Electrochemical Performance of Nitrogen Self-Doping Carbon Materials Prepared by Pyrolysis and Activation of Defatted Microalgae
Abstract
:1. Introduction
2. Results and Conclusions
2.1. Porosity of Microalgae-Based N-Doped Activated Carbon
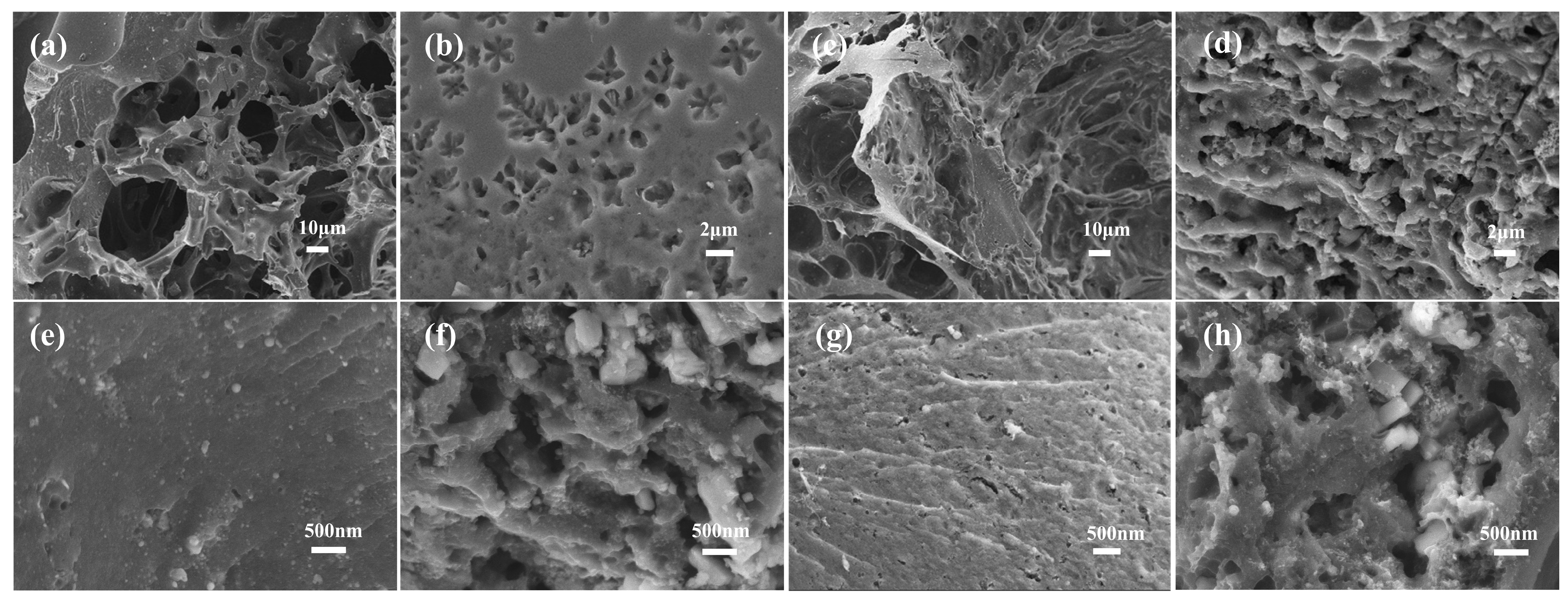
