Preparation and Photoelectric Properties of Pr-Doped p-Cu2O Thin Films Photocatalyst Based on Energy Band Structure Regulation
Abstract
:1. Introduction
2. Results
2.1. Selection of Deposition Potential for Pr-Cu2O
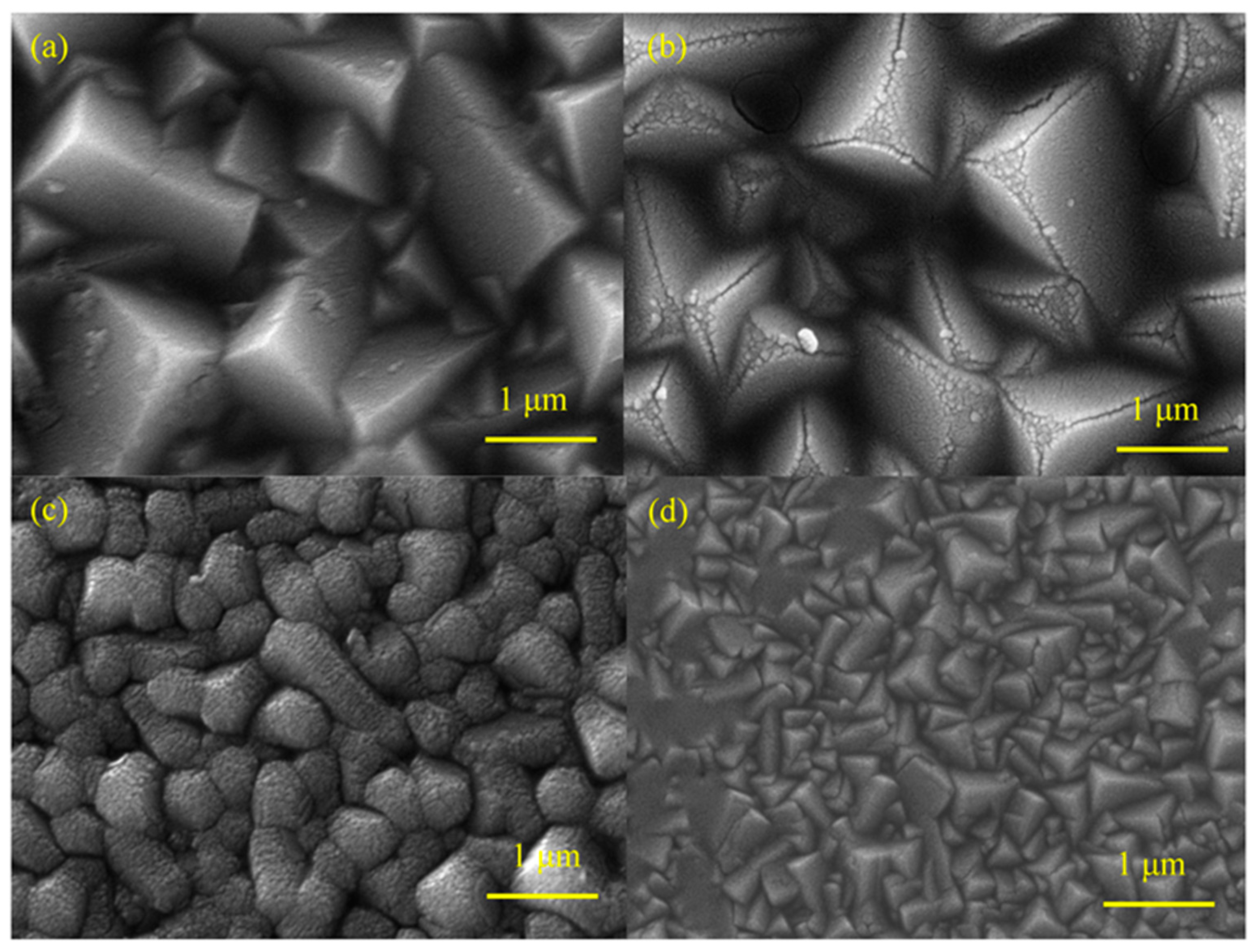
2.2. Microstructure Analysis of Pr-Cu2O Thin Films
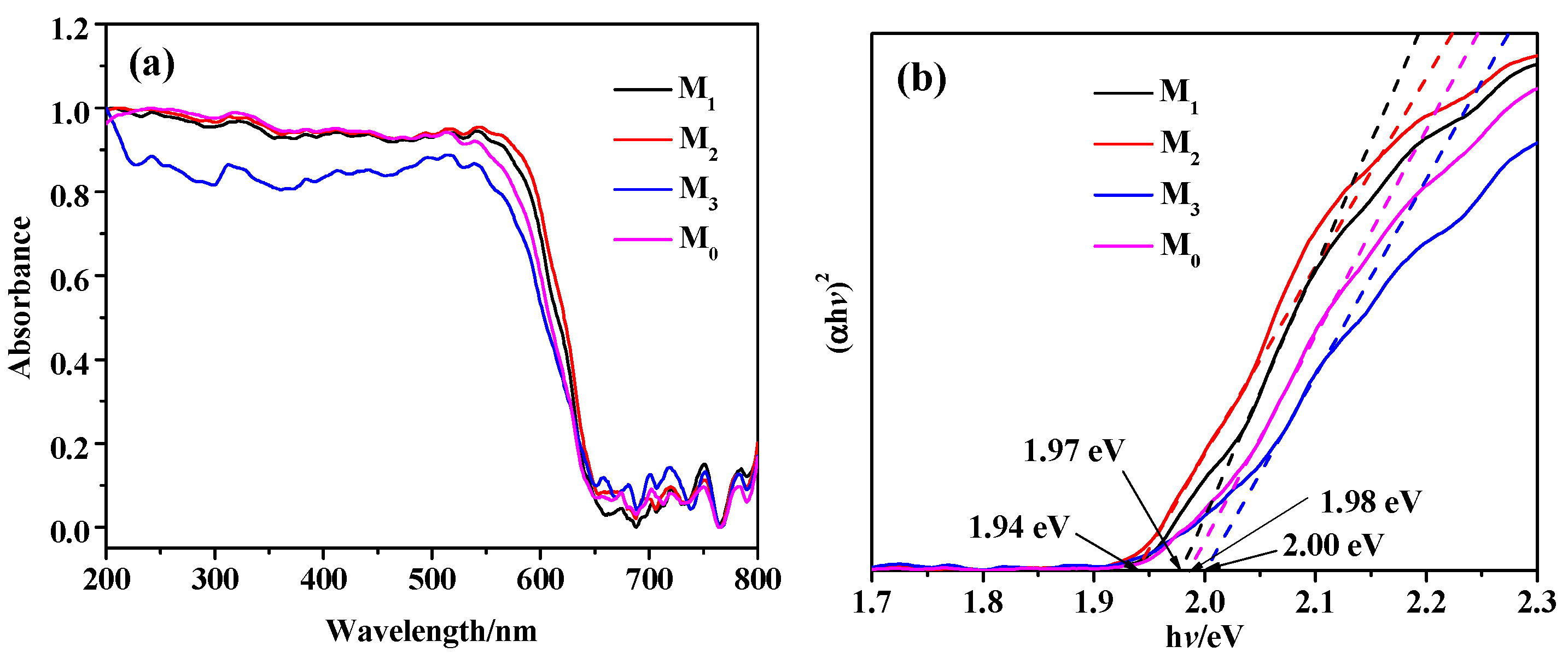
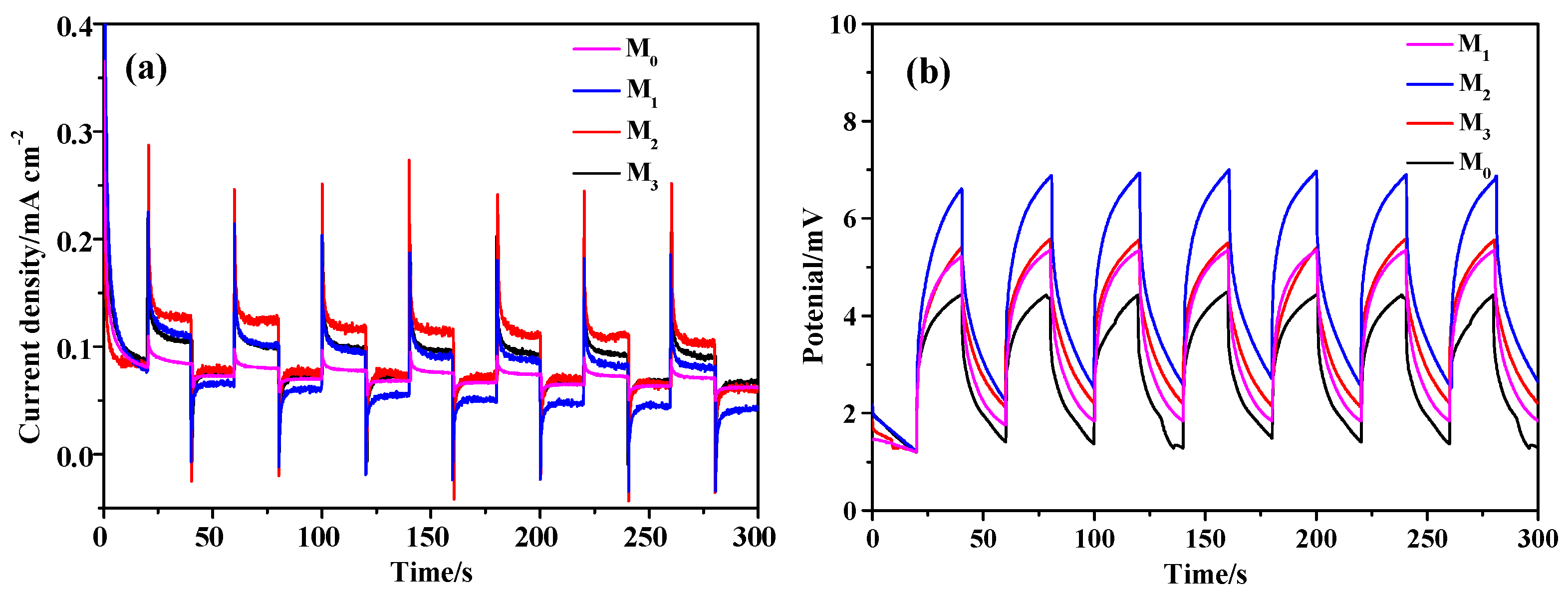
2.3. Photoelectric Properties Analysis of the Prepared Samples
2.4. Analysis of Photocatalytic Properties of Pr-Cu2O Thin Films
2.5. Analysis of Factors for Enhancing Photocatalytic Performance of Pr-Cu2O
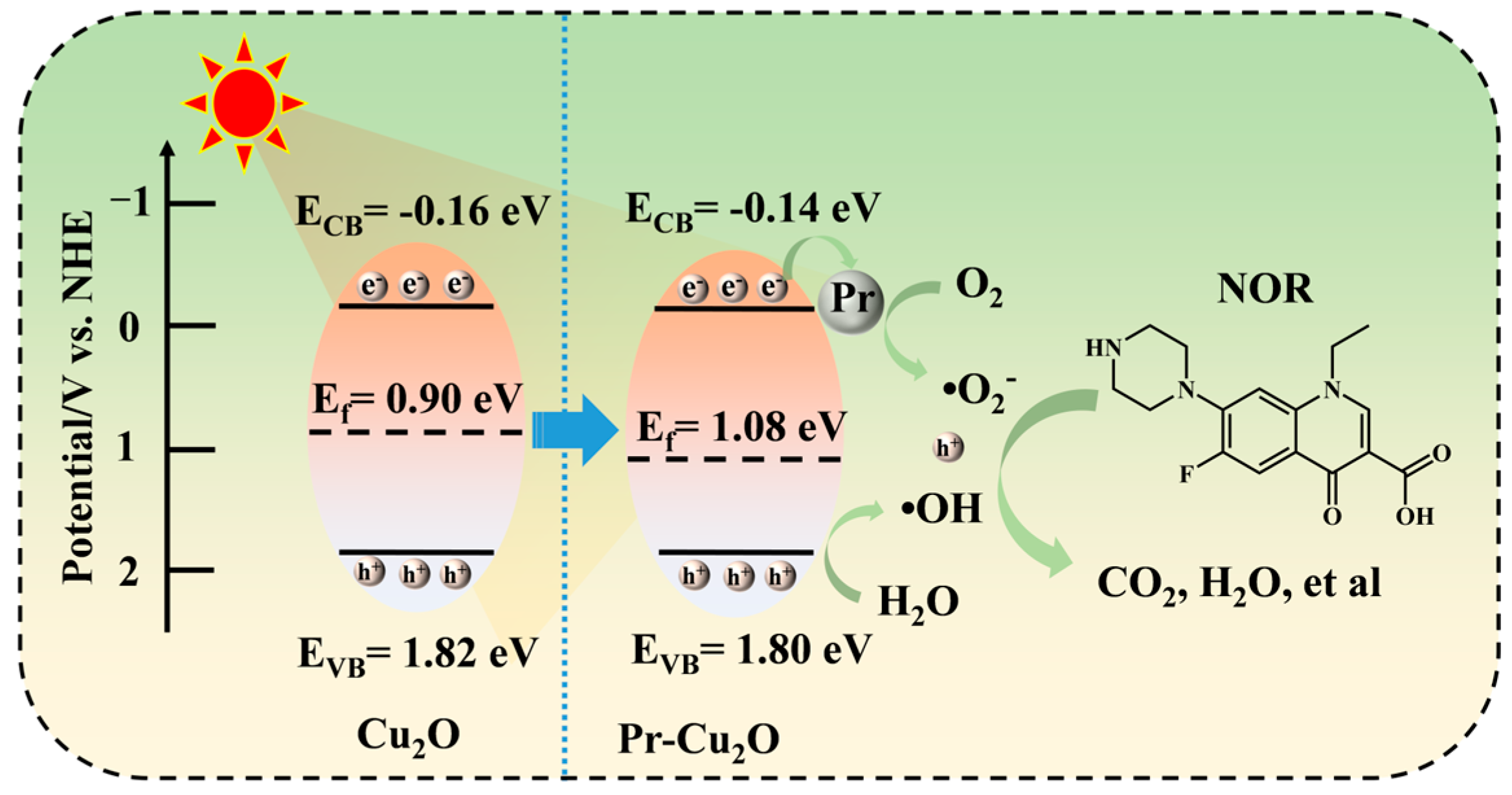
2.6. Photocatalytic Mechanism Analysis of Pr-Cu2O
3. Experimental
3.1. Preparation of Pr-Cu2O Thin Films
3.2. Characterization and Photoelectrochemical Properties Test of the Samples
3.3. Photocatalytic Degradation of Norfloxacin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Zhao, D.; Lu, J.; Liu, J.; Dao, G.; Chen, B.; Huang, B.; Pan, X. Multiple roles of nanomaterials along with their based nanotechnologies in the elimination and dissemination of antibiotic resistance. Chem. Eng. J. 2023, 455, 140927. [Google Scholar] [CrossRef]
- Zuo, S.; Huang, S.; Wang, Y.; Wan, J.; Yan, Z.; Ma, Y.; Wang, S. Surface modify BiOBr/Mn-Ti3C2Tx to enhance the carrier separation efficiency and weak electron utilization for photo-assisted peroxymonosulfate activation to degrade emerging contaminants. Chem. Eng. J. 2023, 475, 146230. [Google Scholar] [CrossRef]
- Wang, K.; Yu, X.; Liu, Z.; Zhang, T.; Ma, Y.; Niu, J.; Yao, B. Interface engineering of 0D/2D Cu2O/BiOBr Z-scheme heterojunction for efficient degradation of sulfamethoxazole: Mechanism, degradation pathway, and DFT calculation. Chemosphere 2024, 346, 140596. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Ke, X.; Qian, W.; Yan, Z.; Luan, J.; Liu, W. Insight into tetracycline photocatalytic degradation mechanism in a wide pH range on BiOI/BiOBr: Coupling DFT/QSAR simulations with experiments. Appl. Catal. B-Environ. 