Influence of Co3O4 Nanostructure Morphology on the Catalytic Degradation of p-Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Co3O4 Nanostructures
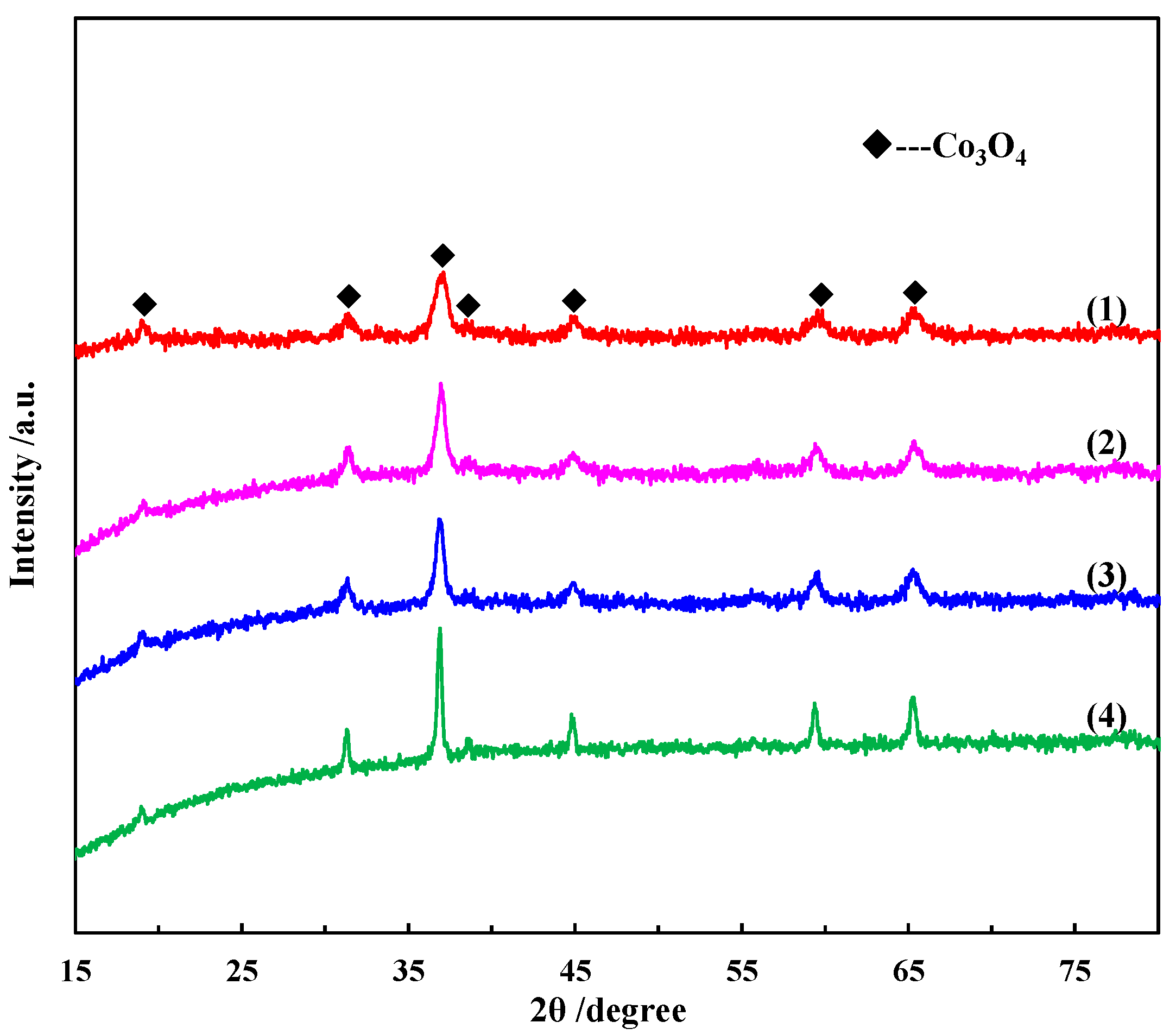
2.1.1. XRD
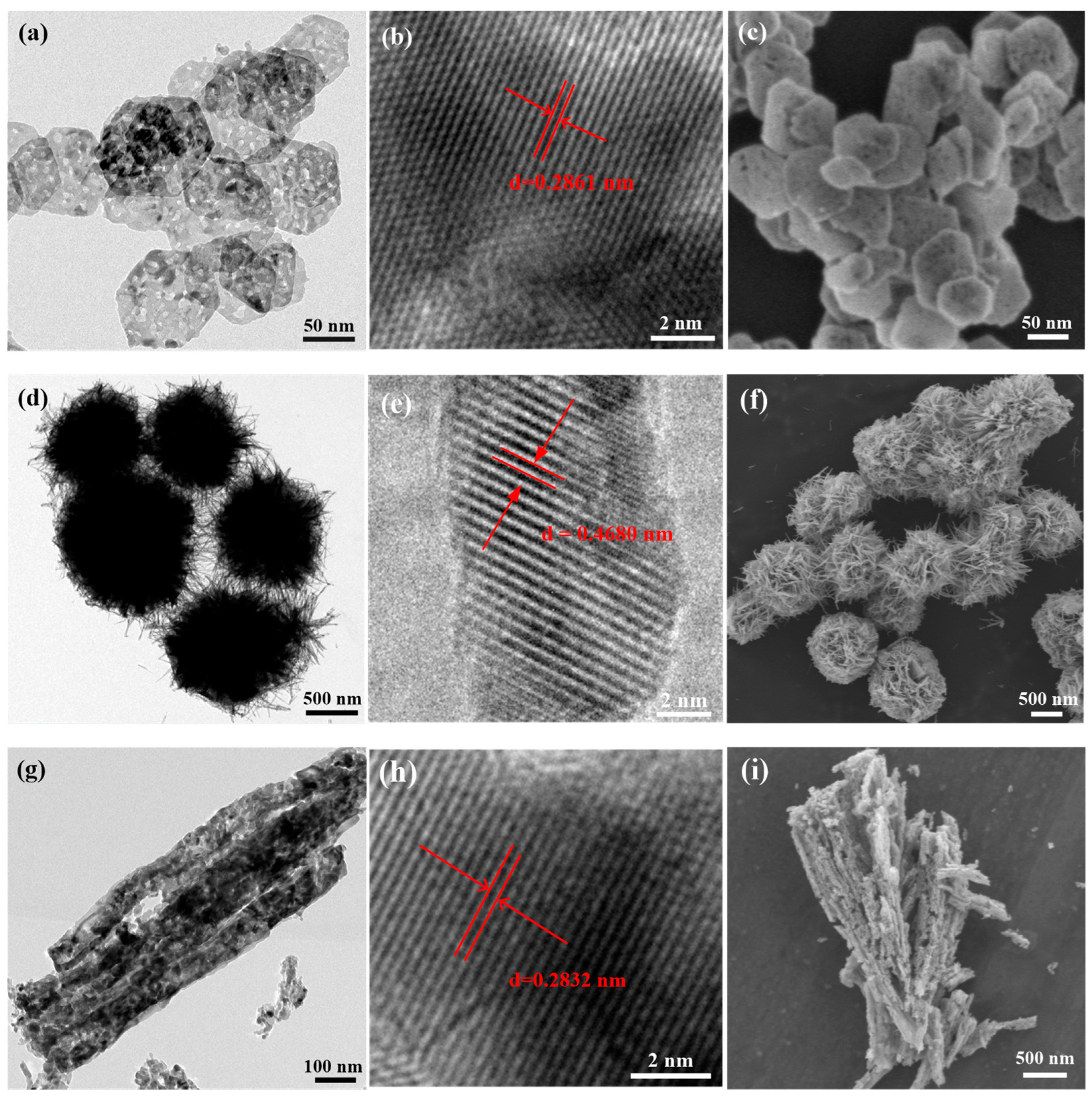
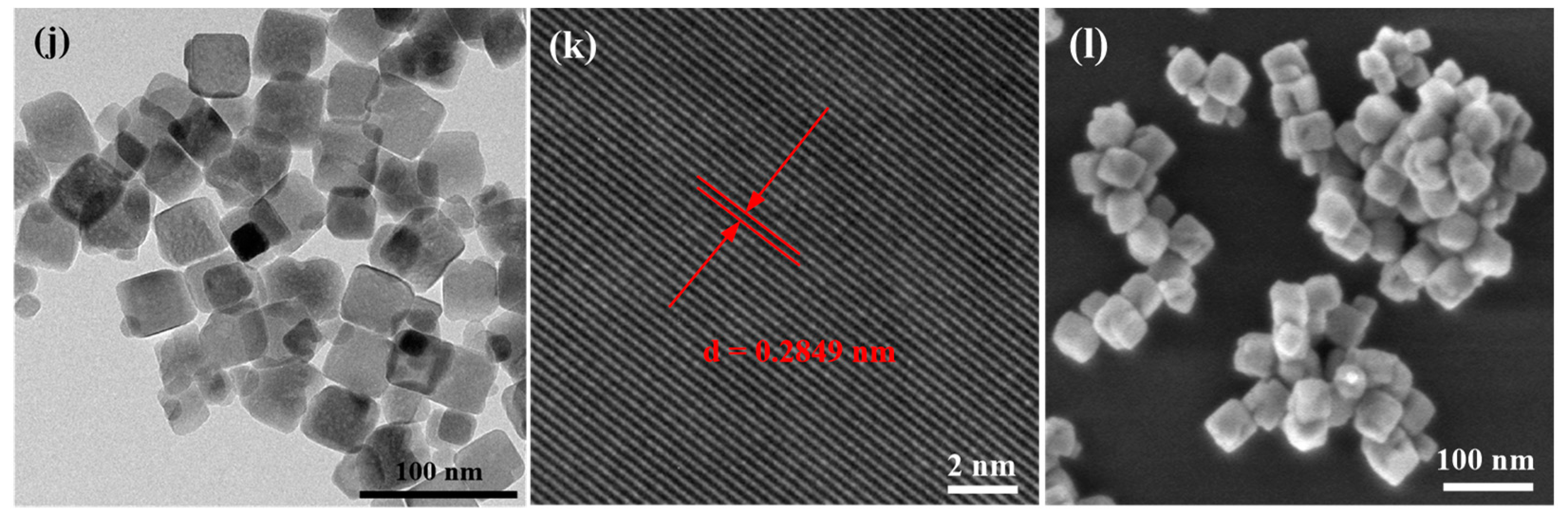
2.1.2. TEM and SEM
2.1.3. N2 Physisorption
2.1.4. H2-TPR
2.1.5. XPS
2.2. Catalytic Performance of Co3O4 Nanostructures
2.2.1. Catalytic Activity Test
2.2.2. Stability Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co(OH)2 Nanoplates
3.3. Preparation of Co(OH)2 Microflowers
3.4. Preparation of CoC2O4 Nanorods
3.5. Preparation of CoC2O4 Nanocubes
3.6. Preparation of Reduced Co3O4
3.7. Catalyst Characterization
3.8. Catalytic Reduction of p-NP to p-AP by Excess NaBH4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Ito, H.; Komori, M.; Kobayashi, R.; Shibuta, M.; Eguchi, T.; Nakajima, A. Liquid-phase catalysis by single-size palladium nanoclusters supported on strontium titanate: Size-specific catalysts for Suzuki-Miyaura coupling. Catal. Sci. Technol. 2018, 8, 5827–5834. [Google Scholar] [CrossRef]
- Gao, Q.X.; Wang, X.F.; Di, J.L.; Wu, X.C.; Tao, Y.R. Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with {110} and {001} planes for methane combustion and CO oxidation. Catal. Sci. Technol. 2011, 1, 574–577. [Google Scholar] [CrossRef]
- Peng, K.; Hou, Y.; Zhang, Y.; Liu, X.; Li, Y.; Li, B.; Zeng, Z.; Huang, Z. Engineering oxygen vacancies in metal-doped MnO2 nanospheres for boosting the low-temperature toluene oxidation. Fuel 2022, 314, 123965. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Mesoporous TiO2: Preparation, doping, and as a composite for photocatalysis. ChemCatChem 2013, 5, 885–894. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Yue, J.; Chen, G. Facile Synthesis of CoOOH nanorings over reduced graphene oxide and their application in the reduction of p-nitrophenol. Materials 2022, 15, 8862. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, J.; Huang, M.; Zhang, J.; Zhou, Y.; Wang, G.; Wang, R.; Chen, J. Remarkable performance of atomically dispersed cobalt catalyst for catalytic removal of indoor formaldehyde. J. Colloid Interface Sci. 2022, 624, 527–536. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Ceria doped CuMnOx as carbon monoxide oxidation catalysts: Synthesis and their characterization. Surf. Interfaces 2020, 18, 100456. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Jin, C.; He, Q.; Wang, Y. Fabrication of ZIF-8@TiO2 micron composite via hydrothermal method with enhanced absorption and photocatalytic activities in tetracycline degradation. J. Alloys Compd. 2020, 825, 154008. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, L.; Zhao, Z.; Zhang, Y.; Zhang, Z.; Lu, G.; Guo, Y.; Guo, Y. Low-temperature CO oxidation over Co3O4-based catalysts: Significant promoting effect of Bi2O3 on Co3O4 catalyst. Appl. Catal. B-Environ. 2014, 146, 43–49. [Google Scholar] [CrossRef]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F. Complete oxidation of methane on Co3O4/CeO2 nanocomposite: A synergistic effect. Catal. Today 2017, 311, 48–55. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Edit. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, M.; Tao, S.; Chen, G. Template-free synthesis of Co3O4 nanorings and their catalytic application. CrystEngComm 2018, 20, 679–688. [Google Scholar] [CrossRef]
- Ma, C.; Mu, Z.; Li, J.; Jin, Y.; Cheng, J.; Lu, G.; Hao, Z.; Qiao, S. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, M.; Tao, S.; Chen, G. Oxygen vacancy enhanced catalytic activity of reduced Co3O4 towards p-nitrophenol reduction. Appl. Catal. B-Environ. 2017, 209, 648–656. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Tao, S.; Ren, M.; Chen, G. Facile Synthesis of Co3O4 with different morphologies via oxidation kinetic control and its application in hydrogen peroxide decomposition. Cryst. Growth Des. 2016, 16, 6286–6293. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Zhao, Q.; Chen, G. Shape-controlled fabrication of the porous Co3O4 nanoflower clusters for efficient catalytic oxidation of gaseous toluene. J. Hazard. Mater. 2012, 209–210, 385–391. [Google Scholar] [CrossRef]
- Xie, X.; Yong, L.; Liu, Z.Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef]
- Li, R.; Shi, X.; Huang, Y.; Chen, M.; Zhu, D.; Ho, W.; Cao, J.; Lee, S. Catalytic oxidation of formaldehyde on ultrathin Co3O4 nanosheets at room temperature effect of enhanced active sites exposure on reaction path. Appl. Catal. B-Environ. 2022, 319, 121902. [Google Scholar] [CrossRef]
- Mejia, Y.R.; Reddy Bogireddy, N.K. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Song, J.; Zhang, J.; Ma, Y. Recent progress in the electrochemical quantification of nitrophenols. J. Electroanal. Chem. 2023, 938, 117375. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, P.; Xu, X.; Yang, J. Removal of p-nitrophenol by adsorption with 2-phenylimidazole-modified ZIF-8. Molecules 2023, 28, 4195. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial degradation of nitrophenols and their derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Gaware, G.J.; Chinthala, M. Heterojunction photocatalysts for the removal of nitrophenol: A systematic review. Chemosphere 2023, 310, 136853. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Asiri, A.M. Electrochemical detection and catalytic removal of 4-nitrophenol using CeO2-Cu2O and CeO2-Cu2O/CH nanocomposites. Appl. Surf. Sci. 2019, 492, 726–735. [Google Scholar] [CrossRef]
- Galyaltdinov, S.; Svalova, A.; Brusko, V.; Kirsanova, M.; Dimiev, A.M. Nickel on oxidatively modified carbon as a promising cost-efficient catalyst for reduction of p-nitrophenol. Molecules 2022, 27, 5637. [Google Scholar] [CrossRef]