2.2. Morphology of Biochar and Microalgae-Based N-Doped Activated Carbon
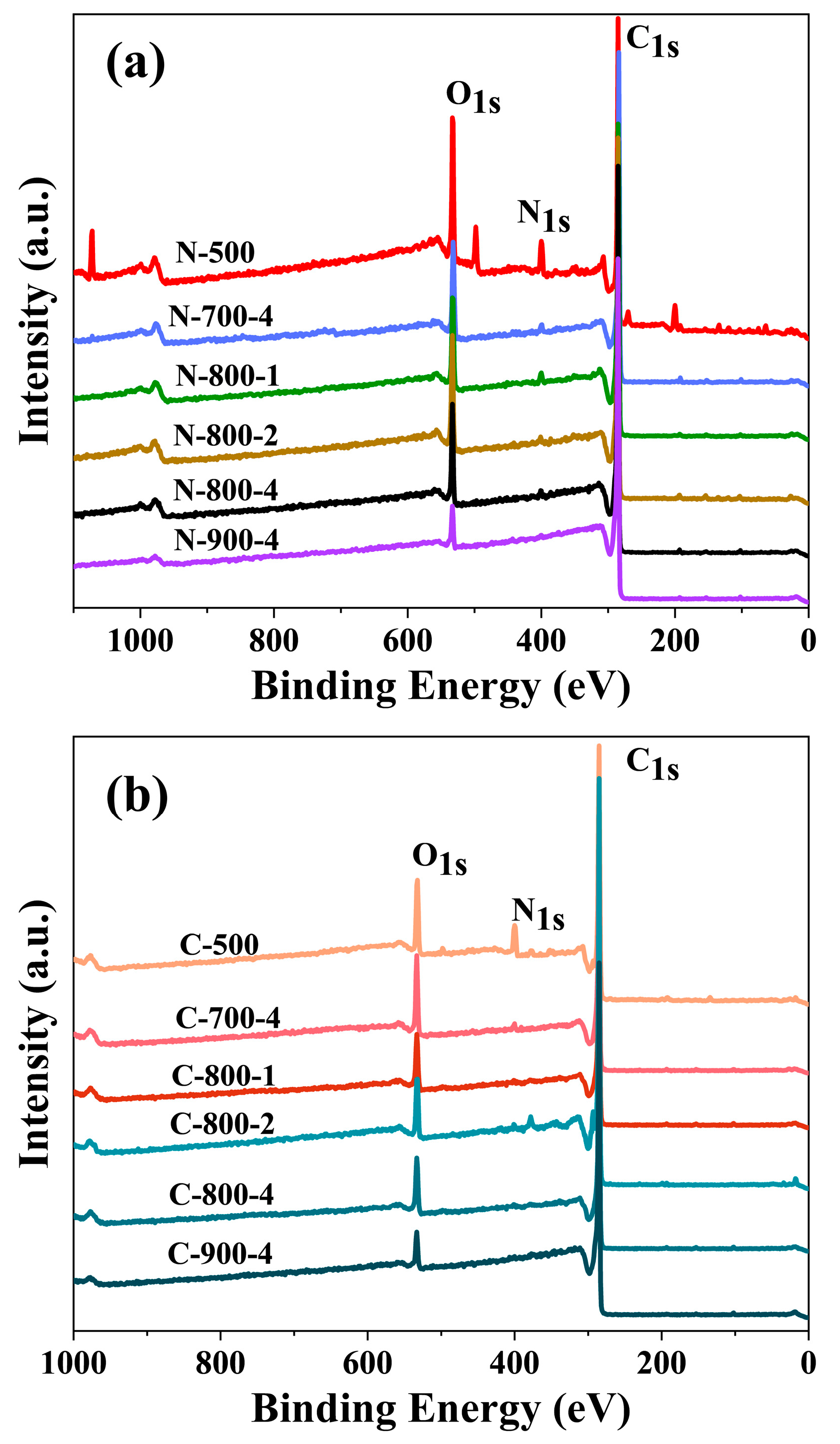
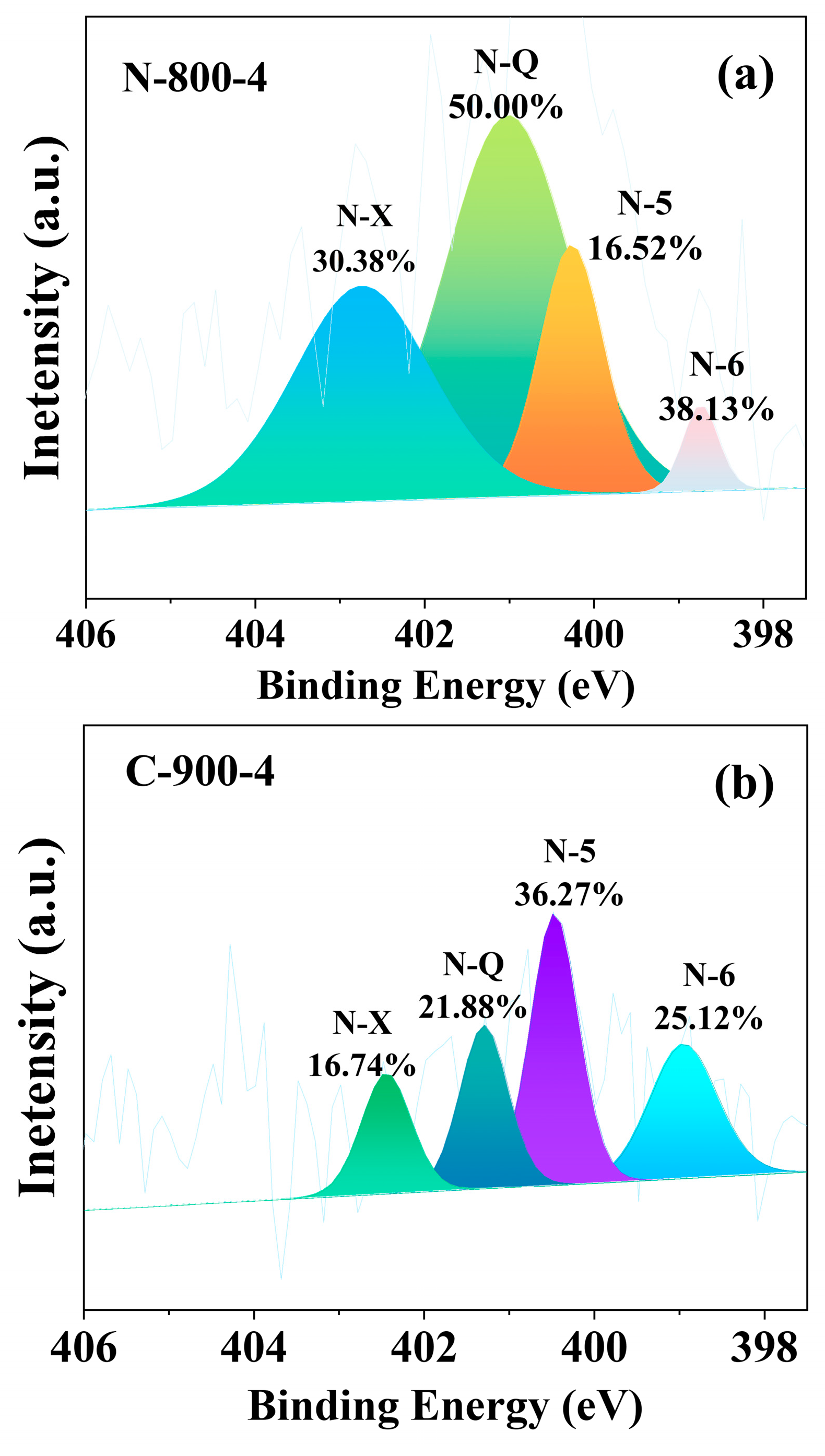
