In Vitro Anti-Tumor and Hypoglycemic Effects of Total Flavonoids from Willow Buds
Abstract
:1. Introduction
2. Results and Discussion
2.1. PTFW Content
2.2. Chemical Component Analysis of PTFW Extract
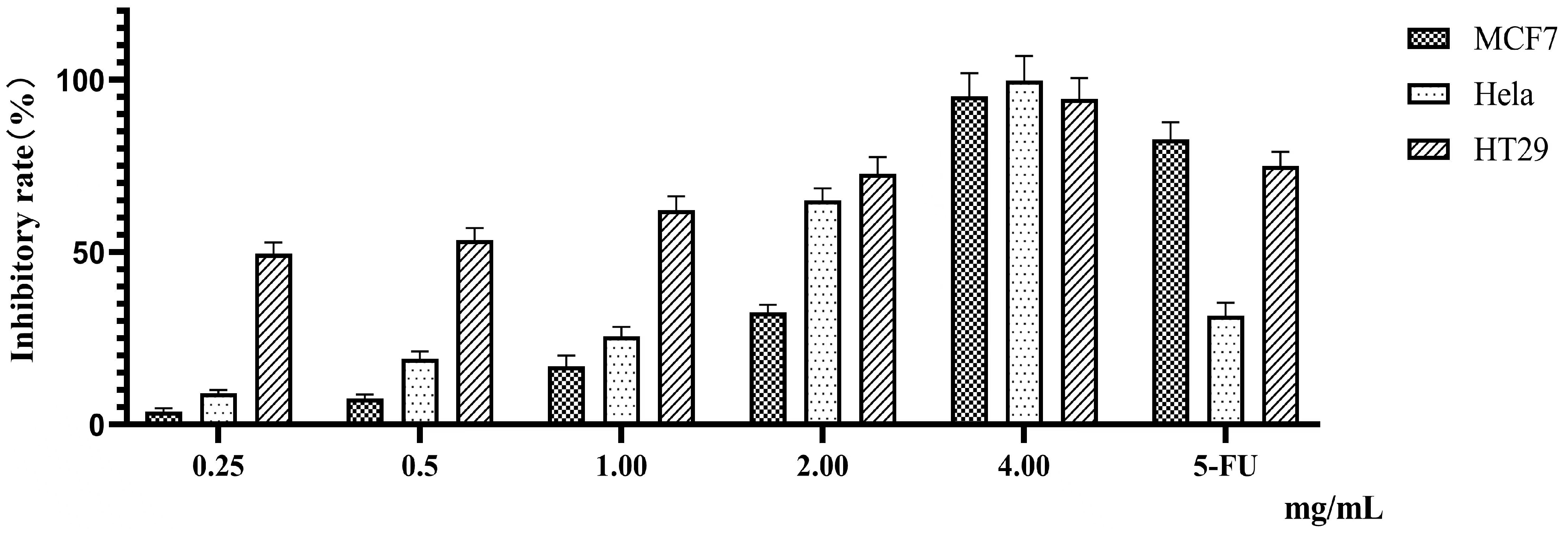
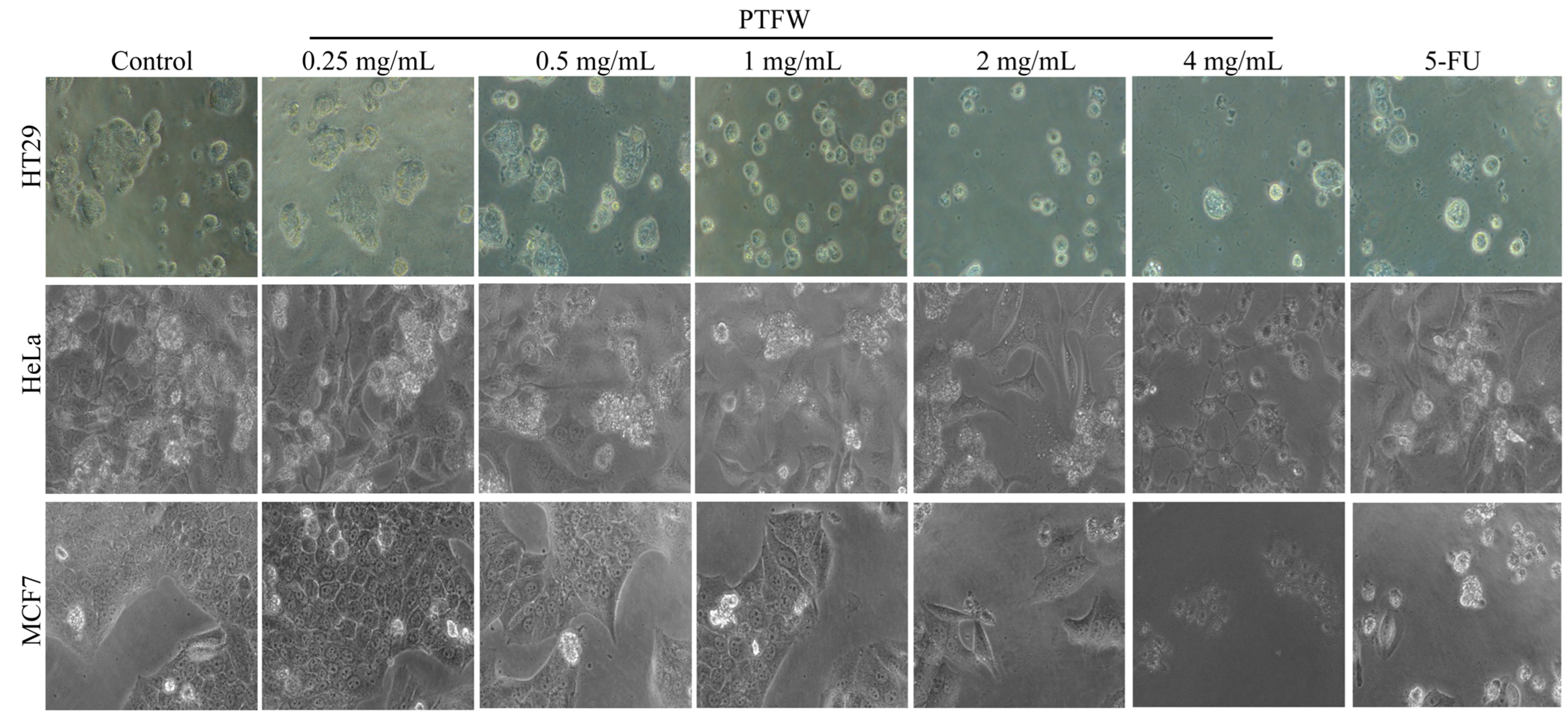
2.3. Inhibitory Effect of PTFW on Three Types of Cancer Cells
2.4. In Vitro Hypoglycemic Test
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of PTFW
3.3. Determination of PTFW Content
3.4. Component Analysis and Detection Conditions
3.5. Cell Culture
3.6. TFW Inhibited Cancer Cell Proliferation
3.7. In Vitro Hypoglycemic Test
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.X.; Affendi, M.M.; Chong, P.P.; Lee, S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer 2022, 74, 3058–3076. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; Xue, Y.M.; Nie, D. The prevalence and treatment of diabetes in China from 2013 to 2018. JAMA 2022, 327, 706. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J. Biomed. Res. 2019, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Song, Y.; Wang, H.; Fu, Y.; Zhang, Y.; Pavlovna, K.I. Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract. Molecules 2022, 27, 5695. [Google Scholar] [CrossRef]
- Zheng, Q.; Tan, W.; Feng, X.; Feng, K.; Zhong, W.; Liao, C.; Liu, Y.; Li, S.; Hu, W. Protective Effect of Flavonoids from Mulberry Leaf on AAPH-Induced Oxidative Damage in Sheep Erythrocytes. Molecules 2022, 27, 7625. [Google Scholar] [CrossRef]