- Feng, J.; Su, L.; Ma, Y.; Ren, C.; Guo, Q.; Chen, X.; Feng, J.; Su, L.; Ma, Y.; Ren, C. CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem. Eng. J. 2013, 221, 16–24. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Elfiad, A.; Galli, F.; Djadoun, A.; Sennour, M.; Chegrouche, S.; Meddour-Boukhobza, L.; Boffito, D.C. Natural alpha-Fe2O3 as an efficient catalyst for the p-nitrophenol reduction. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2018, 229, 126–134. [Google Scholar] [CrossRef]
- Pan, L.; Li, L.; Chen, Y. Synthesis of NiO nanomaterials with various morphologies and their electrocatalytic performances for p-nitrophenol reduction. J. Sol-Gel Sci. Technol. 2012, 62, 364–369. [Google Scholar] [CrossRef]
- Konar, S.; Kalita, H.; Puvvada, N.; Tantubay, S.; Pathak, A. Shape-dependent catalytic activity of CuO nanostructures. J. Catal. 2016, 336, 11–22. [Google Scholar] [CrossRef]
- Che, W.; Ni, Y.; Zhang, Y.; Ma, Y. Morphology-controllable synthesis of CuO nanostructures and their catalytic activity for the reduction of 4-nitrophenol. J. Phys. Chem. Solids 2015, 77, 1–7. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Wi-Afedzi, T.; Liu, Y.T.; Ghanbari, F.; Lin, K.Y.A. Cobalt oxides with various 3D nanostructured morphologies for catalytic reduction of 4-nitrophenol: A comparative study. J. Water Process Eng. 2020, 37, 101379. [Google Scholar] [CrossRef]
- Mogudi, B.M.; Ncube, P.; Meijboom, R. Catalytic activity of mesoporous cobalt oxides with controlled porosity and crystallite sizes: Evaluation using the reduction of 4-nitrophenol. Appl. Catal. B-Environ. 2016, 198, 74–82. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A-Gen. 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Pacchioni, S.; Antonio Ruiz, P.; Gianfranco, P. Role of metal/oxide interfaces in enhancing the local oxide reducibility. Top. Catal. 2019, 62, 1192–1201. [Google Scholar]
