Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation
Abstract
:1. Introduction
2. Results and Discussion
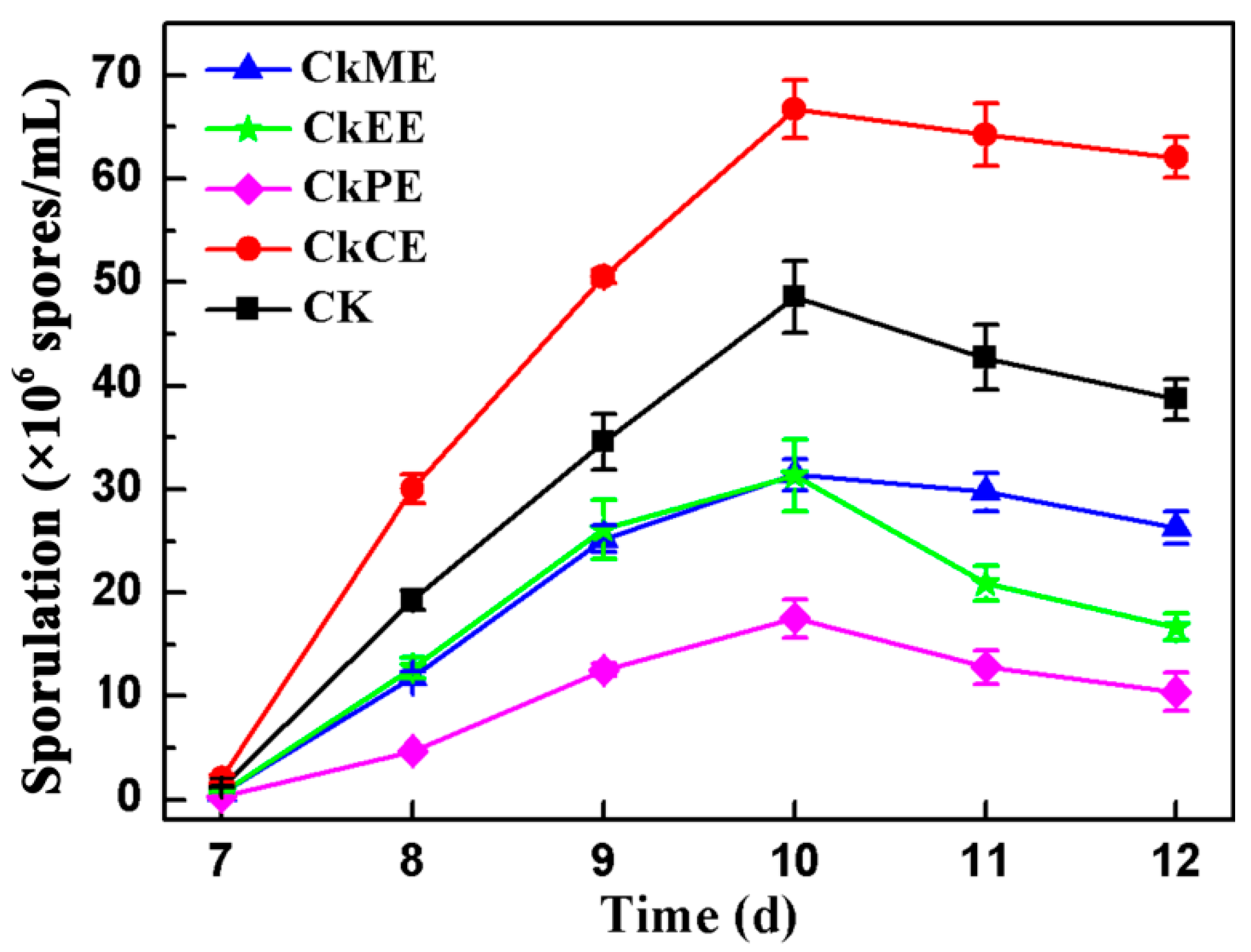
2.1. Effect of C. kanehirae Extracts on the Sporulation of AcSmF
2.2. Composition of CkCE
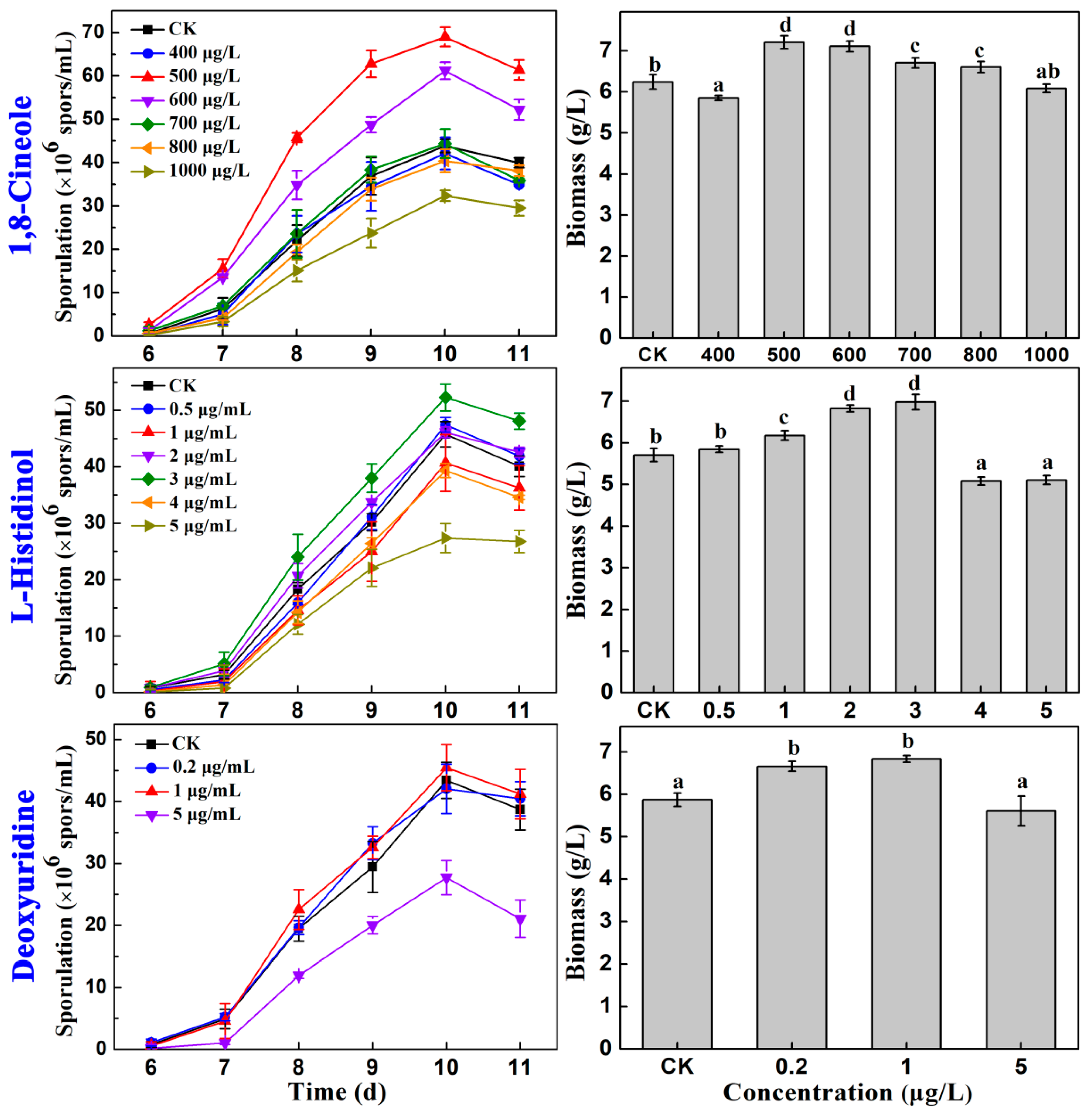
2.3. Effects of the Main Compounds in CkCE on the Sporulation and Biomass of AcSmF
2.4. RNA-Seq and Statistical Analysis
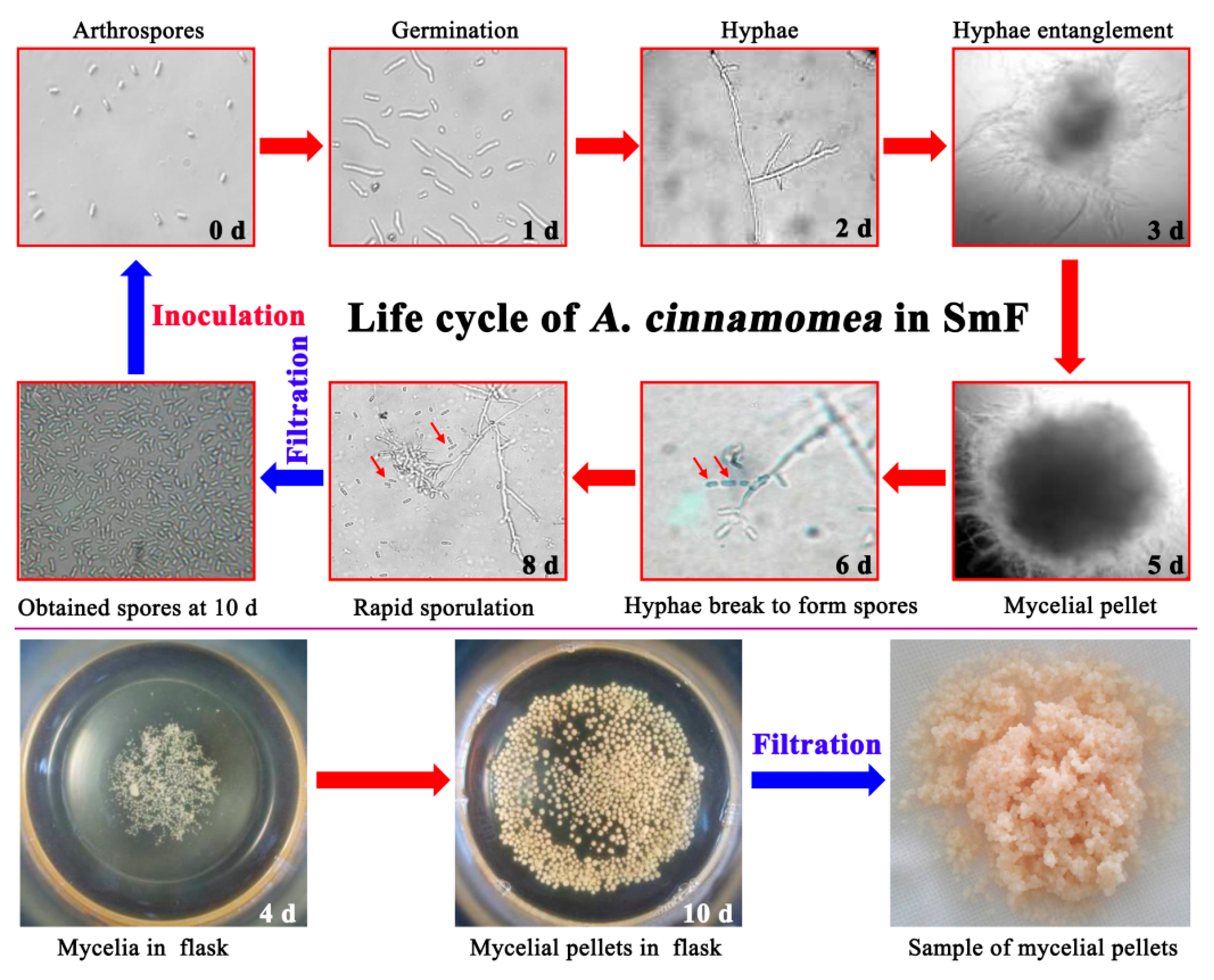
2.4.1. Preparation of Samples for RNA-Seq
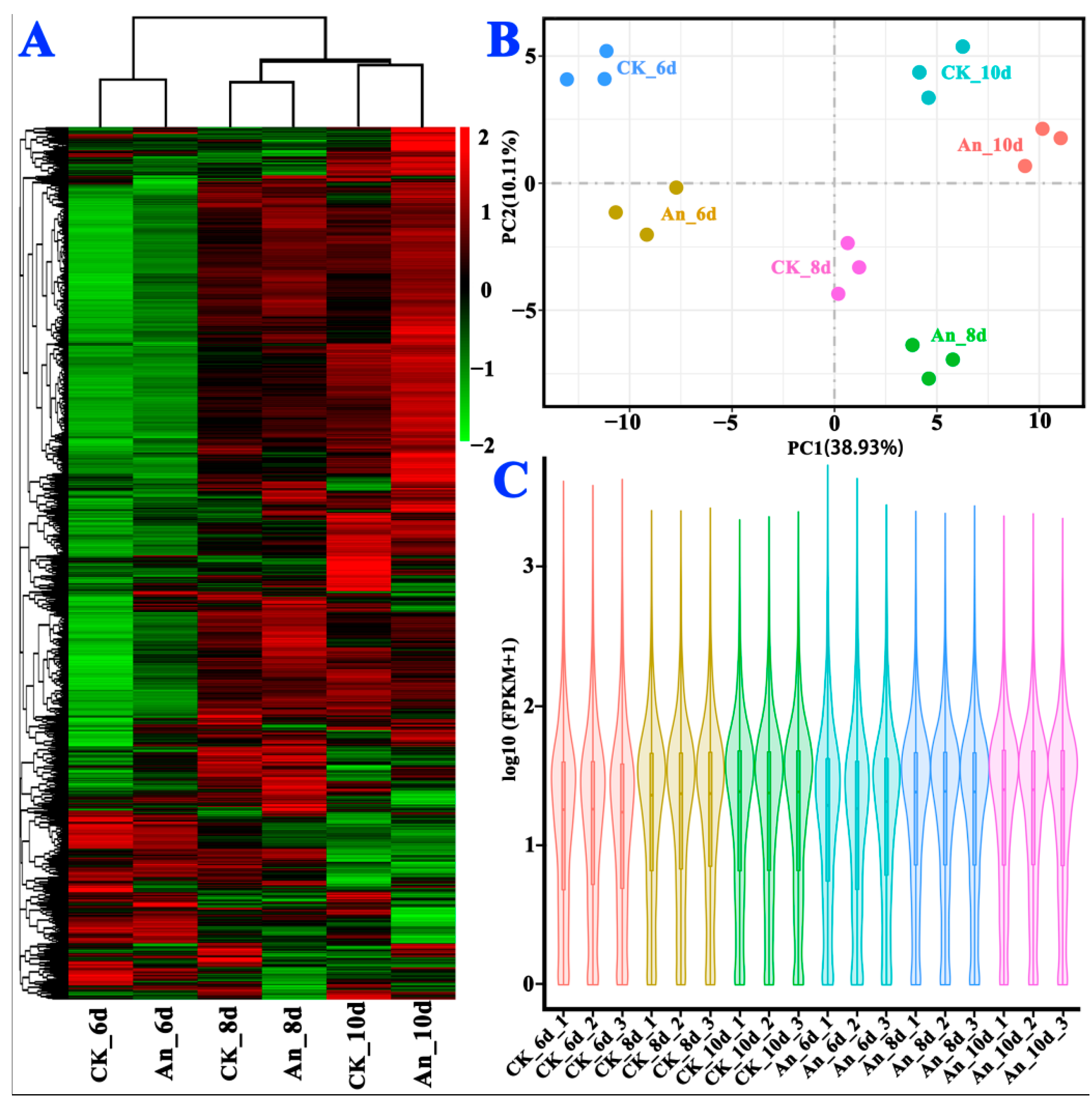
2.4.2. Statistical Analysis of Sample Repeatability and DEGs
2.4.3. Enrichment Analysis of DEGs
2.5. Bioinformatic Analysis
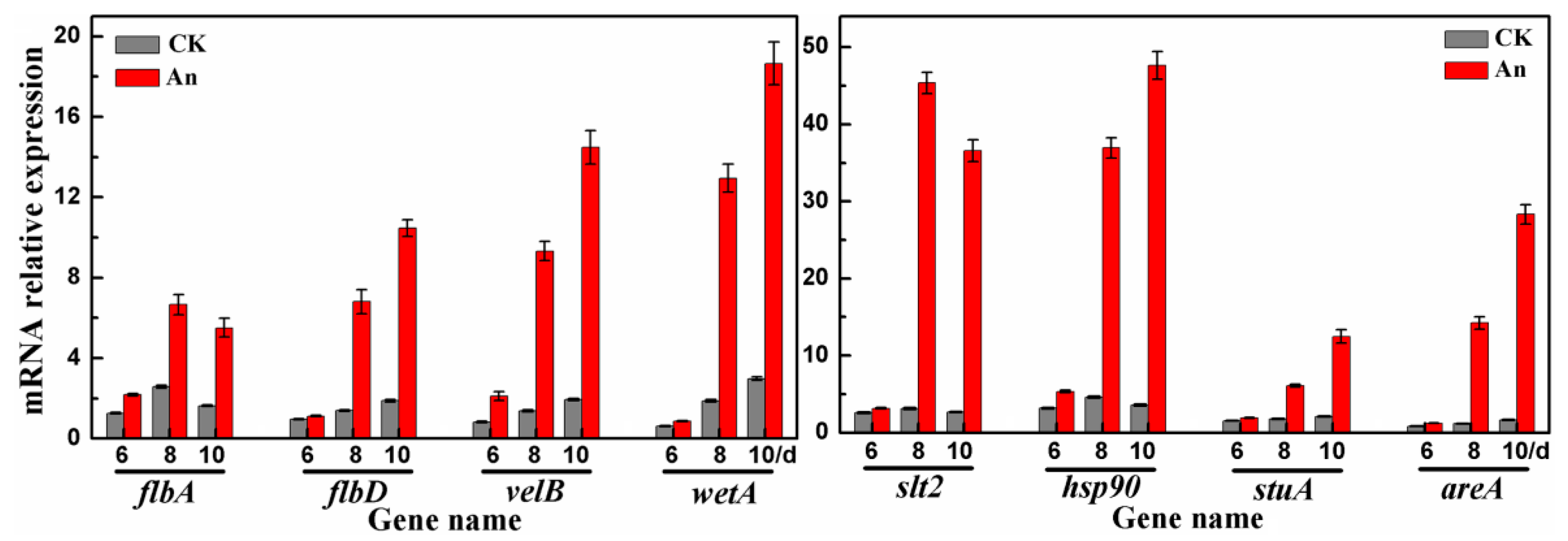
2.6. RT-qPCR Analysis
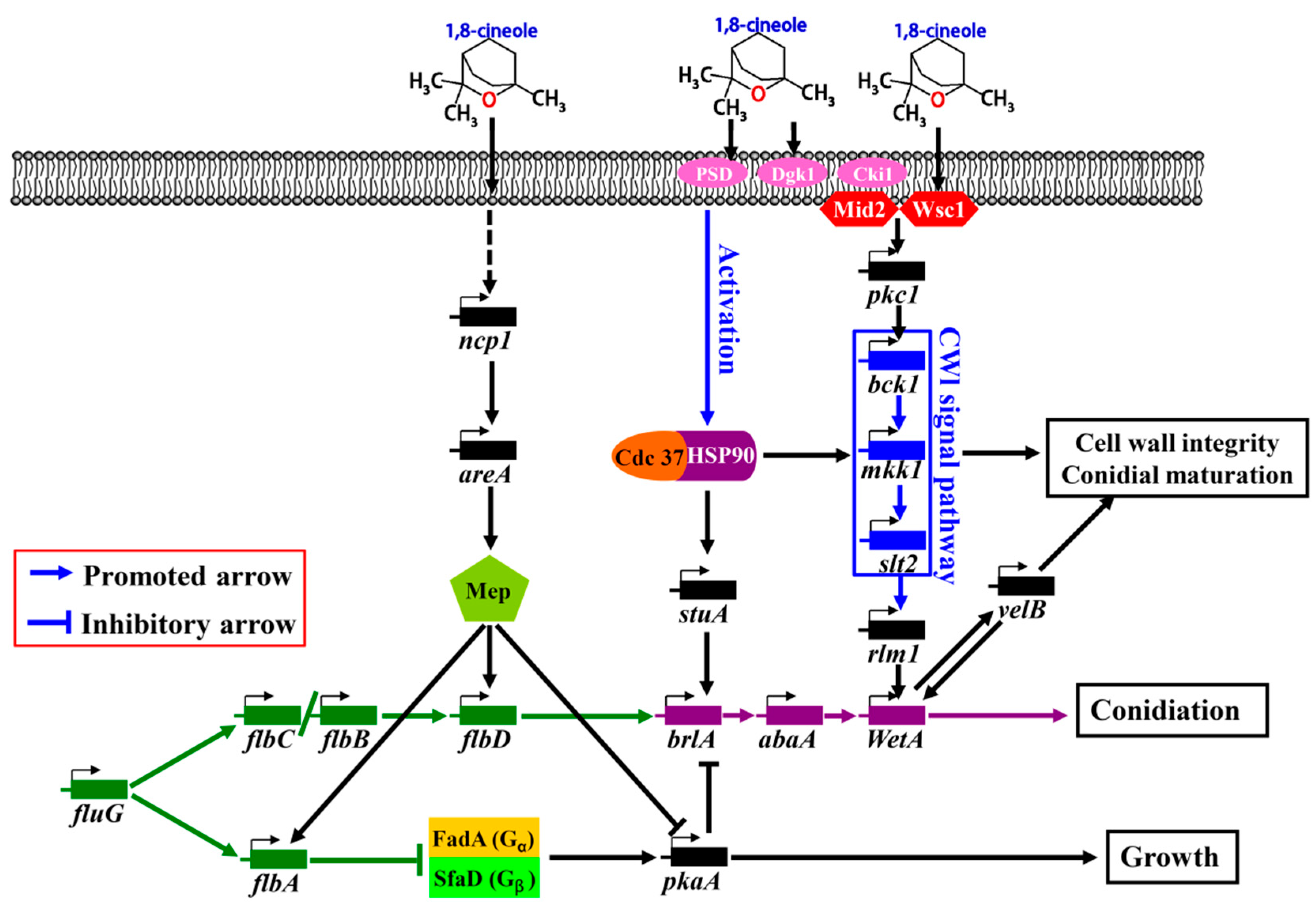
2.7. Putative Signaling Pathway of the Cineole-Promoted Sporulation of A. cinnamomea
3. Materials and Methods
3.1. Materials
3.2. Submerged Fermentation of A. cinnamomea
3.3. Effects of Different Additives on the Sporulation and Biomass of AcSmF
3.3.1. Preparation of C. kanehirae Extracts
3.3.2. Effects of Additives on the Sporulation and Biomass of AcSmF
3.4. LC-MS/MS Analysis for CkCE
3.5. Sample Preparation for RNA-Seq
3.6. RNA-Seq and Bioinformatic Analysis
3.7. RT-qPCR Analysis
3.8. Statistical Analysis of Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.L.; Li, W.C.; Chuang, Y.C.; Liu, H.C.; Huang, C.H.; Lo, K.Y.; Chen, C.Y.; Chang, F.M.; Chang, G.A.; Lin, Y.L.; et al. Sexual crossing, chromosome-level genome sequences, and comparative genomic analyses for the medicinal mushroom Taiwanofungus Camphoratus (Syn. Antrodia Cinnamomea, Antrodia Camphorata). Microbiol. Spectr. 2022, 10, e02032-21. [Google Scholar] [CrossRef]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Therapeut. