Antimicrobial Potency and E. coli β-Carbonic Anhydrase Inhibition Efficacy of Phenazone-Based Molecules
Abstract
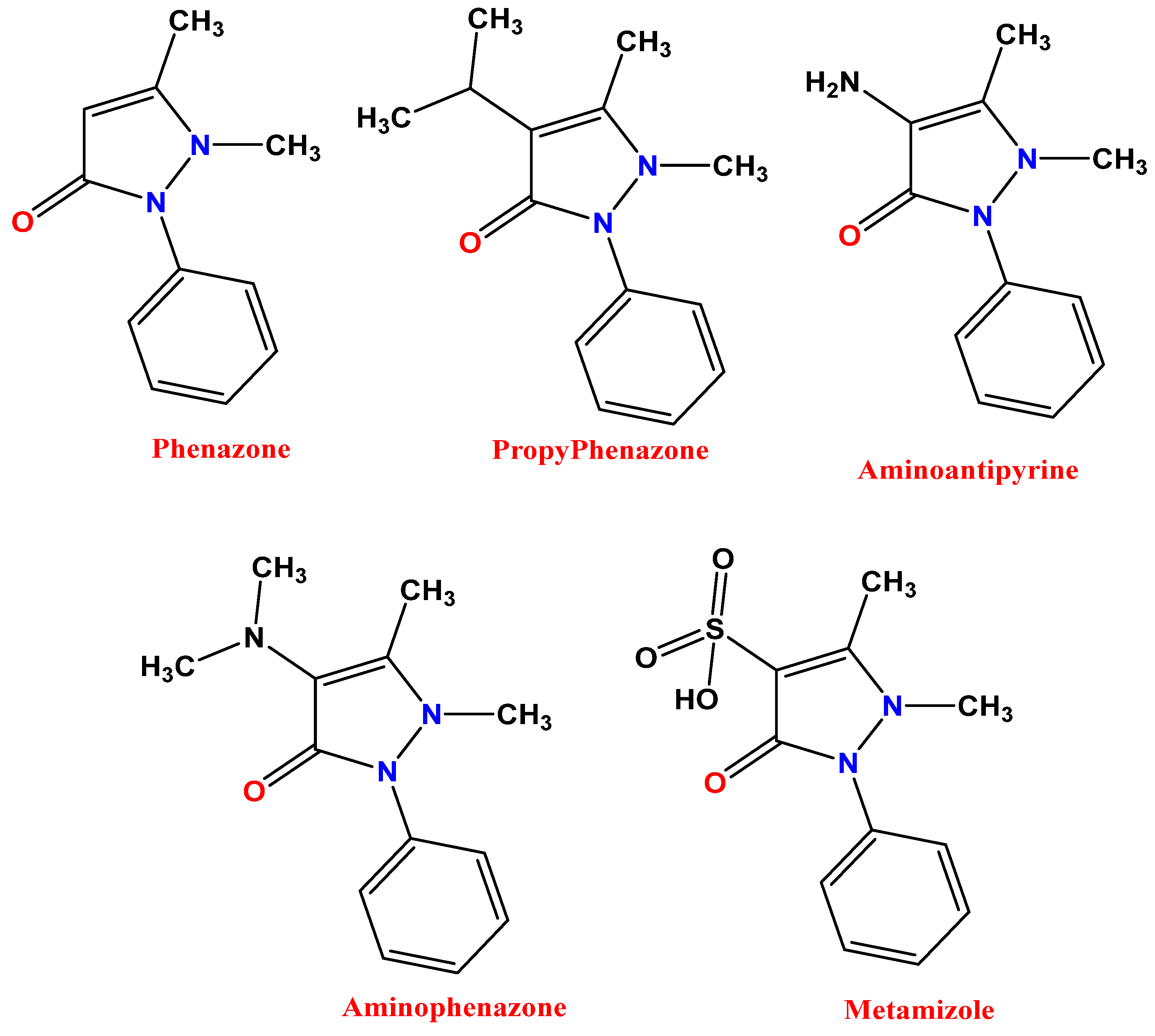
:1. Introduction
2. Results and Discussion
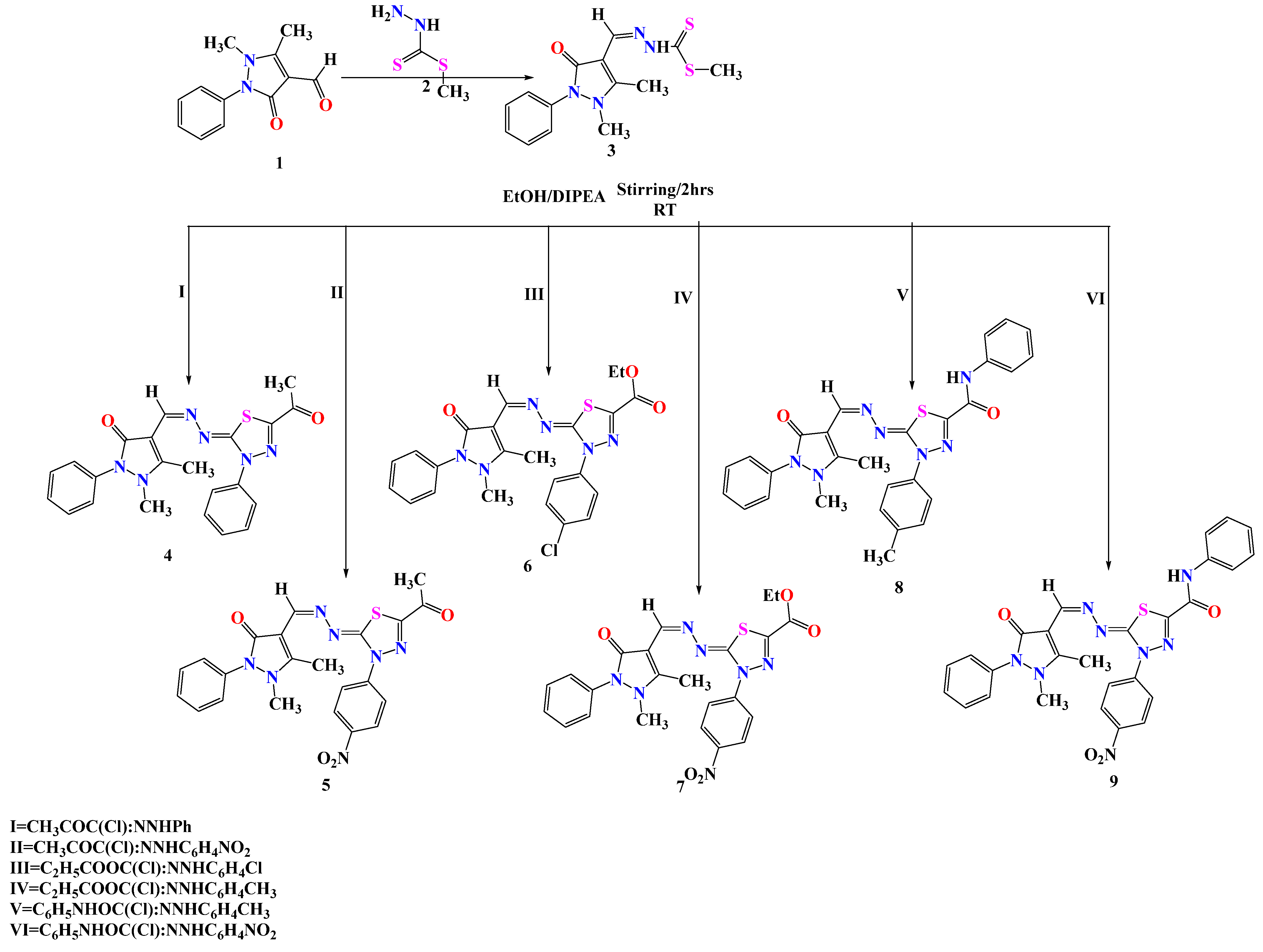
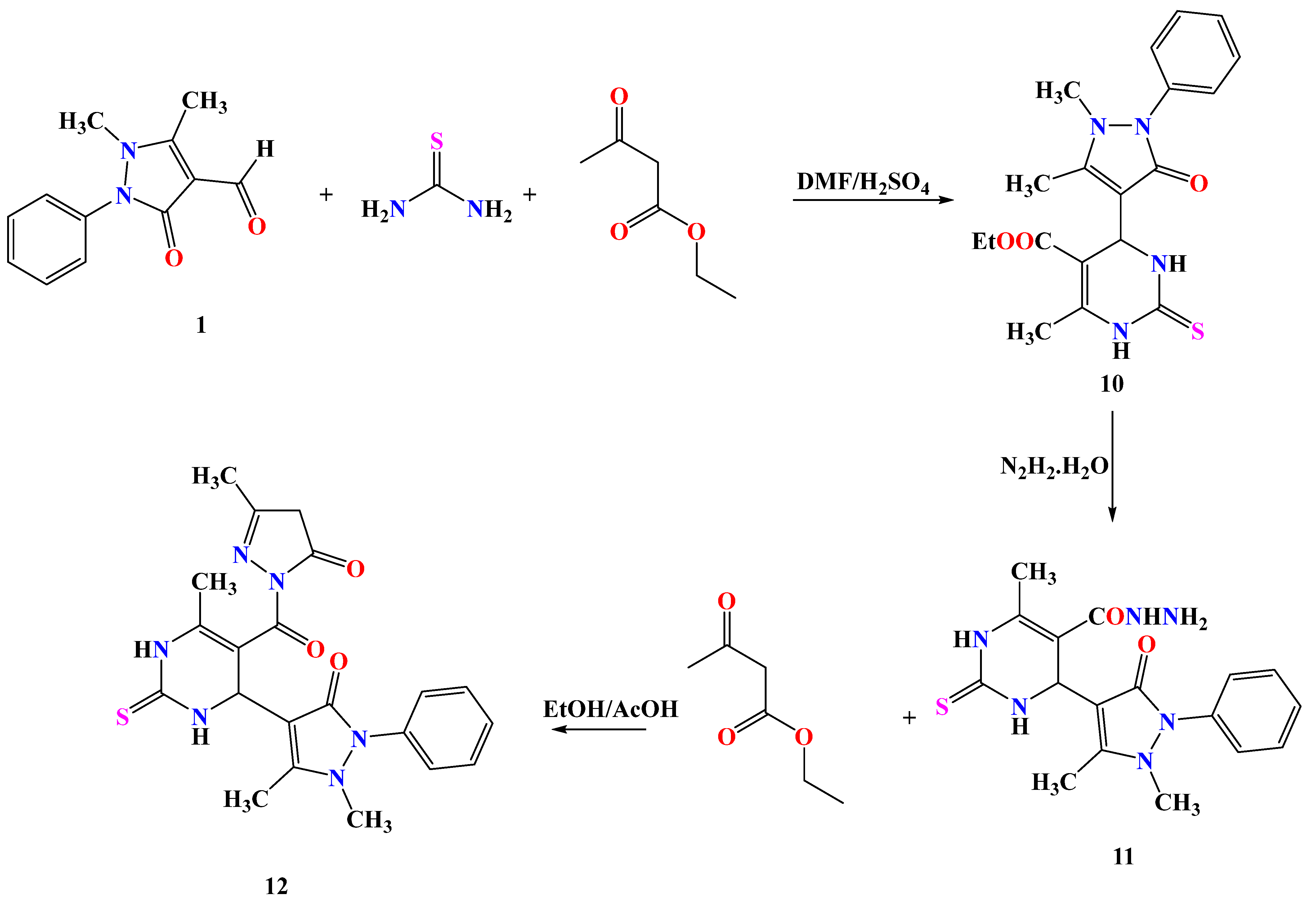
2.1. Chemistry
2.2. Antimicrobial Activity of the Newly Synthesized Compounds
2.3. Kinetics of E. coli Growth (Growth Curve)
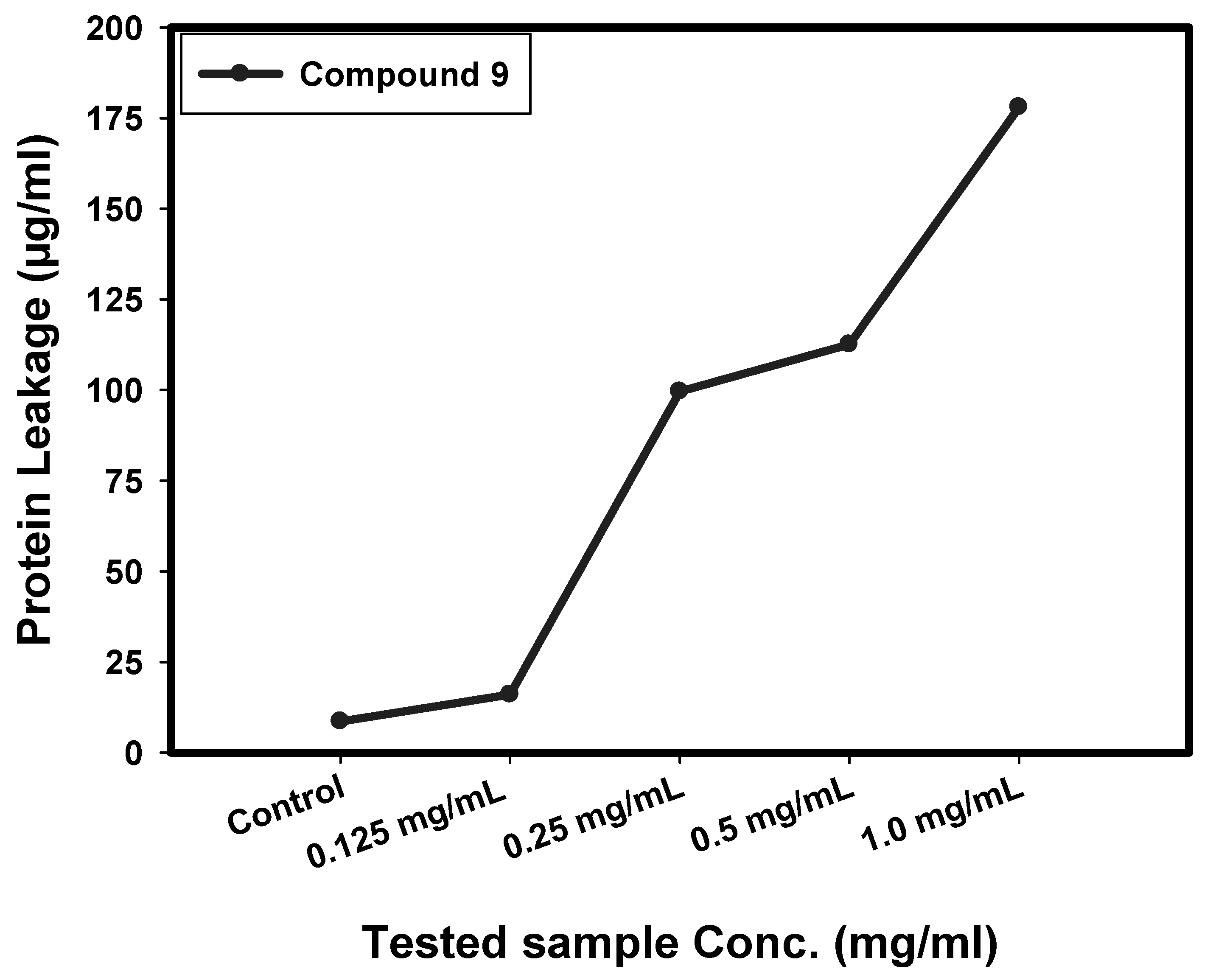
2.4. Determination of Protein Leakage from Bacterial Cell Membranes
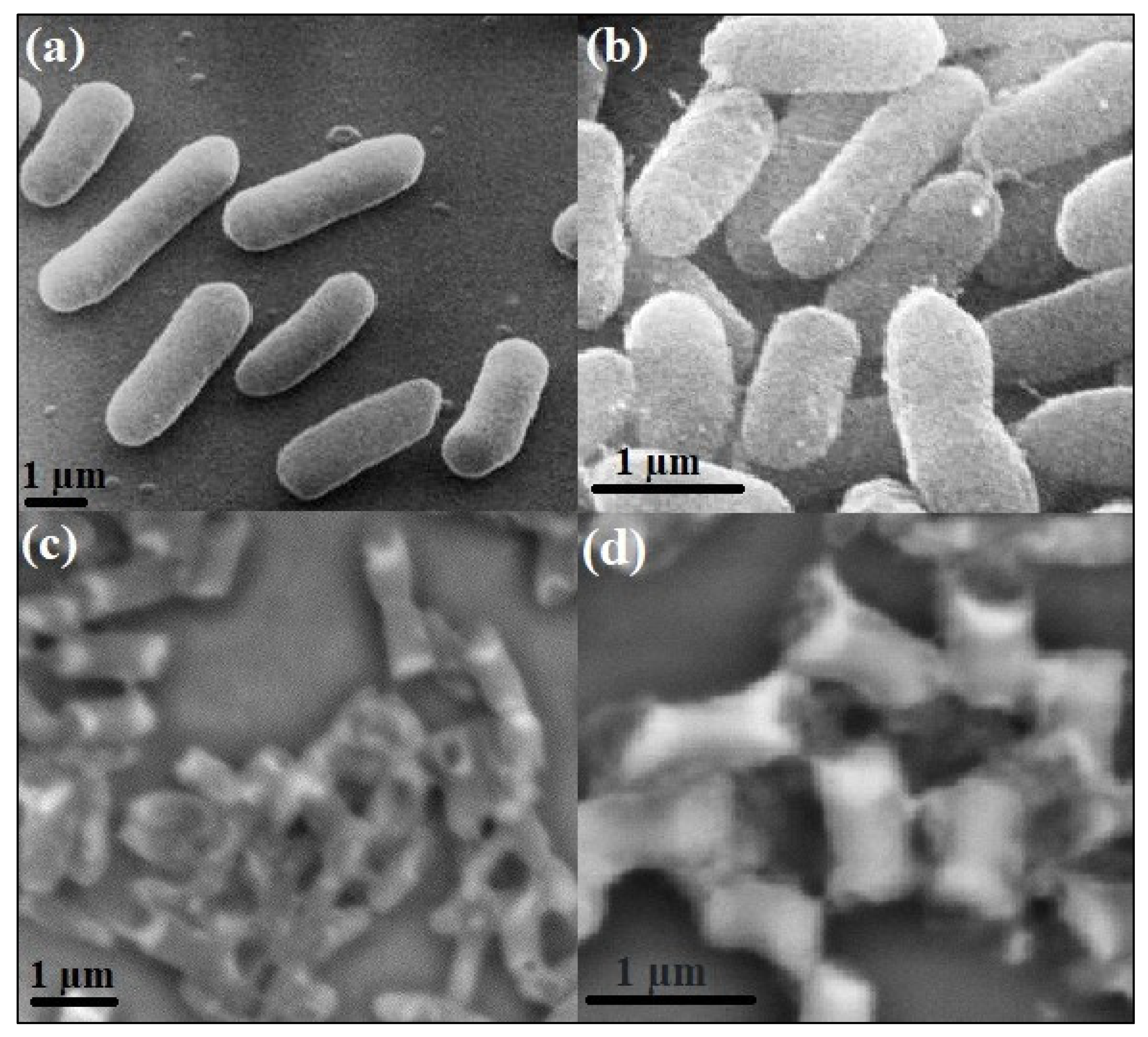
2.5. Mechanism of Biological Action Determination by SEM
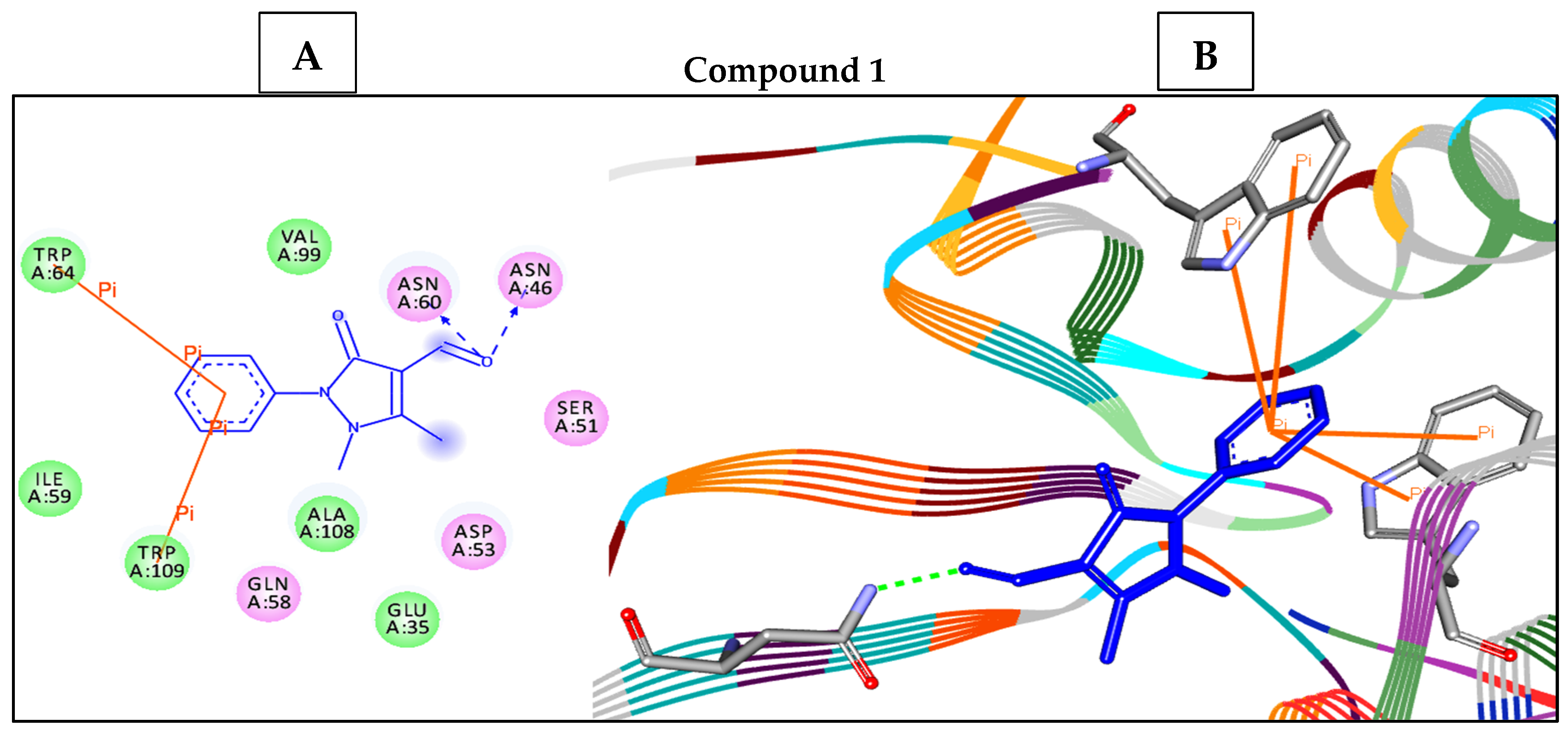
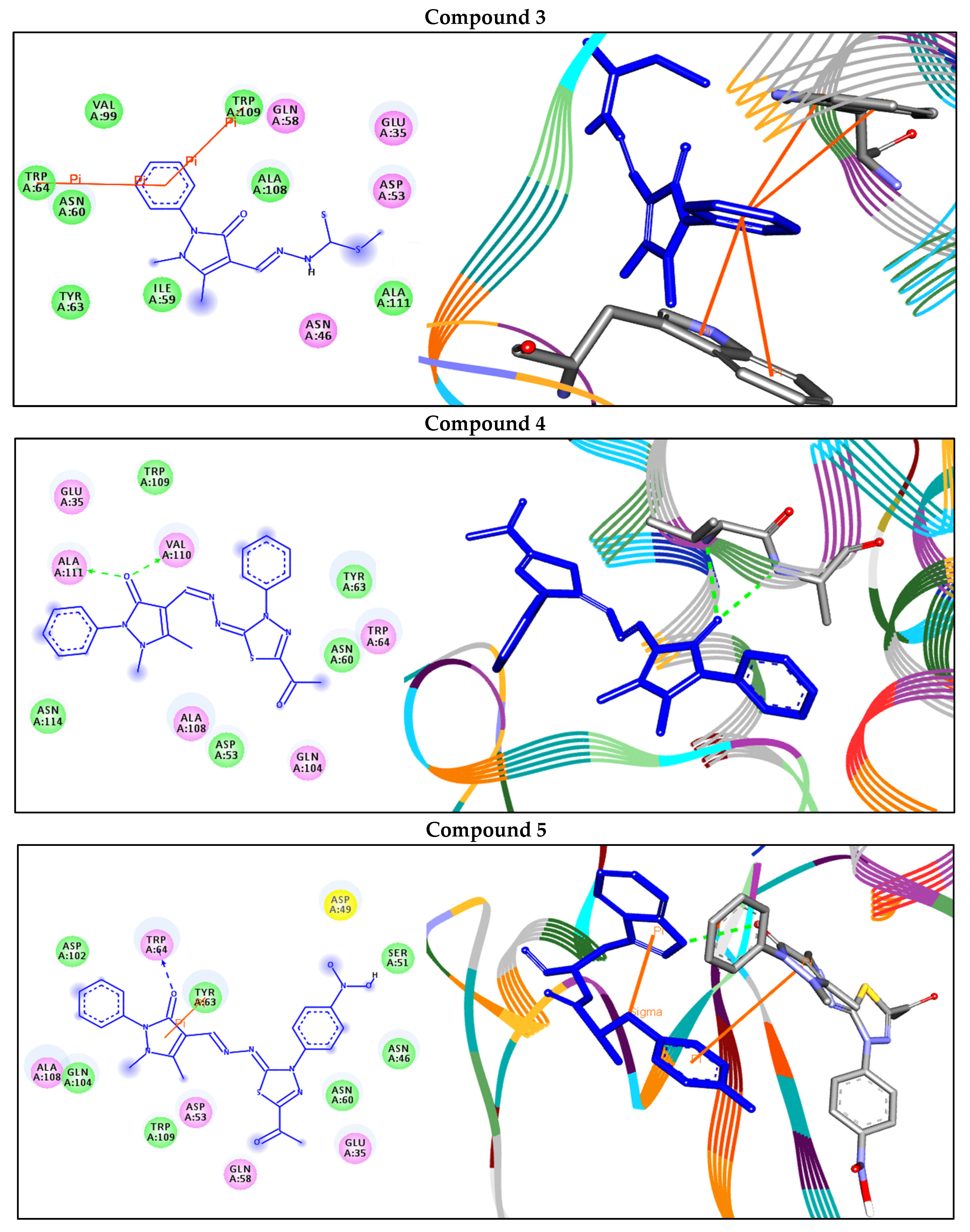
2.6. Molecular Docking Studies and ADMET Analysis
3. Materials and Methods
3.1. Chemistry
3.1.1. Raw Materials
3.1.2. Instrumentation
3.1.3. Synthetic Procedures of the Target Molecules
Synthetic Procedures of the Target Molecule methyl-2-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)methylene)hydrazine-1-carbodithioate (3)
Synthetic Procedures of the Target Molecules (4–9)
3.2. Antimicrobial Activity
3.2.1. Antimicrobial Assay
3.2.2. Growth Curve Assay
3.2.3. Effect of Compound 9 on Protein Leakage from Bacterial Cell Membranes
3.2.4. Mechanism of Biological Action Using SEM Analysis
3.2.5. Statistical Analysis
3.3. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayrak, H.; Cebeci, Y.U.; Karaoğlu, Ş.A. Synthesis of novel antipyrine derivatives possessing remarkable antimicrobial activities. ChemistrySelect 2019, 4, 12906–12908. [Google Scholar] [CrossRef]
- Aly, H.M.; Saleh, N.M.; Elhady, H.A. Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 4566–4572. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Khudhur, S.H.; Nahi, R.J. Synthesis, In Vitro Anticancer Activity Study of Some New Antipyrine Derivatives Containing Thiazolidin-4-One Ring. Int. J. Pharm. Res. 2020, 1, 1662–1666. [Google Scholar]
- Maliyappa, M.R.; Keshavayya, J.; Nazrulla, M.A.; Sudhanva, M.S.; Rangappa, S. Six-substituted benzothiazole based dispersed azo dyes having antipyrine moiety: Synthesis, characterization, DFT, antimicrobial, anticancer and molecular docking studies. J. Iran. Chem. Soc. 2022, 19, 3815–3835. [Google Scholar] [CrossRef]