- Abu Bakar, F.I.; Abu Bakar, M.F.; Abdullah, N.; Endrini, S.; Fatmawati, S. Optimization of Extraction Conditions of Phytochemical Compounds and Anti-Gout Activity of Euphorbia hirta L. (Ara Tanah) Using Response Surface Methodology and Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis. Evid. Based Complement. Altern. Med. 2020, 2020, 4501261. [Google Scholar] [CrossRef]
- Souilah, N.; Ullah, Z.; Bendif, H.; Medjroubi, K.; Hazmoune, T.; Hamel, T.; Öztürk, M.; Nieto, G.; Akkal, S. Phenolic Compounds from An Algerian Endemic Species of Hypochaeris laevigata var. hipponensis and Investigation of Antioxidant Activities. Plants 2020, 9, 514. [Google Scholar] [CrossRef]
- Du, W.J.; Ji, J.; Wang, L.; Lan, X.Y.; Li, J.; Lei, J.Q.; He, X.; Zhang, C.F.; Huang, W.Z.; Wang, Z.Z.; et al. Relationship between the UPLC-Q-TOF-MS fingerprinted constituents from Daphne genkwa and their anti-inflammatory, anti-oxidant activities. Biomed. Chromatogr. 2017, 31, 4012. [Google Scholar] [CrossRef]
- Su, Q.; Peng, M.; Zhang, Y.; Xu, W.; Darko, K.O.; Tao, T.; Huang, Y.; Tao, X.; Yang, X. Quercetin induces bladder cancer cells apoptosis by activation of AMPK signaling pathway. Am. J. Cancer Res. 2016, 6, 498–508. [Google Scholar]
- Lee, J.; Kim, J.H. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F.; Nabavi, S.M. Molecular mechanismsunderlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef]
- Hu, S.; Huang, L.; Meng, L.; Sun, H.; Zhang, W.; Xu, Y. Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt andmitogenactivated protein kinase kinase signaling pathways. Mol. Med. Rep. 2015, 12, 6745–6751. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Z.; Long, X.; Pan, Y.; Wang, R.; Chen, X.; Zhao, X. Inhibitory Effect of Lotus Leaf-Enriched Flavonoid Extract on the Growth of HT-29 Colon Cancer Cells through the Expression of PI3K-Related Molecules. Biomed. Res. Int. 2022, 9, 6770135. [Google Scholar] [CrossRef] [PubMed]