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Yang, H.L.; Chang, Y.H.; Pandey, S.; Bhat, A.A.; Vadivalagan, C.; Lin, K.Y.; Hseu, Y.C. Antrodia camphorata and coenzyme Q(0), a novel quinone derivative of Antrodia camphorata, impede HIF-1 alpha and epithelial-mesenchymal transition/metastasis in human glioblastoma cells. Environ. Toxicol. 2023, 38, 1548–1564. [Google Scholar] [CrossRef]
- Ke, L.Q.; Zhi, M.Y.; Tang, P.; He, D. Preparation of total triterpenoids from Antrodia cinnamomea fermentation mycelium and their in vitro inhibitory effects on hepatocellular carcinoma. Food Sci. Tech. 2023, 43, e005923. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Zhu, Q.; Gong, J.S.; Geng, Y.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Comparative transcriptomic and proteomic analyses reveal a FluG-mediated signaling pathway relating to asexual sporulation of Antrodia camphorata. Proteomics 2017, 17, 1700256. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Kirk, P.M.; Redhead, S.A.; Stalpers, J.A.; Dai, Y.C.; Norvell, L.L.; Yang, Z.L.; Ryvarden, L.; Su, C.H.; Li, Y.; et al. Resolution of the nomenclature for niu-chang-chih (Taiwanofungus camphoratus), an important medicinal polypore. Taxon 2012, 61, 1305–1310. [Google Scholar] [CrossRef]
- Yeh, R.Y.; Shiu, Y.L.; Shei, S.C.; Cheng, S.C.; Huang, S.Y.; Lin, J.C.; Liu, C.H. Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immun. 2009, 27, 26–32. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, Y.K.; Chen, K.H.; Leu, Y.L.; Way, T.D.; Wang, L.W.; Chen, Y.J. Ethanol extracts of Cinnamomum kanehirai Hayata leaves induce apoptosis in human hepatoma cell through caspase-3 cascade. OncoTargets Ther. 2015, 8, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Yeh, H.C.; Chen, C.Y. Secondary metabolites of the leaves of Cinnamomum kanehirai. Chem. Nat. Compd. 2016, 52, 1143–1144. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, C.L.; Li, W.J.; Li, H.T.; Chen, C.Y. Secondary metabolites from the stems of Cinnamomum kanehirai. Chem. Nat. Compd. 