- Cechinel Filho, V.; Corrêa, R.; Vaz, Z.; Calixto, J.B.; Nunes, R.J.; Pinheiro, T.R.; Andricopulo, A.D.; Yunes, R.A. Further studies on analgesic activity of cyclic imides. Il Farmaco 1998, 53, 55–57. [Google Scholar] [CrossRef]
- Bondock, S.; Rabie, R.; Etman, H.A.; Fadda, A.A. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety. Eur. J. Med. Chem. 2008, 43, 2122–2129. [Google Scholar] [CrossRef]
- Chandra, J.N.N.S.; Sadashiva, C.T.; Kavitha, C.V.; Rangappa, K.S. Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis(4-fluorophenyl)-methyl] piperazine. Bioorganic Med. Chem. 2006, 14, 6621–6627. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Khylyuk, D.; Andrzejczuk, S.; Wujec, M. Design, synthesis, antibacterial evaluations and in silico studies of novel thiosemicarbazides and 1,3,4-thiadiazoles. Molecules 2022, 27, 3161. [Google Scholar] [CrossRef] [PubMed]
- Budziak, I.; Karcz, D.; Makowski, M.; Rachwał, K.; Starzak, K.; Matwijczuk, A.; Myśliwa-Kurdziel, B.; Oniszczuk, A.; Combrzyński, M.; Podleśna, A. Non-typical fluorescence effects and biological activity in selected 1,3,4-thiadiazole derivatives: Spectroscopic and theoretical studies on substituent, molecular aggregation, and pH effects. Int. J. Mol. Sci. 2019, 20, 5494. [Google Scholar] [CrossRef]
- Gür, M. Synthesis, Characterization, and Antimicrobial Properties of New 1,3,4-Thiadiazoles Derived from Azo Dyes. J. Heterocycl. Chem. 2019, 56, 980–987. [Google Scholar] [CrossRef]
- Karabasanagouda, T.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of some novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines carrying thioalkyl and sulphonyl phenoxy moieties. Eur. J. Med. Chem. 2007, 42, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.N.; Duran, A.; Rollas, S.; Sener, G.; Uysal, M.K.; Gülen, D. Synthesis of new 2,5-disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorganic Med. Chem. 2002, 10, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.S. Synthesis, charactrisation and antibacterial activity of mercapto 1,2,4-triazole, 1,3,4-thiadiazoles, mercapto benzhydrazones and thiazolidinone derivatives of 4-hydroxybenzhydrazide. Int. J. Pharm. Pharm. Sci. 2012, 4, 164. [Google Scholar]