2024, 340, 123226. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, H.; Zhang, A.; Wu, L.; Fu, Y.; Zhou, Q. Enhancing photocatalytic antibiotics mineralization and water oxidation via constructing interfacial electric field in plate-on-plate BiOCl/WO3 photocatalysts. J. Colloid Interface Sci. 2023, 642, 264–272. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, Z.; Zheng, X.; Chu, C.; Guo, Y. Construction and actual application of In2O3/BiOBr heterojunction for effective removal of ciprofloxacin under visible light: Photocatalytic mechanism, DFT calculation, degradation pathway and toxicity evaluation. J. Alloys Compd. 2024, 971, 172779. [Google Scholar] [CrossRef]
- Ding, X.; Liu, W.; Feng, Y.; Liu, J.; Zeng, X.; Zhou, R.; Zhang, X.; Wang, R.; Guo, Q. Application of WO3 and Zn-doped WO3 prepared by microwave irradiation for photocatalytic degradation of Rhodamine B in water and wastewater. Int. J. Electrochem. Sci. 2022, 17, 221045. [Google Scholar] [CrossRef]
- Yang, F.; Yu, X.; Wang, K.; Liu, Z.; Gao, Z.; Zhang, T.; Niu, J.; Zhao, J.; Yao, B. Photocatalytic degradation of methylene blue over BiVO4/BiPO4/rGO heterojunctions and their artificial neural network model. J. Alloys Compd. 2023, 960, 170716. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, B.; Li, L.; Tian, Z.; Xu, X.; Wu, Z.; Yang, J.; Zhang, Z. Hydrogen-substituted graphdiyne encapsulated cuprous oxide photocathode for efficient and stable photoelectrochemical water reduction. Nat. Commun. 2022, 13, 5770. [Google Scholar] [CrossRef]
- Yu, X.; Chen, H.; Ji, Q.; Chen, Y.; Wei, Y.; Zhao, N.; Yao, B. p-Cu2O/n-ZnO heterojunction thin films with enhanced photoelectrochemical properties and photocatalytic activities for norfloxacin. Chemosphere 2021, 267, 129285. [Google Scholar] [CrossRef]