2018, 54, 762–763. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yuan, X.L.; Luo, Y.N.; Luo, M.N.; Zheng, Y. Effects of culture mechanism of Cinnamomum kanehirae and C. camphora on the expression of genes related to terpene biosynthesis in Antrodia cinnamomea. Mycobiology 2022, 50, 121–131. [Google Scholar] [CrossRef]
- Zeng, W.W.; Chen, T.C.; Liu, C.H.; Wang, S.Y.; Shaw, J.F.; Chen, Y.T. Identification and isolation of an intermediate metabolite with dual antioxidant and anti-proliferative activity present in the fungus Antrodia cinnamomea cultured on an alternative medium with Cinnamomum kanehirai leaf extract. Plants 2021, 10, 737. [Google Scholar] [CrossRef]
- Hsu, F.L.; Chou, C.J.; Chang, Y.C.; Chang, T.T.; Lu, M.K. Promotion of hyphal growth and underlying chemical changes in Antrodia camphorata by host factors from Cinnamomum camphora. Int. J. Food Microbiol. 2006, 106, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.M.; Geng, Y.; Li, H.X.; Sun, Q.; Shi, J.S.; Xu, Z.H. Alpha-terpineol promotes triterpenoid production of Antrodia cinnamomea in submerged culture. FEMS Microbiol. Lett. 2014, 358, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Meskher, H.; Achi, F. Electrochemical sensing systems for the analysis of catechol and hydroquinone in the aquatic environments: A critical review. Crit. Rev. Anal. Chem. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Gong, X.; Hamid, M.I.; Fu, Y.; Jiatao, X.; Cheng, J.; Li, G.; Jiang, D. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet. Biol. 2012, 49, 347–357. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.A.; Menexes, G.; Constantinidou, H.I.A. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur. J. Plant. Pathol. 