- El-Masry, R.M.; Kadry, H.H.; Taher, A.T.; Abou-Seri, S.M. Comparative Study of the Synthetic Approaches and Biological Activities of the Bioisosteres of 1,3,4-Oxadiazoles and 1,3,4-Thiadiazoles over the Past Decade. Molecules 2022, 27, 2709. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Smigon, C.; Ozer, I.; Shova, S.; Gutu, I.; Vornicu, N.; Mangalagiu, I.; D’Ambrosio, M.; Aricu, A. Synthesis and evaluation of biological activity of homodrimane sesquiterpenoids bearing 1,3,4-oxadiazole or 1,3,4-thiadiazole units. Chem. Heterocycl. Compd. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Madhu Sekhar, M.; Yamini, G.; Divya, K.R.G.; Padmavathi, V.; Padmaja, A. Synthesis and bioassay of a new class of disubstituted 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Med. Chem. Res. 2019, 28, 1049–1062. [Google Scholar] [CrossRef]
- Han, X.; Yu, Y.L.; Hu, Y.S.; Liu, X.H. 1,3,4-thiadiazole: A privileged scaffold for drug design and development. Curr. Top. Med. Chem. 2021, 21, 2546–2573. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef]
- Sabt, A.; Abdelrahman, M.T.; Abdelraof, M.; Rashdan, H.R.M. Investigation of Novel Mucorales Fungal Inhibitors: Synthesis, In-Silico Study and Anti-Fungal Potency of Novel Class of Coumarin-6-Sulfonamides-Thiazole and Thiadiazole Hybrids. ChemistrySelect 2022, 7, e202200691. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Rashdan, H.R.M.; Mohamed Ahmed, E.; Shabaan, S.N. Synthesis and cytotoxic activity of some novel benzocoumarin derivatives under solvent free conditions. Green Chem. Lett. Rev. 2019, 12, 9–18. [Google Scholar] [CrossRef]
- Roaiah, H.F.; Rashdan, H.R.; Soliman, A.; Muhammed, Z.; Wietrzyk, J.; Milczarek, M. Design, efficient synthesis, mechanism of reaction and antiproliferative activity against cancer and normal cell lines of a novel class of fused pyrimidine derivatives. Acta Pol. Pharm.-Drug Res. 2018, 75, 679–688. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of some new antimicrobial 5,6,7,8-tetrahydro-pyrimido [4,5-b] quinolone derivatives. Der Pharm. Chem 2014, 6, 23–29. [Google Scholar]
- Zarenezhad, E.; Farjam, M.; Iraji, A. Synthesis and biological activity of pyrimidines-containing hybrids: Focusing on pharmacological application. J. Mol. Struct. 2021, 1230, 129833. [Google Scholar] [CrossRef]