- Almalki, D.A.; Naguib, D.M. Anticancer Activity of Aqueous Fenugreek Seed Extract Against Pancreatic Cancer, Histological Evidence. J. Gastrointest. Cancer 2022, 53, 683–686. [Google Scholar] [CrossRef]
- Fallah, M.; Davoodvandi, A.; Nikmanzar, S.; Aghili, S.; Mirazimi, S.M.A.; Aschner, M.; Rashidian, A.; Hamblin, M.R.; Chamanara, M.; Naghsh, N.; et al. Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed. Pharmacother. 2021, 142, 112024. [Google Scholar] [CrossRef]
- Jagaeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics of Allmania nodiflora (L.) R.Br. ex Wight polyphenols during in vitro simulated digestion. Food Chem. 2022, 372, 131294. [Google Scholar] [CrossRef]
- Li, J.S.; Ji, T.; Su, S.L.; Zhu, Y.; Chen, X.L.; Shang, E.X.; Guo, S.; Qian, D.W.; Duan, J.A. Mulberry leaves ameliorate diabetes via regulating metabolic profiling and AGEs/RAGE a nd p38 MAPK/NF-κB pathway. J. Ethnopharmacol. 2022, 283, 114713. [Google Scholar] [CrossRef]
- Yang, M.L.; Lu, C.; Fan, Z.F.; Zhao, T.R.; Cheng, G.G.; Wang, Y.D.; Cao, J.X.; Liu, Y.P. Hypoglycemic and hypolipidemic effects of Epigynum auritum in high fat diet and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2022, 288, 114986. [Google Scholar] [CrossRef]
- Abdollahi, A.; Adelibahram, F.; Ghassab-Abdollahi, N.; Araj-Khodaei, M.; Parsian, Z.; Mirghafourvand, M. The effect of Salvia officinalis on blood glycemic indexes and blood lipid profile in diabetic patients: A systematic review and meta-analysis. J. Complement. Integr. Med. 2022, 20, 521–529. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, C.; Gao, X.; Wang, H.; Guo, Y.; Liu, M. Extraction Process, Component Analysis, and In Vitro Antioxidant, Antibacterial, and Anti-Inflammatory Activities of Total Flavonoid Extracts from Abutilon theophrasti Medic. Leaves. Mediat. Inflamm. 2018, 2018, 3508506. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, D.; Zhang, J.; Hu, P.; Shen, W.; Fan, B.; Ma, Q.; Wang, X. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complement. Altern. Med. 2016, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Nanok, K.; Sansenya, S. Combination effects of rice extract and five aromatic compounds against α-glucosidase, α-amylase and tyrosinase. J. Biosci. Bioeng. 2021, 132, 9–17. [Google Scholar] [CrossRef] [PubMed]
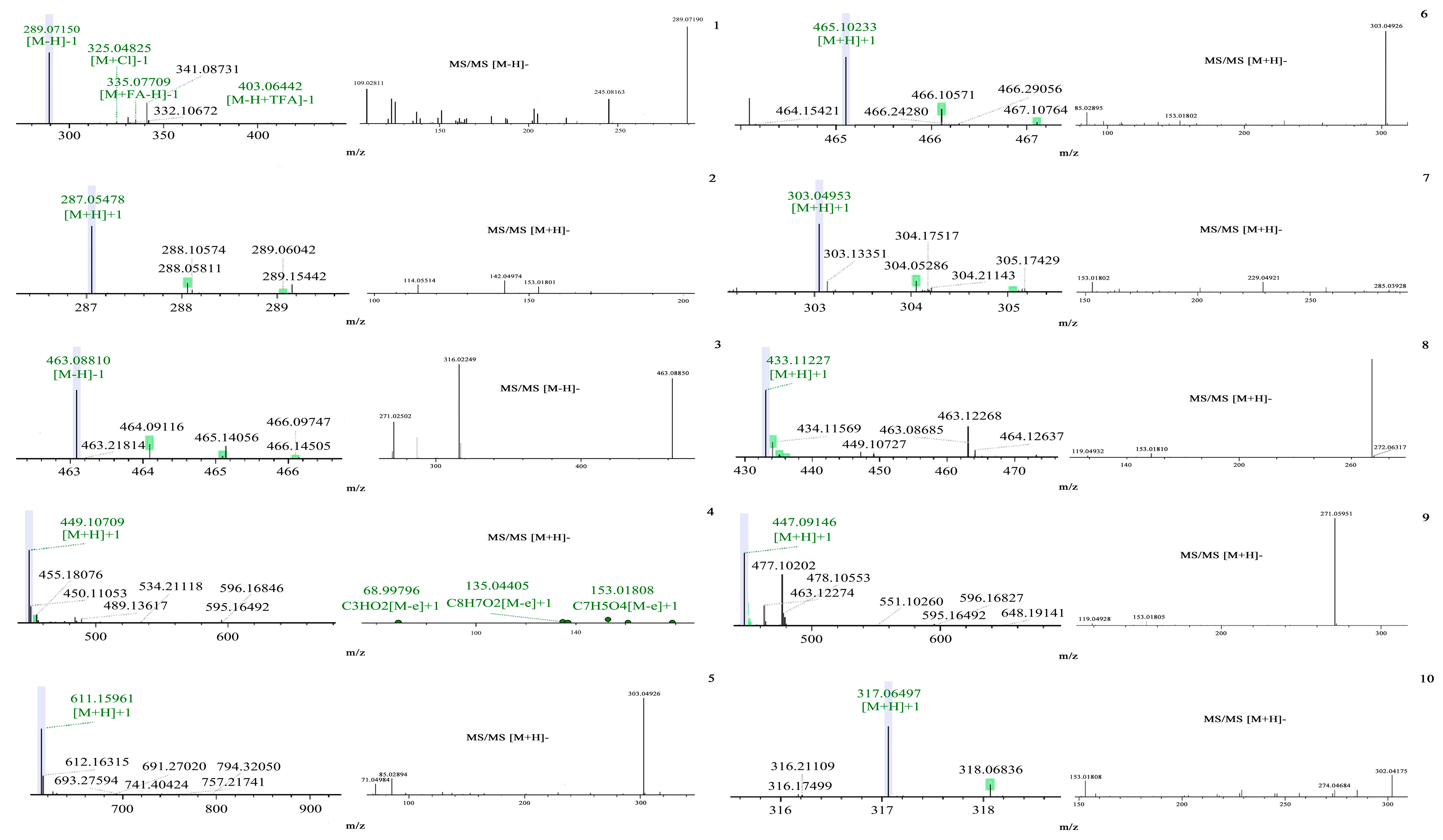

| No. | Rt (min) | [M − H]− | MS/MS [M − H]− | [M + H]− | MS/MS [M + H]− | Calculated Mass | Formula | Proposed Molecule | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.00 | 289.071 | 109.028, 245.081, 289.071 | — | — | 290.1 | C15H14O6 | Catechin | [5] |
| 2 | 12.38 | — | — | 287.055 | 114.055, 142.049, 153.018 | 286.1 | C15H10O6 | Kaempferol | [5] |
| 3 | 12.59 | 463.088 | 271.025, 316.022, 463.088 | — | — | 464.1 | C21H20O12 | Myricitrin | [6] |
| 4 | 12.63 | 449.107 | 68.997, 135.044, 153.018 | 448.1 | C21H20O11 | Cynaroside | [6] | ||
| 5 | 12.81 | — | — | 611.159 | 71.049, 85.028, 303.049 | 610.2 | C27H30O16 | Rutin | [5] |
| 6 | 12.86 | — | — | 465.102 | 85.028, 153.018, 303.049 | 464.1 | C21H20O12 | Isoquercitrin | [7] |