2011, 130, 297–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, X.; Liu, L.; Xing, R.; Song, X.; Zou, Y.; Li, L.; Wan, H.; Jia, R.; et al. Study on the anti-biofilm mechanism of 1,8-cineole against Fusarium solani species complex. Front Pharmacol. 2022, 13, 1010593. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rubio, G.; Fernandez-Acero, T.; Martin, H.; Molina, M. Mitogen-activated protein kinase phosphatases (MKPs) in fungal signaling: Conservation, function, and regulation. Int. J. Mol. Sci. 2019, 20, 1709. [Google Scholar] [CrossRef]
- Zhao, T.; Wen, Z.; Xia, Y.; Jin, K. The transmembrane protein MaSho1 negatively regulates conidial yield by shifting the conidiation pattern in Metarhizium acridum. Appl. Microbiol. Biot. 2020, 104, 4005–4015. [Google Scholar] [CrossRef]
- Philip, B.; Levin, D.E. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell Biol. 2001, 21, 271–280. [Google Scholar] [CrossRef]
- Futagami, T.; Nakao, S.; Kido, Y.; Oka, T.; Kajiwara, Y.; Takashita, H.; Omori, T.; Furukawa, K.; Goto, M. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot. Cell 2011, 10, 1504–1515. [Google Scholar] [CrossRef]
- Futagami, T.; Goto, M. Putative cell wall integrity sensor proteins in Aspergillus nidulans. Communic. Integr. Biol. 2012, 5, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yang, J.; Jiang, K.; Bai, N.; Zhu, M.; Zhu, Y.; Zhang, K.Q. AoBck1 and AoMkk1 are necessary to maintain cell wall integrity, vegetative growth, conidiation, stress resistance, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front. Microbiol. 2021, 12, 649582. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Bai, N.; Yang, X.; Liu, Y.; Zhang, K.Q.; Yang, J. Fus3 regulates asexual development and trap morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora. iScience 2023, 26, 107404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. Three mitogen-activated protein kinases required for cell wall integrity contribute greatly to biocontrol potential of a fungal entomopathogen. PLoS ONE 2014, 9, e87948. [Google Scholar] [CrossRef]