- Mohana Roopan, S.; Sompalle, R. Synthetic chemistry of pyrimidines and fused pyrimidines: A review. Synth. Commun. 2016, 46, 645–672. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Pang, L.-P.; Wang, B.; Zhang, M.; Hu, B.; Xue, D.-Q.; Shao, K.-P.; Zhang, B.-L.; Liu, Y.; Zhang, E. Design and synthesis of novel 1,2,3-triazole-pyrimidine hybrids as potential anticancer agents. Eur. J. Med. Chem. 2014, 86, 368–380. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Iannazzo, D.; Romeo, R.; Giofrè, S.V.; Legnani, L. Pyridine and pyrimidine derivatives as privileged scaffolds in biologically active agents. Curr. Med. Chem. 2019, 26, 7166–7195. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kaur, T. Pyrimidine-based antimalarials: Design strategies and antiplasmodial effects. MedChemComm 2016, 7, 749–768. [Google Scholar] [CrossRef]
- Manohar, S.; Pavan, V.S.; Taylor, D.; Kumar, D.; Ponnan, P.; Wiesner, L.; Rawat, D.S. Highly active 4-aminoquinoline–pyrimidine based molecular hybrids as potential next generation antimalarial agents. RSC Adv. 2015, 5, 28171–28186. [Google Scholar] [CrossRef]
- Yavuz, S.Ç.; Akkoç, S.; Tüzün, B.; Şahin, O.; Saripinar, E. Efficient synthesis and molecular docking studies of new pyrimidine-chromeno hybrid derivatives as potential antiproliferative agents. Synth. Commun. 2021, 51, 2135–2159. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, S.I.; Tekwani, B.L.; Ponnan, P.; Rawat, D.S. 4-Aminoquinoline-pyrimidine hybrids: Synthesis, antimalarial activity, heme binding and docking studies. Eur. J. Med. Chem. 2015, 89, 490–502. [Google Scholar] [CrossRef]
- Sahoo, J.; Sahoo, C.R.; Sarangi, P.K.N.; Prusty, S.K.; Padhy, R.N.; Paidesetty, S.K. Molecules with versatile biological activities bearing antipyrinyl nucleus as pharmacophore. Eur. J. Med. Chem. 2020, 186, 111911. [Google Scholar] [CrossRef]
- Nehra, B.; Rulhania, S.; Jaswal, S.; Kumar, B.; Singh, G.; Monga, V. Recent advancements in the development of bioactive pyrazoline derivatives. Eur. J. Med. Chem. 2020, 205, 112666. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, A.G.; Sekeroglu, V.; Yildirim, E.; Dindaroglu, G.; Sekeroglu, Z.A. Antipyrine derived-Schiff base copper complex: Synthesis, characterization, and in vitro evaluation. Inorganica Chim. Acta 2022, 543, 121146. [Google Scholar] [CrossRef]