- Sehrawat, P.; Rana, S.; Mehta, S.K.; Kansal, S.K. Optimal synthesis of MoS2/Cu2O nanocomposite to enhance photocatalytic performance towards indigo carmine dye degradation. Appl. Surf. Sci. 2022, 604, 154482. [Google Scholar] [CrossRef]
- Xu, L.; An, H.; Wu, Z.; Ju, W.; Wang, Y.; Wang, X.; Wang, X. Sonocatalytic degradation of tetracycline by Cu2O/MgFe2O4 nanocomposites: Operational parameters, sonocatalytic mechanism, degradation pathways, and environmental toxicity. Surf. Interfaces 2023, 41, 103202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhang, Y.; Li, Y.; Yuan, Y. Shape-dependent synthesis and photocatalytic degradation by Cu2O nanocrystals: Kinetics and photocatalytic mechanism. J. Colloid Interface Sci. 2023, 651, 117–127. [Google Scholar] [CrossRef]
- Nie, J.; Yu, X.; Liu, Z.; Wei, Y.; Zhang, J.; Zhao, N.; Yu, Z.; Yao, B. Boosting principles for the photocatalytic performance of Cr-doped Cu2O crystallites and mechanisms of photocatalytic oxidation for levofloxacin. Appl. Surf. Sci. 2022, 576, 151842. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Chen, Y.; Ji, Q.; Wei, Y.; Niu, J.; Yu, Z.; Yao, B. Ag-Cu2O composite films with enhanced photocatalytic activities for methylene blue degradation: Analysis of the mechanism and the degradation pathways. J. Environ. Chem. Eng. 2021, 9, 106161. [Google Scholar] [CrossRef]
- Guo, S.T.; Tang, Z.Y.; Du, Y.W.; Liu, T.; Ouyang, T.; Liu, Z.Q. Chlorine anion stabilized Cu2O/ZnO photocathode for selective CO2 reduction to CH4. Appl. Catal. B-Environ. 2023, 321, 122035. [Google Scholar] [CrossRef]
- Nie, J.; Yu, X.; Liu, Z.; Zhang, J.; Ma, Y.; Chen, Y.; Ji, Q.; Zhao, N.; Chang, Z. Energy band reconstruction mechanism of Cl-doped Cu2O and photocatalytic degradation pathway for levofloxacin. J. Clean. Prod. 2022, 363, 132593. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Guo, Y.; Jia, J.; Wang, S.; Duan, X.; Cui, F.; Gao, S.; Tian, J. Iron-doped cuprous oxides toward accelerated nonradical oxidation: Doping induced controlled facet transformation and optimized electronic structure. Chem. Eng. J. 2021, 407, 127172. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Kumar, N.; Limbu, S.; Tripathi, S.K.; Chauhan, R.N. Effect of Na ion on Cu2O:Co nanocrystals and subsequent attributes in structural and optical characteristics. Mater. Lett. 2023, 335, 133814. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdel-wahab, M.S.; Elfayoumi, M.A.K.; Tawfik, W.Z. Highly efficient sputtered Ni-doped Cu2O photoelectrodes for solar hydrogen generation from water-splittin. Int. J. Hydrogen Energy 2023, 48, 1863–1876. [Google Scholar] [CrossRef]
- Gu, Y.; Bao, A.; Zhang, X.; Yan, J.; Du, Q.; Zhang, M.; Qi, X. Facile fabrication of sulfur-doped Cu2O and g-C3N4 with Z-scheme structure for enhanced photocatalytic water splitting performance. Mater. Chem. Phys. 2021, 266, 124542. [Google Scholar] [CrossRef]
- Ding, Y.; Li, D.; Zuo, S.; Guan, Z.; Ding, S. Boron-doping accelerated Cu(II)/Cu(I) cycle for enhancing peroxymonosulfate activation. Sep. Purif. Technol. 2022, 282, 120086. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, X.; Wei, Y.; Wang, J.; Zhao, N.; Yang, Q.; Yu, Z.; Niu, J. Preparation of novel Cu/Cu2O composite thin films by pulse deposition method and their enhanced photocatalytic performance for methylene blue. J. Electrochem. Soc. 2022, 169, 072505. [Google Scholar] [CrossRef]
- Verma, A.; Hong, Y.A.; Hu, A.; Fu, Y.P. Insights into Cu2O morphology, facet etching, and its hybridized carbonaceous construction for photocatalytic antibiotic pollutant removal. Appl. Surf. Sci. 2023, 611, 155712. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Deng, S.; Hou, M.; Zhang, X.; Jiang, Z.; Lai, N.C. Morphology-controlled synthesis of Cu2O encapsulated phase change materials: Photothermal conversion and storage performance in visible light regime. Chem. Eng. J. 2023, 454, 140089. [Google Scholar] [CrossRef]