| 7 | 12.87 | — | — | 303.049 | 153.018, 229.049, 285.123 | 302.0 | C15H10O7 | Quercetin | [5] |
| 8 | 13.34 | — | — | 433.112 | 119.049, 153.018, 272.063 | 432.1 | C21H20O10 | Apigetrin | [8] |
| 9 | 13.55 | — | — | 447.091 | 119.049, 153.018, 271.059 | 446.1 | C21H18O11 | Apigenin 7-O-glucuronide | [9] |
| 10 | 13.63 | — | — | 317.064 | 153.018, 274.048, 302.041 | 316.1 | C16H12O7 | Isorhamnetin | [5] |
| Sample | IC50 mg/mL | |
|---|---|---|
| α-Amylase | α-Glucosidase | |
| Acarbose | 2.124 | 0.01542 |
| Rutin | 6.730 | 0.1895 |
| PTFW | 2.943 | 1.874 |
| Time (min) | Water Phase Ratio (%) | Organic Phase Ratio (%) |
|---|---|---|
| 1 | 98 | 2 |
| 5 | 80 | 20 |
| 10 | 50 | 50 |
| 15 | 20 | 80 |
| 20 | 5 | 95 |
| 27 | 5 | 95 |
| 28 | 98 | 2 |
| 30 | 98 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Fan, L.; Zhang, D.; Zhang, Z.; Wang, W. In Vitro Anti-Tumor and Hypoglycemic Effects of Total Flavonoids from Willow Buds. Molecules 2023, 28, 7557. https://doi.org/10.3390/molecules28227557
Zhang P, Fan L, Zhang D, Zhang Z, Wang W. In Vitro Anti-Tumor and Hypoglycemic Effects of Total Flavonoids from Willow Buds. Molecules. 2023; 28(22):7557. https://doi.org/10.3390/molecules28227557
Chicago/Turabian StyleZhang, Peng, Lulu Fan, Dongyan Zhang, Zehui Zhang, and Weili Wang. 2023. "In Vitro Anti-Tumor and Hypoglycemic Effects of Total Flavonoids from Willow Buds" Molecules 28, no. 22: 7557. https://doi.org/10.3390/molecules28227557
APA StyleZhang, P., Fan, L., Zhang, D., Zhang, Z., & Wang, W. (2023). In Vitro Anti-Tumor and Hypoglycemic Effects of Total Flavonoids from Willow Buds. Molecules, 28(22), 7557. https://doi.org/10.3390/molecules28227557