- Saber, R.W.; Abou-Melha, K.; El-Metwaly, N. Synthesis of new Cr (III) complexes derived from antipyrine-based ligands: Elucidation, conformation, cytotoxicity and genotoxicity via in-vitro and in-silico approaches. J. Mol. Liq. 2022, 359, 119361. [Google Scholar] [CrossRef]
- Abbas, S.Y.; Abd El-Aziz, M.M.; Awad, S.M.; Mohamed, M.S. Synthesis and evaluation of antipyrine derivatives bearing a thiazole moiety as antibacterial and antifungal agents. Synth. Commun. 2023, 53, 1812–1822. [Google Scholar] [CrossRef]
- Yasar, Q.; Zaheer, Z. 4-Aminoantipyrine Analogs as Anti-inflammatory and Antioxidant agents: Synthesis, Biological Evaluation and Molecular Docking Studies. Int. J. Pharm. Investig. 2021, 11, 14–22. [Google Scholar] [CrossRef]
- Cebeci, Y.U.; Bayrak, H.; Karaoğlu, Ş.A.; Fahim, A.M. Synthesis of novel antipyrine-azole-S-alkyl derivatives antimicrobial activity, molecular docking, and computational studies. J. Mol. Struct. 2022, 1260, 132810. [Google Scholar] [CrossRef]
- Fratoni, E.; Theindl, L.C.; da Rosa, J.S.; dos Nascimento, M.V.P.S.; da Maciel, T.R.G.; de Campos-Buzzi, F.; Dalmarco, E.M. The in vitro anti-inflammatory activity of N-antipyrine-3,4-dichloromaleimide derivatives is due to an immunomodulatory effect on cytokines environment. Immunopharmacol. Immunotoxicol. 2023, 45, 224–233. [Google Scholar] [CrossRef]
- Reisner, A.; Maierl, M.; Jörger, M.; Krause, R.; Berger, D.; Haid, A.; Tesic, D.; Zechner, E.L. Type 1 fimbriae contribute to catheter-associated urinary tract infections caused by Escherichia coli. J. Bacteriol. 2014, 196, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef]
- Hemdan, B.A.; Radwan, E.K.; Rashdan, H.R.M. Design and solvent free synthesis of novel phenazone based molecule for water disinfection and rapid removal of the anionic direct fast blue B2RL dye. J. Water Process Eng. 2023, 54, 103861. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, B.; Tao, L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules 2022, 27, 7886. [Google Scholar] [CrossRef]