- Penn, T.J.; Wood, M.E.; Soanes, D.M.; Csukai, M.; Corran, A.J.; Talbot, N.J. Protein kinase C is essential for viability of the rice blast fungus Magnaporthe oryzae. Mol. Microbiol. 2015, 98, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Deng, J.L.; Zhang, F.; Zhu, Z.; Yan, L.J.; Zhang, M.J.; Yuan, J.; Wang, S.H. CWI pathway participated in vegetative growth and pathogenicity through a downstream effector AflRlm1 in Aspergillus flavus. iScience 2021, 24, 103159. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.C.; Lee, Y.; Lim, J.Y.; Fu, M.; Kim, J.C.; Choi, G.J.; Son, H.; Lee, Y.W. Heat shock protein 90 is required for sexual and asexual development, virulence, and heat shock response in Fusarium graminearum. Sci. Rep. 2016, 6, 28154. [Google Scholar] [CrossRef] [PubMed]
- Hawle, P.; Horst, D.; Bebelman, J.P.; Yang, X.X.; Siderius, M.; van der Vies, S.M. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p). Eukaryot. Cell 2007, 6, 521–532. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Li, Y.; Bi, Y.; Li, R.; Yuan, J.; Xu, W.; Prusky, D. AaHog1 regulates infective structural differentiation mediated by physicochemical signals from pear fruit cuticular wax, stress response, and Alternaria alternata pathogenicity. J. Fungi. 2022, 8, 266. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, J.; Zhao, Y.; Wang, K. CtPMK1, a mitogen-activated-protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Colletotrichum truncatum on soybean. Ann. Appl. Biol. 2015, 167, 63–74. [Google Scholar] [CrossRef]