- Motamedi, M.; Ramezanzadeh, B.; Mahdavian, M. Corrosion inhibition properties of a green hybrid pigment based on Pr-Urtica Dioica plant extract. J. Ind. Eng. Chem. 2018, 66, 116–125. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vessaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B-Environ. 2020, 268, 118310. [Google Scholar] [CrossRef]
- Kayani, Z.; Chaudhry, W.; Sagheer, R.; Riaz, S.; Naseem, S. Effect of Ce doping on crystallite size, band gap, dielectric and antibacterial properties of photocatalyst copper oxide Nano-structured thin films. Mater. Sci. Eng. B 2022, 283, 115799. [Google Scholar] [CrossRef]
- Jarosiński, Ł.; Pawlak, J.; Al-Ani, S.K.J. Inverse logarithmic derivative method for determining the energy gap and the type of electron transitions as an alternative to the Tauc method. Opt. Mater. 2019, 88, 667–673. [Google Scholar] [CrossRef]
- Hussain, S.; Cao, C.; Nabi, G.; Khan, W.S.; Usman, Z.; Mahmood, T. Effect of electrodeposition and annealing of ZnO on optical and photovoltaic properties of the p-Cu2O/n-ZnO solar cells. Electrochim. Acta 2011, 56, 8342–8346. [Google Scholar] [CrossRef]
- Tang, C.; Ning, X.; Li, J.; Guo, H.L.; Yang, Y. Modulating conductivity type of cuprous oxide (Cu2O) films on copper foil in aqueous solution by comproportionation. J. Mater. Sci. Technol. 2019, 35, 1570–1577. [Google Scholar] [CrossRef]
- Zhu, J.; He, J.; Hu, L. A novel Cu2O@HNbWO6 heterojunction photocatalyst with enhanced photocatalytic performance. Mater. Lett. 2019, 254, 297–300. [Google Scholar] [CrossRef]