- Seal, N.; Neogi, S. Lewis acid-base integrated robust metal-organic framework and reconfigurable composite for solvent-free Biginelli condensation and tandem catalysis with size selectivity. Mater. Today Chem. 2022, 26, 101064. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Rashdan, H.U.A.R.M.; Abdel-Aziem, A.N.A.; El-Naggar, D.I.A.H.; Nabil, S.A.A. Synthesis and biological evaluation of some new pyridines, isoxazoles and isoxazolopyridazines bearing 1,2,3-triazole moiety. Acta Pol. Pharm.-Drug Res. 2019, 76, 469–482. [Google Scholar]
- Paul, D.; Maiti, S.; Sethi, D.P.; Neogi, S. Bi-functional NiO-ZnO nanocomposite: Synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 2021, 32, 131–143. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Soares Filipe, J.F.; Martino, P.A. In vitro antibacterial activity of biological-derived silver nanoparticles: Preliminary data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int. J. Nanomed. 2013, 1645–1652. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Angeli, A.; Kartsev, V.; Petrou, A.; Lichitsky, B.; Komogortsev, A.; Pinteala, M.; Geronikaki, A.; Supuran, C.T. Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies. Pharmaceuticals 2022, 15, 316. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1263, pp. 243–250. ISBN 9780123944474. [Google Scholar]
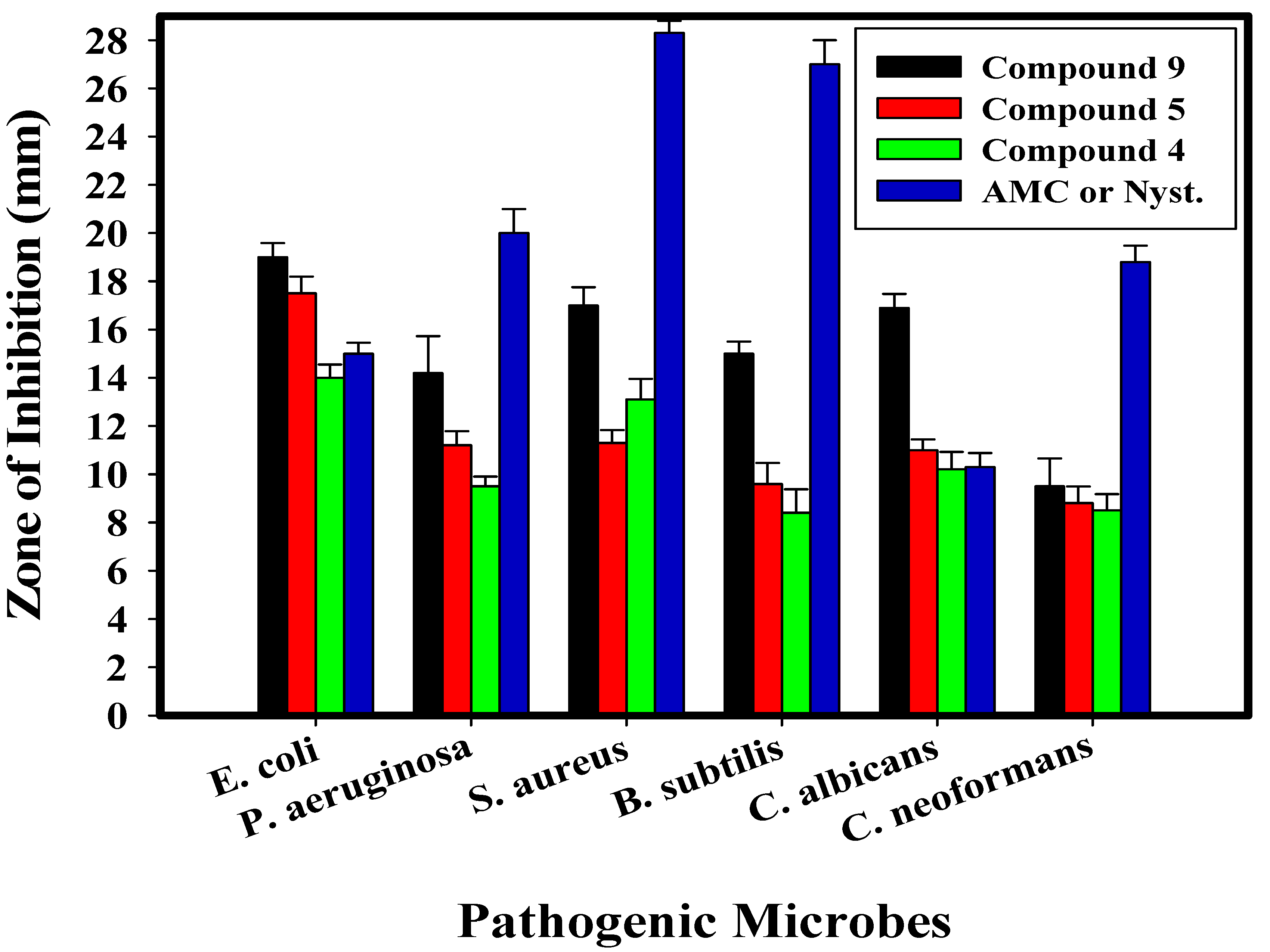



| Test Organism | Compound 4 | Compound 5 | Compound 9 | AMC/Nystatin | ||||
|---|---|---|---|---|---|---|---|---|
| IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | |
| E. coli | 14.0 ± 0.55 e | 125 | 17.5 ± 0.76 e | 31.25 | 19.0 ± 0.58 f | 15.62 | 15.0 ± 0.46 d | 250 |
| P. aeruginosa | 9.5 ± 0.40 b | 250 | 11.2 ± 0.58 d | 125 | 14.2 ± 1.53 b,c,d | 125 | 20.0 ± 1.00 c | 500 |
| S. aureus | 13.1 ± 0.85 d | 125 | 11.3 ± 0.53 d | 125 | 17.0 ± 0.76 d,e | 31.25 | 28.3 ± 0.50 b | 250 |
| B. subtilis | 8.4 ± 0.98 b,c | 500 | 9.6 ± 0.87 b,c | 500 | 15.0 ± 0.50 a | 31.25 | 27.0 ± 1.00 a | 31.25 |
| C. albicans | 10.2 ± 0.72 f | 250 | 11.0 ± 0.45 f | 125 | 16.9.0 ± 0.58 f | 31.25 | 10.3 ± 0.58 f | 125 |
| C. neoformans | 8.5 ± 0.68 b,c,d | 500 | 8.8 ± 0.69 c | 500 | 9.5 ± 1.15 c,d,e | 500 | 18.8 ± 0.68 c | 250 |
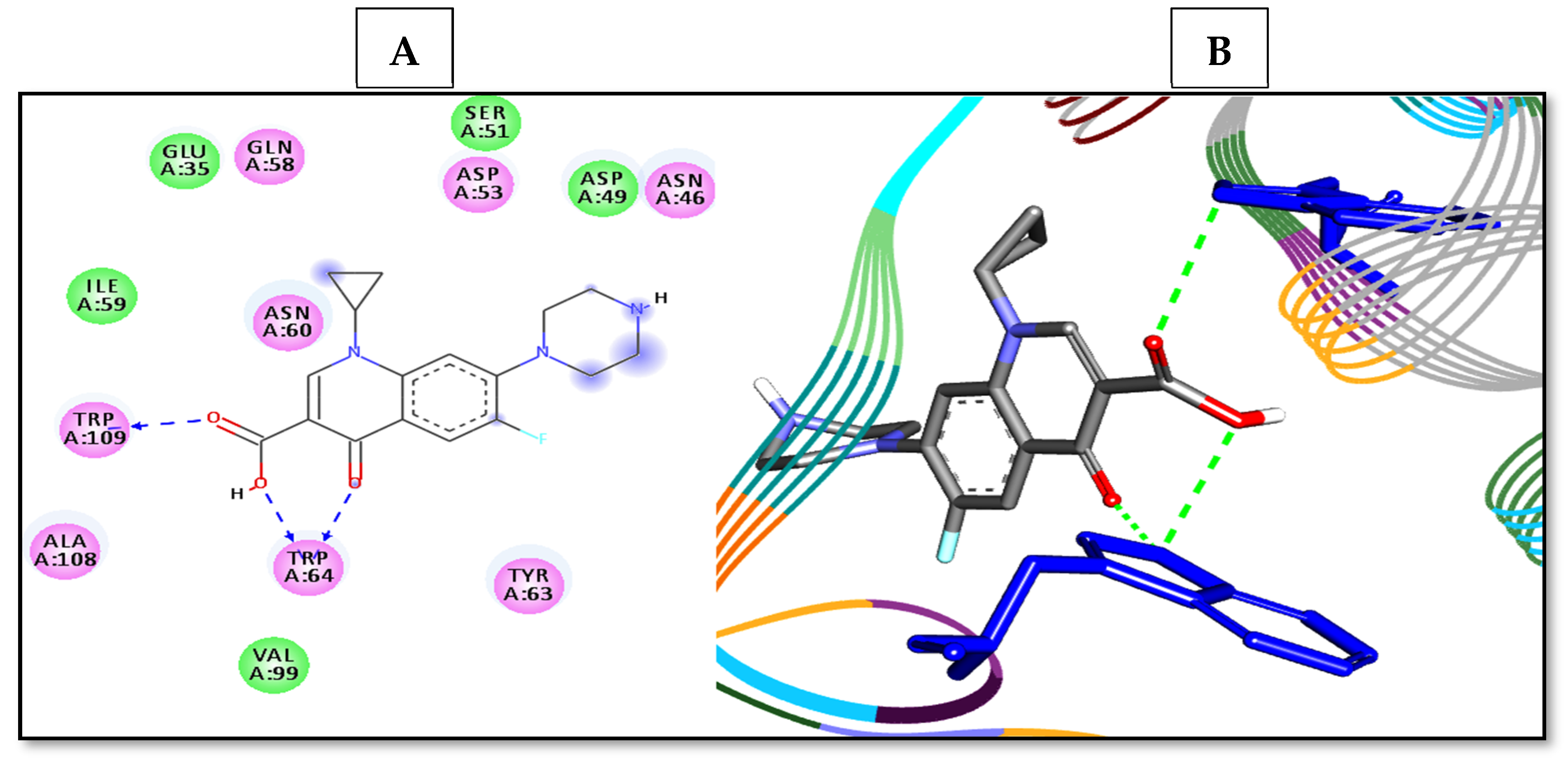