- Hou, R.; Jiang, C.; Zheng, Q.; Wang, C.; Xu, J.R. The AreA transcription factor mediates the regulation of deoxynivalenol (DON) synthesis by ammonium and cyclic adenosine monophosphate (cAMP) signalling in Fusarium graminearum. Mol. Plant Pathol. 2015, 16, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Hu, M.; Zhang, Q.; Xia, Y.; Jin, K. MaNCP1, a C2H2 zinc finger protein, governs the conidiation pattern shift through regulating the reductive pathway for nitric oxide synthesis in the filamentous fungus Metarhizium acridum. Microbiol. Spectr. 2022, 10, e0053822. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.A.; Xu, H.; Gray, J.V. The synthetic genetic network around PKC1 identifies novel modulators and components of protein kinase C signaling in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef]
- Rodriguez-Pena, J.M.; Garcia, R.; Nombela, C.; Arroyo, J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between MAPK routes. Yeast 2010, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Jung, U.S.; Piotrowski, J.; Levin, D.E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995, 9, 1559–1571. [Google Scholar] [CrossRef]
- Ketela, T.; Green, R.; Bussey, H. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 1999, 181, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Mensonides, F.I.; Brul, S.; Klis, F.M.; Hellingwerf, K.J.; Teixeira de Mattos, M.J. Activation of the protein kinase C1 pathway upon continuous heat stress in Saccharomyces cerevisiae is triggered by an intracellular increase in osmolarity due to trehalose accumulation. Appl. Environ. Microbiol. 2005, 71, 4531–4538. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. analysis 2020, 31, 204–214. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Metod. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| No. | Name | Formula | CAS NO. | Content |
|---|---|---|---|---|
| 1 | 1,8-Cineole | C10H18O | 470-82-6 | 20.49% |
| 2 | L-Histidinol | C6H11N3O | 1596-64-1 | 9.95% |
| 3 | Deoxyuridine | C9H12N2O5 | 54-42-2 | 9.45% |