- Mogudi, B.M.; Ncube, P.; Bingwa, N.; Mawila, N.; Mathebula, S.; Meijboom, R. Promotion effects of alkali- and alkaline earth metals on catalytic activity of mesoporous Co3O4 for 4-nitrophenol reduction. Appl. Catal. B-Environ. 2017, 218, 240–248. [Google Scholar] [CrossRef]
- Rabee, A.I.M.; Gaid, C.B.A.; Mekhemer, G.A.H.; Zaki, M.I. Combined TPR, XRD, and FTIR studies on the reduction behavior of Co3O4. Mater. Chem. Phys. 2022, 289, 126367. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladium/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742–1748. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Chen, K.; Zhang, X.; Shen, C.; Yuan, L. A rod-like Co3O4 with high efficiency and large specific surface area for lean methane catalytic oxidation. Mol. Catal. 2022, 522, 112229. [Google Scholar] [CrossRef]
- Hu, L.; Liu, X.; Guo, A. Cobalt with porous carbon architecture: Towards of 4-nitrophenol degradation and reduction. Sep. Purif. Technol. 2022, 288, 120595. [Google Scholar] [CrossRef]
- Triveni, R.M.; Buvaneswari, G. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J. Mol. Catal. A-Chem. 2011, 350, 9–15. [Google Scholar]
- Hu, W.; Shao, Z.J.; Cao, X.M.; Hu, P. Multi sites vs. single site for catalytic combustion of methane over Co3O4: A first-principles kinetic Monte Carlo study. Chin. J. Catal. 2020, 41, 1369–1377. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic assemblies for catalytic reduction of nitrophenols: A critical review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, H.; He, Z.; Chen, J.; Song, S. In situ formation of nitrogen-doped carbon-wrapped Co3O4 enabling highly efficient and stable catalytic reduction of p-nitrophenol. Chem. Commun. 2020, 56, 770–773. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Xiang, X.; Sun, K.; Liu, F.; Cai, C.; Han, S.; Xie, Y.; Li, S.; Zu, X. From Ni(OH)2/Graphene composite to Ni@Graphene core-shell: A self-catalyzed epitaxial growth and enhanced activity for nitrophenol reduction. Carbon 2017, 117, 192–200. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, S.; Naskar, M.K. Synthesis of morphology controllable porous Co3O4 nanostructures with tunable textural properties and their catalytic application. Dalton Trans. 2014, 43, 10248–10257. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Ben Tahar, L.; Harrath, A.H. Synthesis of ultra-small gold nanoparticles by polyphenol extracted from Salvia officinalis and efficiency for catalytic reduction of p-nitrophenol and methylene blue. Green Chem. Lett. Rev. 2020, 13, 18–26. [Google Scholar] [CrossRef]
- Swain, S.; Bhavya, M.B.; Kandathil, V.; Bhol, P.; Patil, S.A. Controlled synthesis of palladium nanocubes as an efficient nanocatalyst for Suzuki-Miyaura cross-coupling and reduction of p-nitrophenol. Langmuir 2020, 36, 5208–5218. [Google Scholar] [CrossRef] [PubMed]
- Harika, V.K.; Sadhanala, H.K.; Perelshtein, I.; Gedanken, A. Sonication-assisted synthesis of bimetallic Hg/Pd alloy nanoparticles for catalytic reduction of nitrophenol and its derivatives. Ultrason. Sonochem. 2019, 60, 104804. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Ma, Y.; Xu, C.; Zhou, S. Au-CuxOy nanoparticles encapsulated in hollow porous silica nanospheres as efficient catalysts for nitrophenol reduction. ACS Appl. Nano Mater. 2022, 6, 461–468. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Bustamante, T.M.; Torres, C.C.; Campos, C.H. Gold nanoparticles supported on mesostructured oxides for the enhanced catalytic reduction of 4-nitrophenol in water. Catal. Today 2020, 388–389, 383–393. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, X. PdCu alloy prepared by ultrasonic method catalyzes the degradation of p-nitrophenol. Environ. Sci. Pollut. Res. 2023, 30, 48449–48459. [Google Scholar] [CrossRef]