| Entry | Binding Energy (kcal/mol) | Docked Complex (Amino Acid–Ligand) Interactions | Distance (Å) |
|---|---|---|---|
| 1 | −7.1 | H-bonds | |
| ASN46:ND2—compound 1 | 2.99 | ||
| ASN60:ND2—compound 1 | 3.07 | ||
| Arene–arene | |||
| TRP109—compound 1 | 3.93 | ||
| TRP64—compound 1 | 4.94 | ||
| 3 | −7.0 | Arene–arene | |
| TRP109—compound 3 | 3.84 | ||
| TRP64—compound 3 | 4.64 | ||
| 4 | −8.1 | H-bonds | |
| VAL110:N—compound 4 | 2.80 | ||
| ALA111:N—compound 4 | 2.85 | ||
| 5 | −9.2 | H-bonds | |
| TRP64:NE1—compound 5 | 2.81 | ||
| Arene–arene | |||
| TYR63—compound 5 | 5.71 | ||
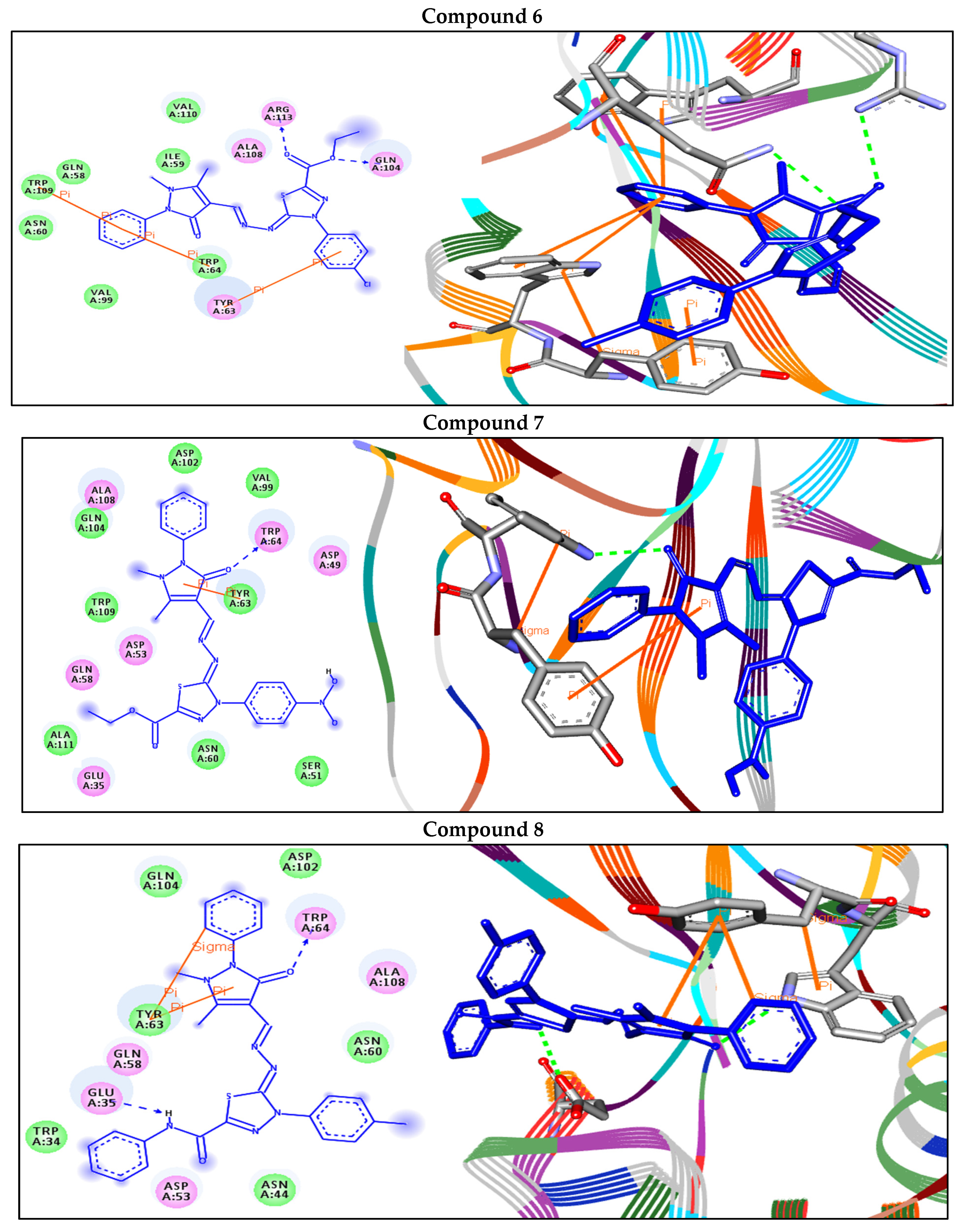
| 6 | −8.1 | H-bonds | |
| GLN104:NE2—compound 6 | 2.94 | ||
| ARG113:NH2—compound 6 | 2.99 | ||
| Arene–arene | |||
| TRP109—compound 6 | 4.80 | ||
| TRP64—compound 6 | 5.82 | ||
| TYR63—compound 6 | 3.84 | ||
| 7 | −8.8 | H-bonds | |
| TRP64:NE1—compound 7 | 2.86 | ||
| Arene–arene | |||
| TYR63—compound 7 | 5.60 | ||
| 8 | −9.3 | H-bonds | |
| TRP64:NE1—compound 8 | 2.44 | ||
| GLU35:OE1—compound 8 | 2.49 | ||
| Arene–arene | |||
| TYR63—compound 8 | 4.18 | ||
| Arene–sigma | |||
| TYR63—compound 8 | 3.50 | ||
| 9 | −9.7 | H-bonds | |
| TRP64:NE1—compound 9 | 2.91 | ||
| Arene–sigma | |||
| TYR63—compound 9 | 3.70 | ||
| 10 | −7.7 | H-bonds | |
| TRP64:NE1—compound 10 | 2.79 | ||
| 11 | −7.5 | H-bonds | |
| TRP64:NE1—compound 11 | 3.00 | ||
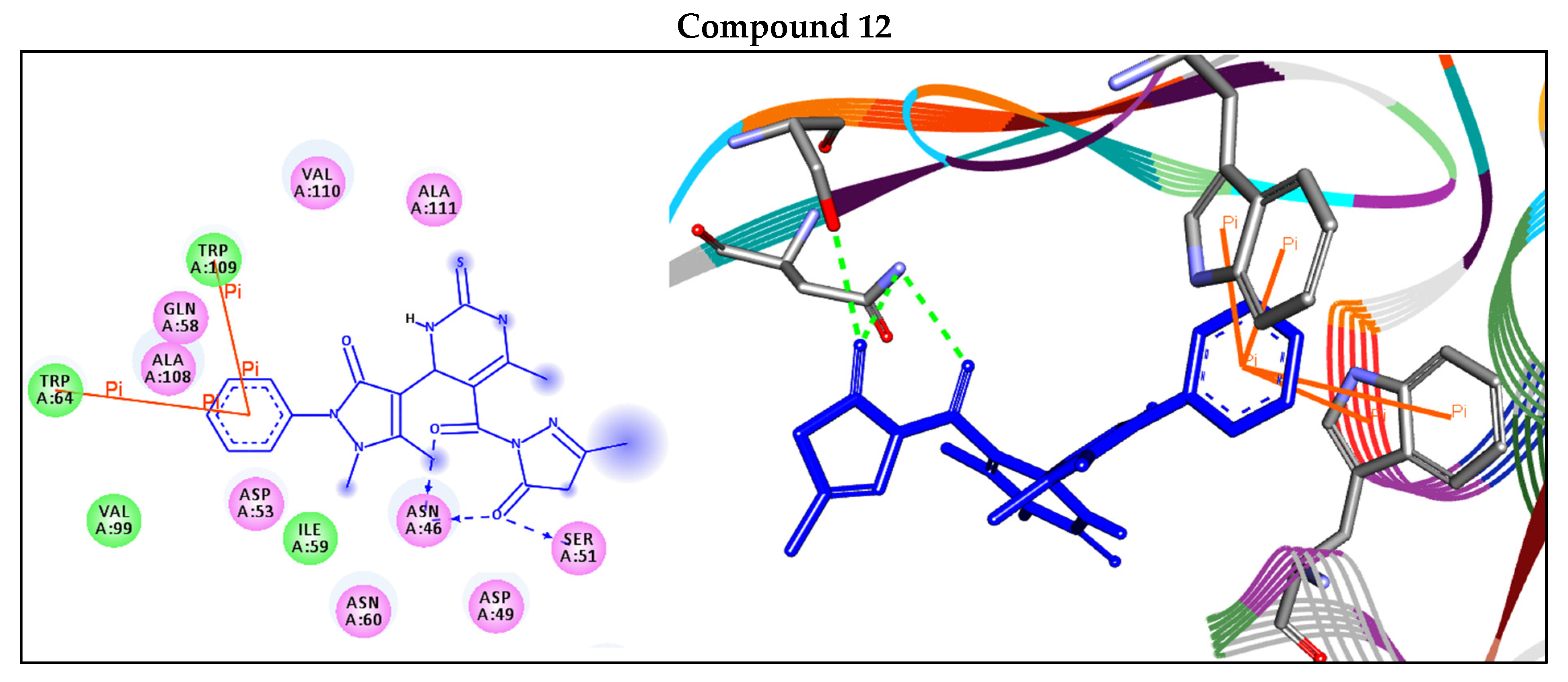
| 12 | −8.8 | H-bonds | |
| TRP64:ND2—compound 12 | 3.12 | ||
| TRP64:ND2—compound 12 | 3.01 | ||
| SER51:OG—compound 12 | 2.78 | ||
| Arene–arene | |||
| TRP109—compound 12 | 3.85 | ||
| TRP64—compound 12 | 4.64 | ||
| Ciprofloxacin | −7.6 | H-bonds | |
| TRP64:NE1—ciprofloxacin | 3.10 | ||
| TRP64:NE1—ciprofloxacin | 3.19 | ||
| TRP109:NE1—ciprofloxacin | 2.78 |
| Molecular Weight (g/mol) | BBB Permeant | GI Absorption | %Human Intestinal Absorption (HIA+) | logp | TPSA A2 | HBA | HBD | N Rotatable | N Violations | Bioavailability Score | AMES Toxicity | Carcinogenicity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. Range | 130–500 | <25 poor >80 high | ≤5 | ≤140 | 2.0–20.0 | 0.0–6.0 | ≤10 | ≤1 | Nontoxic | Noncarcinogenic | |||