| 4 | Catechol | C6H6O2 | 120-80-9 | 8.84% |
| 5 | (-)-beta-Pinene | C10H16 | 18172-67-3 | 4.97% |
| 6 | 2-Chloro-L-phenylalanine | C9H10C1NO2 | 54793-54-3 | 4.36% |
| 7 | Phenyl acetate | C8H8O2 | 122-79-2 | 3.36% |
| 8 | Hydroquinone | C6H6O2 | 123-31-9 | 2.34% |
| 9 | m-chlorophenylpiperazine | C10H13C1N2 | 6640-24-0 | 2.06% |
| 10 | Fomepizole | C4H6N2 | 7554-65-6 | 2.03% |
| 11 | D-Ribose | C5H10O5 | 50-69-1 | 1.46% |
| 12 | Thiabendazole | C10H7N3S | 148-79-8 | 1.43% |
| 13 | Genipin | C11H14O5 | 6902-77-8 | 1.17% |
| 14 | Baicalein | C15H10O5 | 491-67-8 | 0.99% |
| 15 | 1-Aminocyclopropanecarboxylic Acid | C4H7NO2 | 22059-21-8 | 0.89% |
| 16 | Trans-Cinnamate | C9H8O2 | 1754-627-7 | 0.82% |
| 17 | Dihydroxyfumaric acid hydrate | C4H4O6 | 19926-38-0 | 0.77% |
| 18 | Picolinic acid | C6H5NO2 | 98-98-6 | 0.70% |
| 19 | Sinapyl alcohol | C11H14O4 | 537-33-7 | 0.66% |
| 20 | Phytosphingosine | C18H39NO3 | 554-62-1 | 0.65% |
| Unigene ID | Genome ID | Gene Name | Accession Number | E Value | Score |
|---|---|---|---|---|---|
| Cluster-196.3786 | ACg006274 | velB | B0CXQ2.1 | 1 × 10−102 | 359 |
| Cluster-196.2321 | ACg005708 | flbA | P38093.1 | 3 × 10−5 | 47 |
| Cluster-196.4288 | ACg006986 | pkaA | ETI83732.1 | 4 × 10−28 | 122 |
| Cluster-196.2514 | ACg008440 | flbD | JAC66949.1 | 1 × 10−13 | 74 |
| Cluster-196.908 | ACg000535 | wetA | XP_016271747.1 | 6 × 10−5 | 42 |
| Cluster-196.1773 | ACg008470 | abaA | EAA66521.1 | 6 × 10−13 | 73 |
| Cluster-196.3966 | ACg004592 | stuA | KKF92291.1 | 6 × 10−11 | 65 |
| Cluster-196.3292 | ACg003227 | brlA | A0A0F0I5G4.1 | 3 × 10−9 | 60 |
| Cluster-196.3585 | ACg007355 | slt2 | XM_027756054.1 | 1 × 10−162 | 571 |
| Cluster-196.4221 | ACg003505 | areA | CP021224.1 | 2 × 10−120 | 446 |
| Cluster-196.3970 | ACg006851 | pmk1 | PCH35243.1 | 2 × 10−24 | 106 |
| Cluster-196.3144 | ACg008555 | hog1 | OCH90560.1 | 5 × 10−34 | 138 |