- Chernykh, M.; Mikheeva, N.; Zaikovskii, V.; Salaev, M.; Mamontov, G. Room-temperature nitrophenol reduction over Ag-CeO2 catalysts: The role of catalyst preparation method. Catalysts 2020, 10, 580. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Xu, Z.; Hu, J.; Tang, A.; Zuo, X. Mineral-modulated Co catalyst with enhanced adsorption and dissociation of BH4- for hydrogenation of p-nitrophenol to p-aminophenol. Chemosphere 2022, 291, 132871. [Google Scholar] [CrossRef]
- Dou, S.; Zhou, S.; Huang, H.; Yan, P.; Shoko, E.; Isimjan, T.T.; Yang, X. Metal-organic framework (MOF)-derived electron-transfer enhanced homogeneous PdO-rich Co3O4 as a highly efficient bifunctional catalyst for sodium borohydride hydrolysis and 4-nitrophenol reduction. Chemistry 2020, 26, 16923–16931. [Google Scholar] [CrossRef]
- Zoltowska, S.; Minambres, J.F.; Piasecki, A.; Mertens, F.; Jesionowski, T. Three-dimensional commercial-sponge-derived Co3O4@C catalysts for effective treatments of organic contaminants. J. Environ. Chem. Eng. 2021, 9, 105631. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H.; Fujishima, A.; Terashima, C. Nanoflakes-like nickel cobaltite as active electrode material for 4-nitrophenol reduction and supercapacitor applications. J. Hazard. Mater. 2021, 419, 126453. [Google Scholar] [CrossRef] [PubMed]
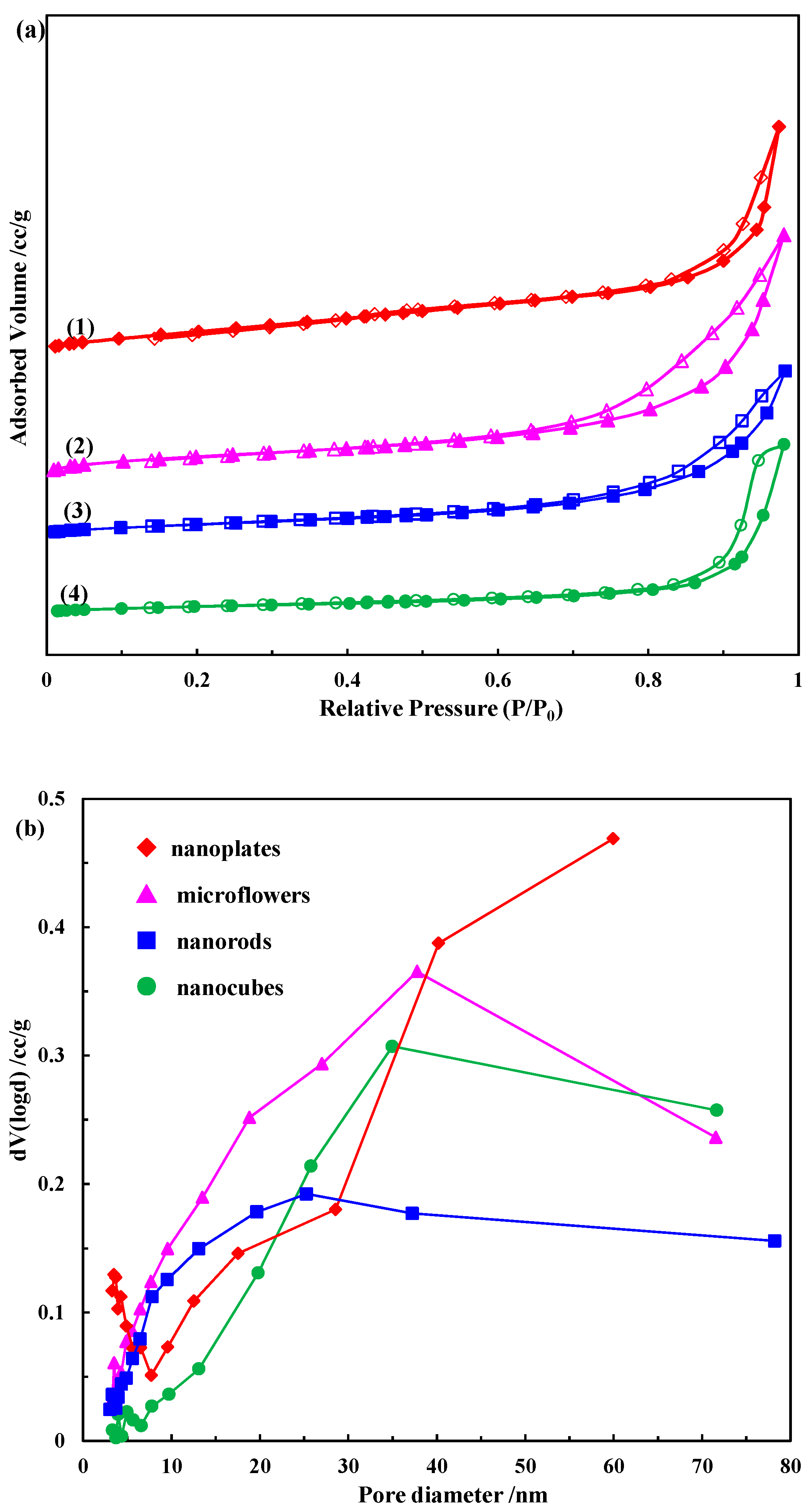


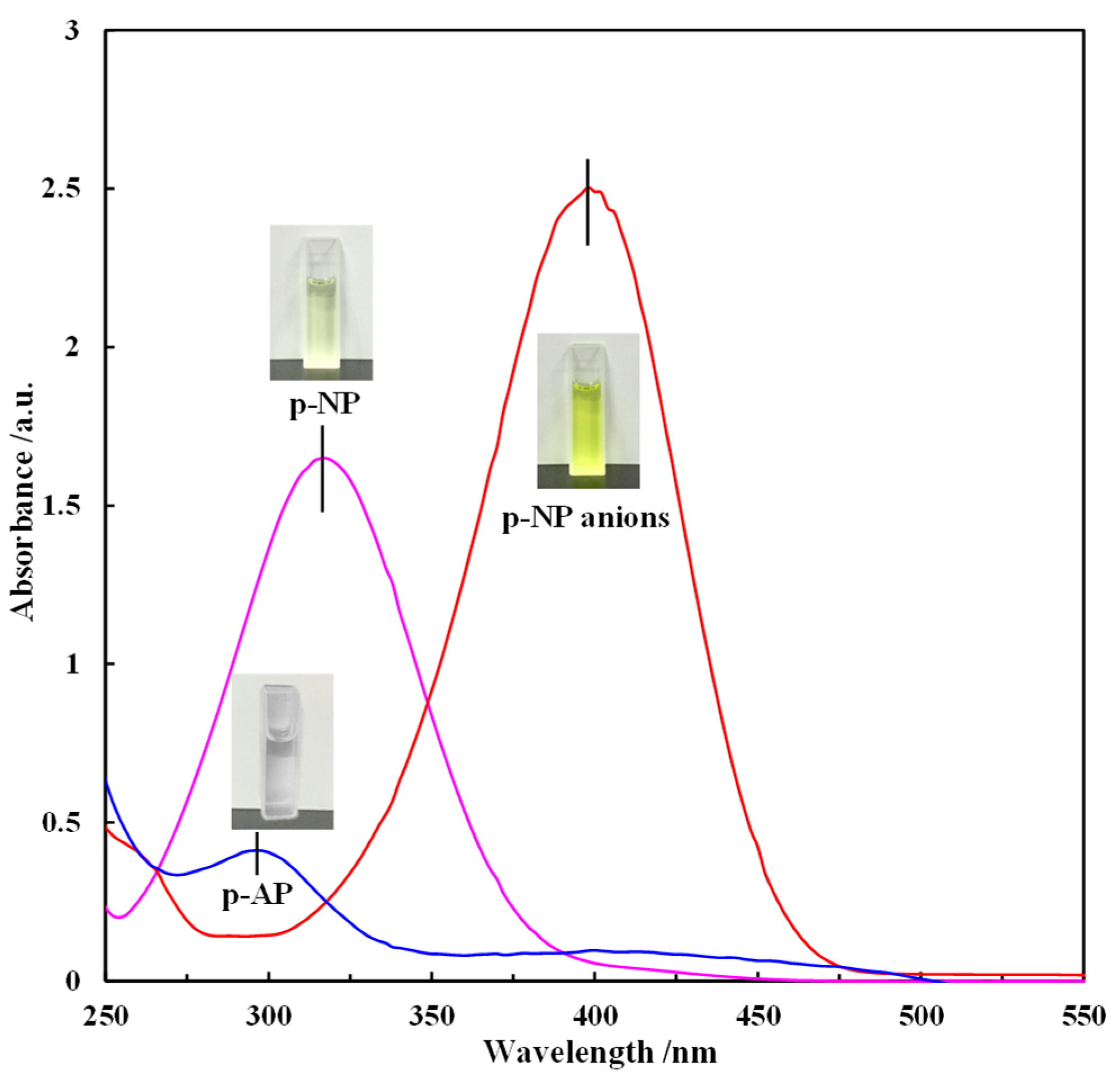
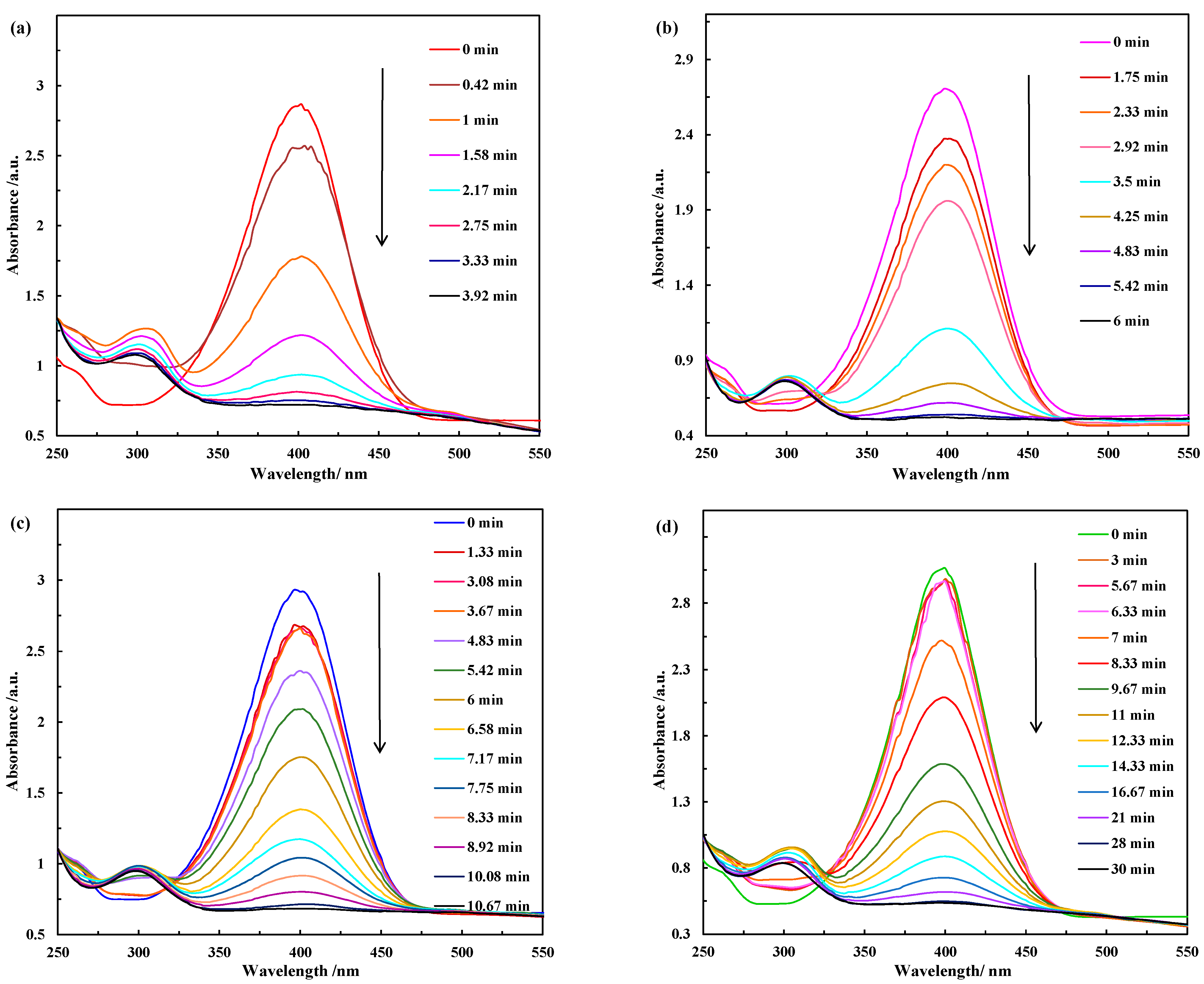
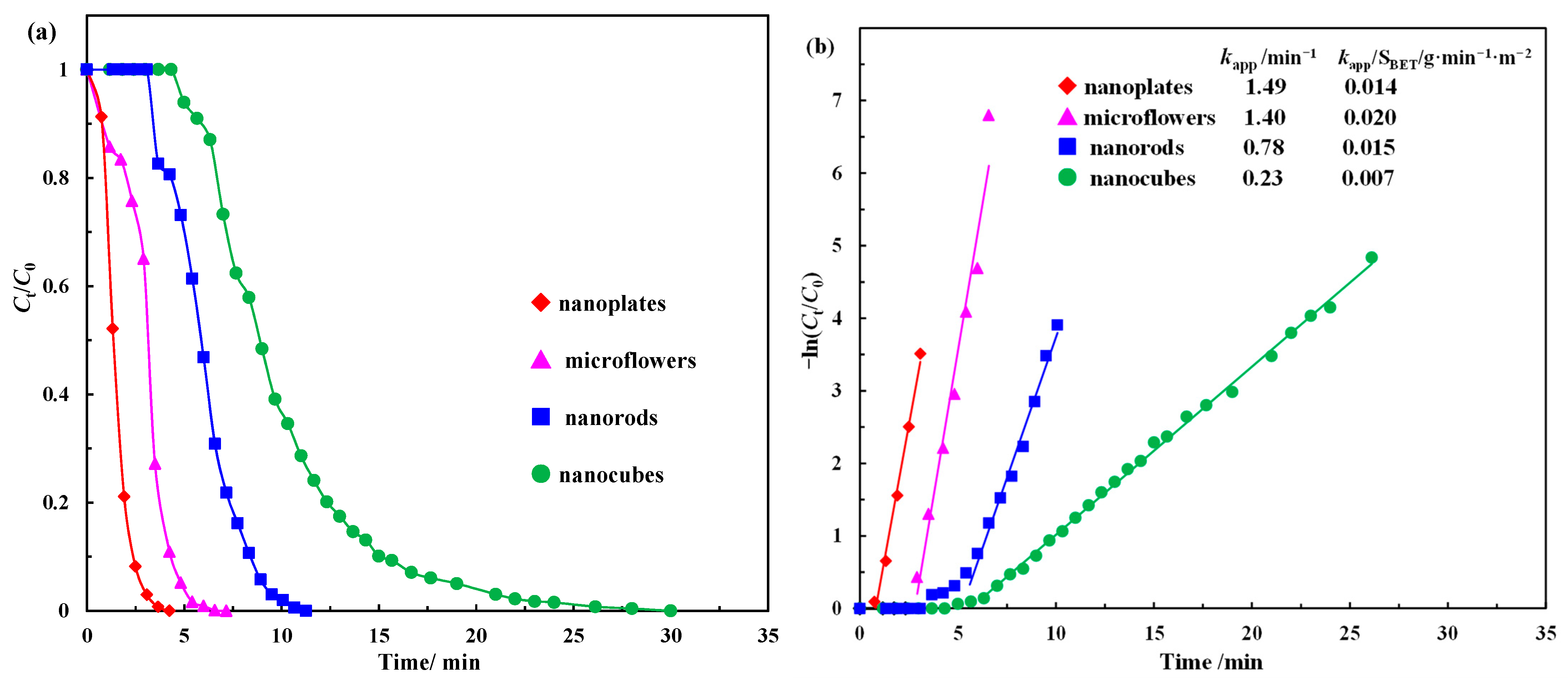
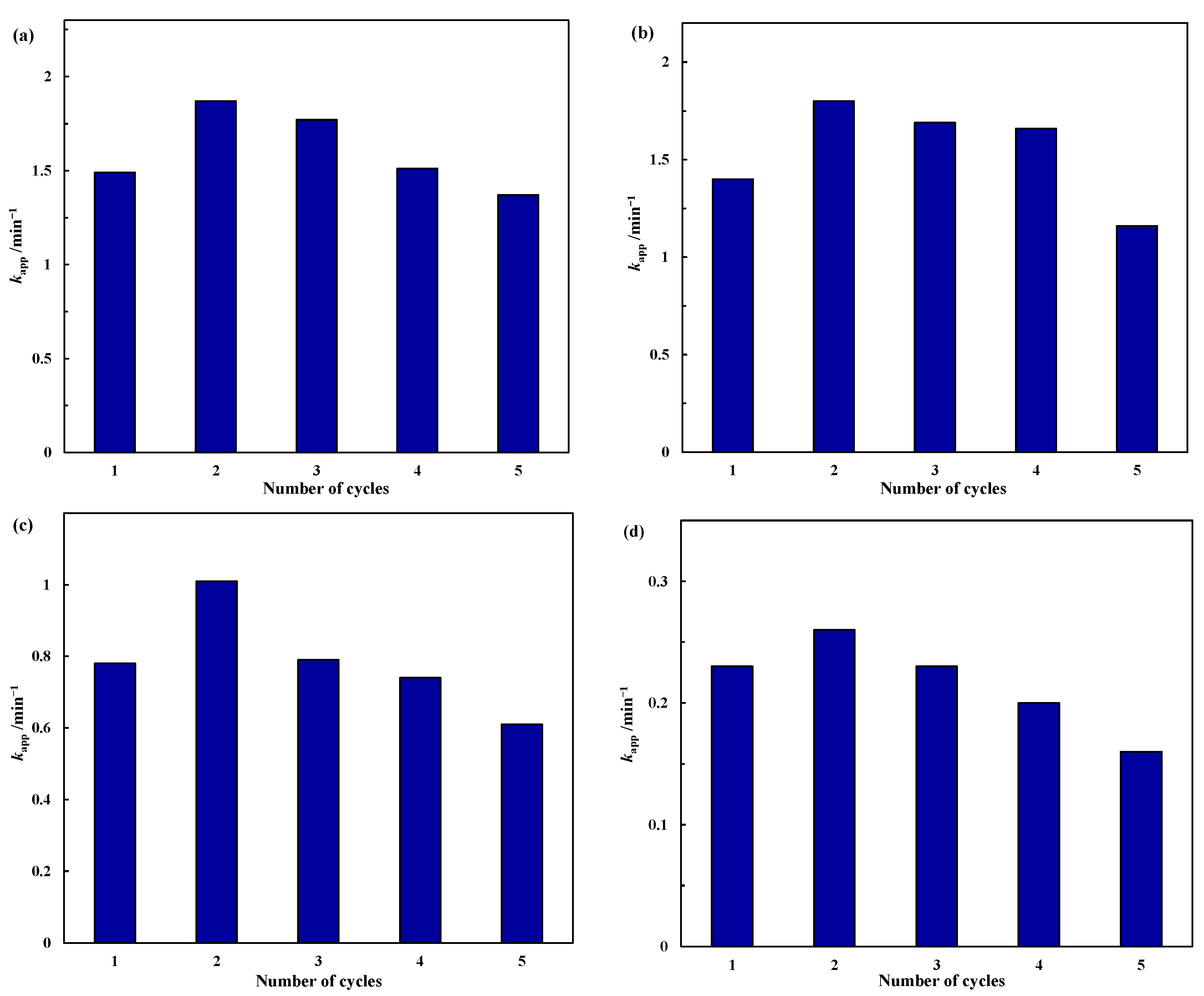
| Catalyst | BET Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Nanoplates | 106.3 | 0.27 | 3.5 |
| Microflowers | 69.3 | 0.30 | 3.5 |
| Nanorods | 51.2 | 0.21 | 7.8 |
| Nanocubes | 31.7 | 0.21 | 3.5 |
| Catalyst | Co2+ (mol%) | Co3+ (mol%) | Co3+/Co2+ |
|---|---|---|---|
| Nanoplates | 34.1 | 65.9 | 1.93 |
| Microflowers | 33.8 | 66.2 | 1.96 |
| Nanorods | 33.9 | 66.1 | 1.95 |
| Nanocubes | 33.5 | 66.5 | 1.99 |
| Catalyst | t0/min |
|---|---|
| Nanoplates | t0 < 0.42 |
| Microflowers | t0 < 1.75 |
| Nanorods | 4.25 < t0 < 4.83 |
| Nanocubes | 6.33 < t0 < 7 |
| Catalyst | mcat a | kapp | knor b | SBET | kapp/SBET | Reference |