- Wu, L.; Tsui, L.K.; Swami, N.; Zangari, G. Photoelectrochemical stability of electrodeposited Cu2O films. J. Phys. Chem. C 2010, 114, 11551–11556. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Cembrero, J.; Orozco-Messana, J.; Manjón, F.J. Performance of graphene oxide-modified electrodeposited ZnO/Cu2O heterojunction solar cells. Bol. Soc. Esp. Ceram. V. 2019, 58, 263–273. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhang, J.N.; Ye, J.H.; Ma, X.N.; Ke, J. Construction of Cu2O/In2O3 hybrids with p-n heterojunctions for enhanced photocatalytic performance. J. Nanosci. Nanotechno. 2019, 19, 7689–7695. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Gu, B.; Tang, Q.; Cao, Q.; Fang, W. Sunlight-driven photocatalytic oxidation of 5-hydroxymethylfurfural over a cuprous oxide-anatase heterostructure in aqueous phase. Appl. Catal. B-Environ. 2023, 320, 122006. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Li, J.; Zhang, J.; Kou, S.; Zhao, J.; Yao, B. Nucleation mechanism and optoelectronic properties of Cu2O onto ITO electrode in the electrochemical deposition process. J. Electrochem. Soc. 2017, 164, D999–D1005. [Google Scholar] [CrossRef]
- Lin, L.; Lin, P.; Song, J.; Zhang, Z.; Wang, X.; Su, W. Boosting the photocatalytic activity and stability of Cu2O for CO2 conversion by LaTiO2N. J. Colloid Interface Sci. 2023, 630, 352–362. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.Q.; Bi, W.J.; Zhao, X.F.; Liu, G.D. Z-scheme Fe2O3-doped Cu2O as an efficient photo-Fenton-like catalyst for degradation of phenol. Mater. Lett. 2019, 234, 13–16. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Dong, Y.; Cui, H.; Sun, H.; Yin, S. Fabrication of Cu2O/MTiO3 (M=Ca, Sr, and Ba) p-n heterojunction for highly enhanced photocatalytic hydrogen generation. J. Alloys Compd. 2023, 930, 167333. [Google Scholar] [CrossRef]
- Norouzi, A.; Nezamzadeh-Ejhieh, A. Investigation of the simultaneous interactions of experimental variables and mechanism pathway in the photodegradation of methylene blue by binary ZnO/Cu2O photocatalyst. Mater. Res. Bull. 2023, 164, 112237. [Google Scholar] [CrossRef]