| 1 | 216.24 | Yes | high | 98.26 | 1.12 | 44.00 | 2 | 0 | 2 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 3 | 336.48 | No | high | 94.12 | 2.69 | 108.71 | 2 | 1 | 5 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 4 | 432.50 | No | high | 98.59 | 2.89 | 114.78 | 5 | 0 | 5 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 5 | 477.50 | No | low | 92.77 | 2.06 | 160.60 | 7 | 0 | 6 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 6 | 496.97 | No | high | 98.42 | 3.88 | 124.01 | 7 | 0 | 6 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 7 | 507.52 | No | low | 89.60 | 2.60 | 169.83 | 8 | 0 | 8 | 1 | 0.17 | Nontoxic | Noncarcinogenic |
| 8 | 523.61 | No | low | 98.53 | 4.09 | 126.81 | 5 | 1 | 7 | 1 | 0.55 | Nontoxic | Noncarcinogenic |
| 9 | 554.58 | No | low | 93.03 | 3.09 | 172.63 | 7 | 1 | 8 | 1 | 0.17 | Nontoxic | Noncarcinogenic |
| 10 | 386.47 | No | high | 95.42 | 1.91 | 109.38 | 3 | 2 | 5 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 11 | 372.44 | No | low | 88.71 | 1.05 | 138.20 | 3 | 4 | 4 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
| 12 | 438.50 | No | high | 92.90 | 1.46 | 132.80 | 4 | 2 | 4 | 0 | 0.55 | Nontoxic | Noncarcinogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashdan, H.R.M.; El-Sayyad, G.S.; Shehadi, I.A.; Abdelmonsef, A.H. Antimicrobial Potency and E. coli β-Carbonic Anhydrase Inhibition Efficacy of Phenazone-Based Molecules. Molecules 2023, 28, 7491. https://doi.org/10.3390/molecules28227491
Rashdan HRM, El-Sayyad GS, Shehadi IA, Abdelmonsef AH. Antimicrobial Potency and E. coli β-Carbonic Anhydrase Inhibition Efficacy of Phenazone-Based Molecules. Molecules. 2023; 28(22):7491. https://doi.org/10.3390/molecules28227491
Chicago/Turabian StyleRashdan, Huda R. M., Gharieb S. El-Sayyad, Ihsan A. Shehadi, and Aboubakr H. Abdelmonsef. 2023. "Antimicrobial Potency and E. coli β-Carbonic Anhydrase Inhibition Efficacy of Phenazone-Based Molecules" Molecules 28, no. 22: 7491. https://doi.org/10.3390/molecules28227491
APA StyleRashdan, H. R. M., El-Sayyad, G. S., Shehadi, I. A., & Abdelmonsef, A. H. (2023). Antimicrobial Potency and E. coli β-Carbonic Anhydrase Inhibition Efficacy of Phenazone-Based Molecules. Molecules, 28(22), 7491. https://doi.org/10.3390/molecules28227491