|---|---|---|---|---|---|---|
| g/L | min−1 | L·min−1·g−1 | m2/g | g·min−1·m−2 | ||
| Au | 0.0034 | 0.1540 | 44.814 | - | - | [51] |
| Pd | 0.0462 | 0.7300 | 15.801 | - | - | [52] |
| Hg/Pd | 1.4286 | 3.5040 | 2.453 | - | - | [53] |
| Au-CuxOy in HPSNs | 0.2500 | 0.9600 | 3.840 | 86.7 | 0.0111 | [54] |
| Au/TNT | 0.3750 | 0.0610 | 0.163 | 124.0 | 0.0005 | [55] |
| PdCu-LDHs | 0.3107 | 1.1000 | 3.540 | - | - | [56] |
| Ag-CeO2 | 0.1200 | 0.6560 | 5.467 | 5.6 | 0.1171 | [57] |
| Co/Eatp@C | 0.0500 | 0.6900 | 13.80 | 236.5 | 0.0029 | [58] |
| PdO-Co3O4 | 0.8333 | 1.3100 | 1.572 | 67.3 | 0.0195 | [59] |
| Co3O4@C | 1.6667 | 0.7550 | 0.453 | 5.1 | 0.1480 | [60] |
| NiCo2O4 | 0.5000 | 0.1260 | 0.252 | 68.4 | 0.0018 | [61] |
| Co3O4 nanoplates | 0.0714 | 1.4900 | 20.868 | 106.3 | 0.0140 | This work |
| Co3O4 microflowers | 0.0714 | 1.4000 | 19.600 | 69.3 | 0.0202 | This work |
| Co3O4 nanorods | 0.0714 | 0.7800 | 10.920 | 51.2 | 0.0152 | This work |
| Co3O4 nanocubes | 0.0714 | 0.2300 | 3.221 | 31.7 | 0.0073 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yang, M.; Liu, Y.; Yue, J.; Chen, G. Influence of Co3O4 Nanostructure Morphology on the Catalytic Degradation of p-Nitrophenol. Molecules 2023, 28, 7396. https://doi.org/10.3390/molecules28217396
Chen H, Yang M, Liu Y, Yue J, Chen G. Influence of Co3O4 Nanostructure Morphology on the Catalytic Degradation of p-Nitrophenol. Molecules. 2023; 28(21):7396. https://doi.org/10.3390/molecules28217396
Chicago/Turabian StyleChen, Huihui, Mei Yang, Yuan Liu, Jun Yue, and Guangwen Chen. 2023. "Influence of Co3O4 Nanostructure Morphology on the Catalytic Degradation of p-Nitrophenol" Molecules 28, no. 21: 7396. https://doi.org/10.3390/molecules28217396
APA StyleChen, H., Yang, M., Liu, Y., Yue, J., & Chen, G. (2023). Influence of Co3O4 Nanostructure Morphology on the Catalytic Degradation of p-Nitrophenol. Molecules, 28(21), 7396. https://doi.org/10.3390/molecules28217396