- Alizadeh, M.; Tong, G.B.; Qadir, K.W.; Mehmood, M.S.; Rasuli, R. Cu2O/InGaN heterojunction thin films with enhanced photoelectrochemical activity for solar water splitting. Renew. Energ. 2020, 156, 602–609. [Google Scholar] [CrossRef]
- Ansari, F.; Sheibani, S.; Fernandez-García, M. Surface modification of Cu2O-CuO photocatalyst on Cu wire through decorating with TiO2 nanoparticles for enhanced visible light photocatalytic activity. J. Alloys Compd. 2022, 919, 165864. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Directly electrospinning synthesized Z-scheme heterojunction TiO2@Ag@Cu2O nanofibers with enhanced photocatalytic degradation activity under solar light irradiation. J. Environ. Chem. Eng. 2021, 9, 106133. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Ji, Q.; Wang, K.; Zhang, J.; Niu, J.; Yu, X. Preparation and Photoelectric Properties of Pr-Doped p-Cu2O Thin Films Photocatalyst Based on Energy Band Structure Regulation. Molecules 2023, 28, 7560. https://doi.org/10.3390/molecules28227560
Wei Y, Ji Q, Wang K, Zhang J, Niu J, Yu X. Preparation and Photoelectric Properties of Pr-Doped p-Cu2O Thin Films Photocatalyst Based on Energy Band Structure Regulation. Molecules. 2023; 28(22):7560. https://doi.org/10.3390/molecules28227560
Chicago/Turabian StyleWei, Yuchen, Qinggong Ji, Kai Wang, Jian Zhang, Jinfen Niu, and Xiaojiao Yu. 2023. "Preparation and Photoelectric Properties of Pr-Doped p-Cu2O Thin Films Photocatalyst Based on Energy Band Structure Regulation" Molecules 28, no. 22: 7560. https://doi.org/10.3390/molecules28227560
APA StyleWei, Y., Ji, Q., Wang, K., Zhang, J., Niu, J., & Yu, X. (2023). Preparation and Photoelectric Properties of Pr-Doped p-Cu2O Thin Films Photocatalyst Based on Energy Band Structure Regulation. Molecules, 28(22), 7560. https://doi.org/10.3390/molecules28227560





