medicinal Excipients on Intestinal Absorption of Alkaloids from Coptis Petrolatum. Nanjing University of Chinese Medicine 2013. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017276878.nh (accessed on 28 August 2022).
- Stadmiller, S.S.; Gorensek-Benitez, A.H.; Guseman, A.J.; Pielak, G.J. Osmotic Shock Induced Protein Destabilization in Living Cells and Its Reversal by Glycine Betaine. J. Mol. Biol. 2017, 429, 1155–1161. [Google Scholar] [CrossRef]
- Lu, J.-F.; Luo, S.; Jin, T.-C.; Wang, L.-C.; Yang, G.-J.; Lu, X.-J.; Chen, J. Betaine protects ayu (Plecoglossus altivelis) against Vibrio anguillarum infection in salinity by regulating the immunomodulatory activity of monocytes/macrophages. Aquaculture 2021, 536, 736482. [Google Scholar] [CrossRef]
- Chen, R.; Wen, C.; Gu, Y.; Wang, C.; Chen, Y.; Zhuang, S.; Zhou, Y. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food Agric. 2020, 100, 2656–2663. [Google Scholar] [CrossRef]
- Yang, Z.; Asare, E.; Yang, Y.; Yang, J.; Yang, H.; Wang, Z. Dietary supplementation of betaine promotes lipolysis by regulating fatty acid metabolism in geese. Poult. Sci. 2021, 100, 101460. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef]
- Eichinger, A.; Danecka, M.K.; Möglich, T.; Borsch, J.; Woidy, M.; Büttner, L.; Muntau, A.C.; Gersting, S.W. Secondary BH4 deficiency links protein homeostasis to regulation of phenylalanine metabolism. Hum. Mol. Genet. 2018, 27, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Xiao, H.-L.; Wang, G.-X.; Lu, Z.-Q.; Xie, M.-R.; Li, C.-S. Predictive value of presepsin and acylcarnitines for severity and biliary drainage in acute cholangitis. World J. Gastroenterol. 2023, 29, 2502–2514. [Google Scholar] [CrossRef]
- Srinivas, S.R.; Prasad, P.D.; Umapathy, N.S.; Ganapathy, V.; Shekhawat, P.S. Transport of butyryl-l-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB0,+. Am. J. Physiol. Liver Physiol. 2007, 293, G1046–G1053. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.; Cai, X.; Wang, C.; Cao, Y.; Xiao, J. Rosmarinic Acid Restores Colonic Mucus Secretion in Colitis Mice by Regulating Gut Microbiota-Derived Metabolites and the Activation of Inflammasomes. J. Agric. Food Chem. 2023, 71, 4571–4585. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, H.; Ke, L.; Gao, G.; Zhu, L. Human Intestinal Flora Metabolite Short-chain Fatty Acids Produced from Amino Acids and Effect on Cell permeability. J. Chin. Inst. Food Sci. Technol. 2021, 21, 60–67. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Wang, P.; Sun, X.; Yang, D.; Zhang, S.; Meng, X.; Zhang, M.; Gao, X. Detrimental Impacts of Pharmaceutical Excipient PEG400 on Gut Microbiota and Metabolome in Healthy Mice. Molecules 2023, 28, 7562. https://doi.org/10.3390/molecules28227562
Zhao M, Wang P, Sun X, Yang D, Zhang S, Meng X, Zhang M, Gao X. Detrimental Impacts of Pharmaceutical Excipient PEG400 on Gut Microbiota and Metabolome in Healthy Mice. Molecules. 2023; 28(22):7562. https://doi.org/10.3390/molecules28227562
Chicago/Turabian StyleZhao, Mei, Pengjiao Wang, Xiaodong Sun, Dan Yang, Shuo Zhang, Xiaoxia Meng, Min Zhang, and Xiuli Gao. 2023. "Detrimental Impacts of Pharmaceutical Excipient PEG400 on Gut Microbiota and Metabolome in Healthy Mice" Molecules 28, no. 22: 7562. https://doi.org/10.3390/molecules28227562
APA StyleZhao, M., Wang, P., Sun, X., Yang, D., Zhang, S., Meng, X., Zhang, M., & Gao, X. (2023). Detrimental Impacts of Pharmaceutical Excipient PEG400 on Gut Microbiota and Metabolome in Healthy Mice. Molecules, 28(22), 7562. https://doi.org/10.3390/molecules28227562