| Cluster-196.3943 | ACg007303 | wsc1 | NC_007198.1 | 2 × 10−9 | 59 |
| Cluster-196.2321 | ACg005707 | mid2 | NP_013436.1 | 3 × 10−4 | 37 |
| Cluster-196.3873 | ACg008261 | bck1 | NP_012440.1 | 2 × 10−4 | 35 |
| Cluster-196.4632 | ACg006849 | mkk1 | NW_007930837.1 | 8 × 10−52 | 203 |
| Cluster-196.1593 | ACg002614 | pkc1 | NP_009445.2 | 3 × 10−6 | 44 |
| Cluster-196.4376 | ACg002705 | rlm1 | XP_001387522.2 | 2 × 10−9 | 54 |
| Cluster-196.4865 | ACg003577 | dgk1 | KAI0930476.1 | 1 × 10−8 | 56 |
| Cluster-196.4571 | ACg008481 | cki1 | XP_024338459.1 | 1 × 10−35 | 145 |
| Cluster-196.4962 | ACg007312 | mep1 | NP_011636.3 | 6 × 10−16 | 76 |
| Cluster-196.4302 | ACg002703 | cdc37 | NP_595752.1 | 4 × 10−14 | 70 |
| Cluster-196.940 | ACg008458 | psd | KAI0635493.1 | 8 × 10−50 | 193 |
| Cluster-196.2224 | - | hsp90 | KZT67759.1 | 2 × 10−54 | 210 |
| Cluster-196.397 | ACg003414 | ncp1 | EJP65478.1 | 1 × 10−4 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Dai, J.; Wang, J.; Lu, C.; Luo, Z.; Zheng, X.; Lu, Z.; Yang, Z. Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation. Molecules 2023, 28, 7511. https://doi.org/10.3390/molecules28227511
Li H, Dai J, Wang J, Lu C, Luo Z, Zheng X, Lu Z, Yang Z. Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation. Molecules. 2023; 28(22):7511. https://doi.org/10.3390/molecules28227511
Chicago/Turabian StyleLi, Huaxiang, Jianing Dai, Juanjuan Wang, Chunlei Lu, Zhishan Luo, Xiangfeng Zheng, Zhenming Lu, and Zhenquan Yang. 2023. "Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation" Molecules 28, no. 22: 7511. https://doi.org/10.3390/molecules28227511
APA StyleLi, H., Dai, J., Wang, J., Lu, C., Luo, Z., Zheng, X., Lu, Z., & Yang, Z. (2023). Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation. Molecules, 28(22), 7511. https://doi.org/10.3390/molecules28227511