2.3. Elemental Composition of Carbon Materials and Valence States of Nitrogen Atom
2.4. Electrochemical Performance of Microalgae-Based N-Doped Activated Carbon
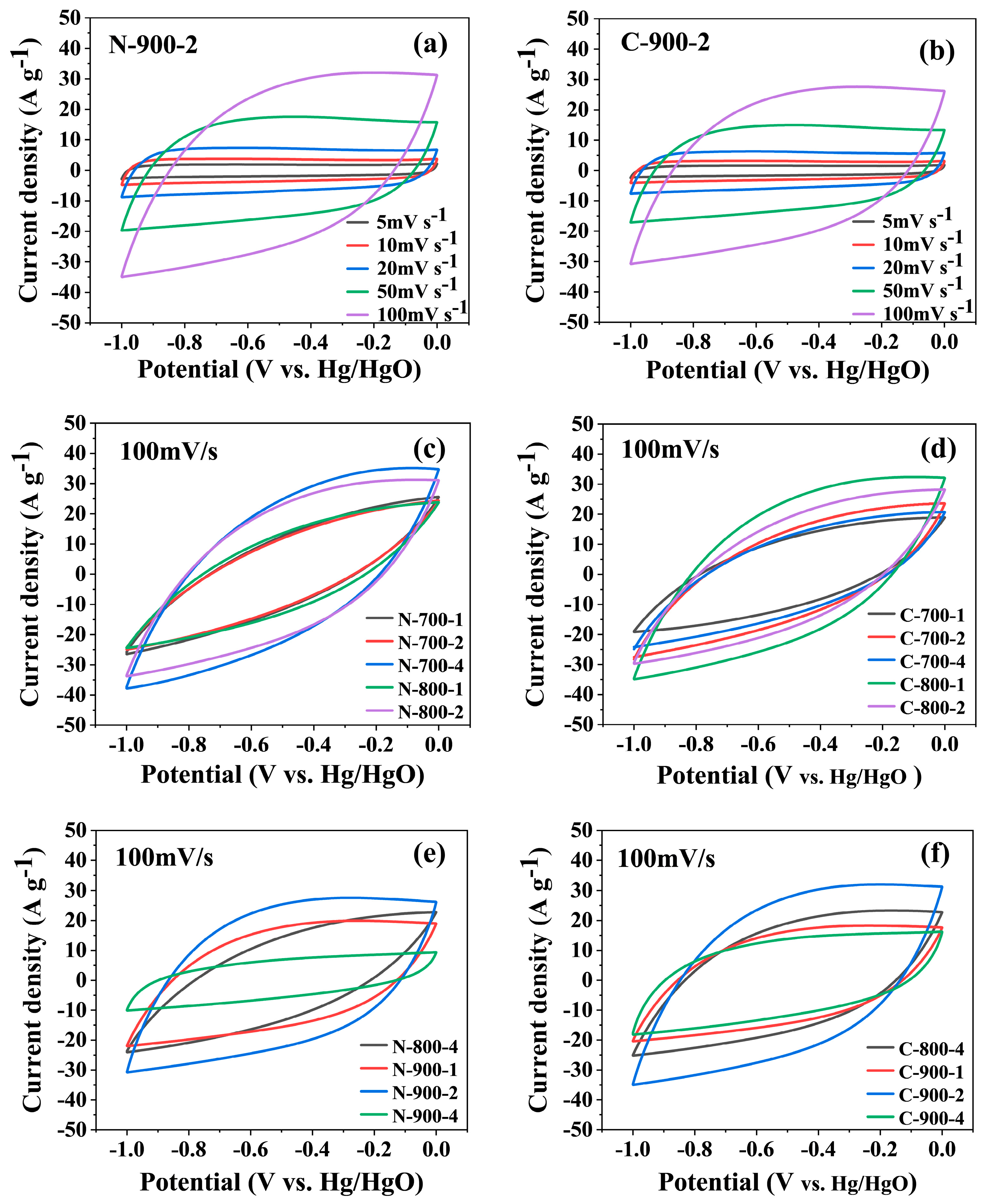
2.4.1. Electrochemical Performance of the Materials in the Three-Electrode System
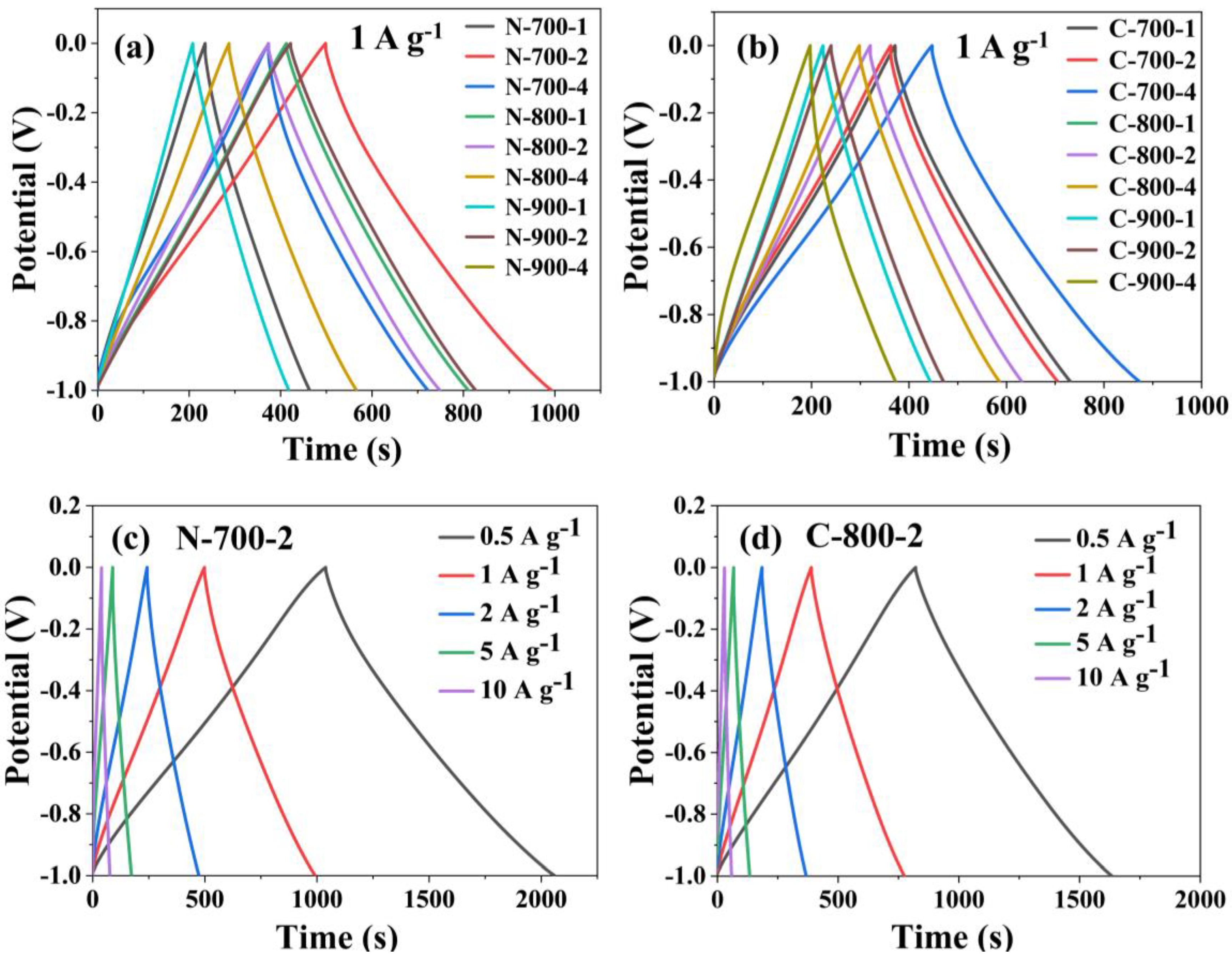
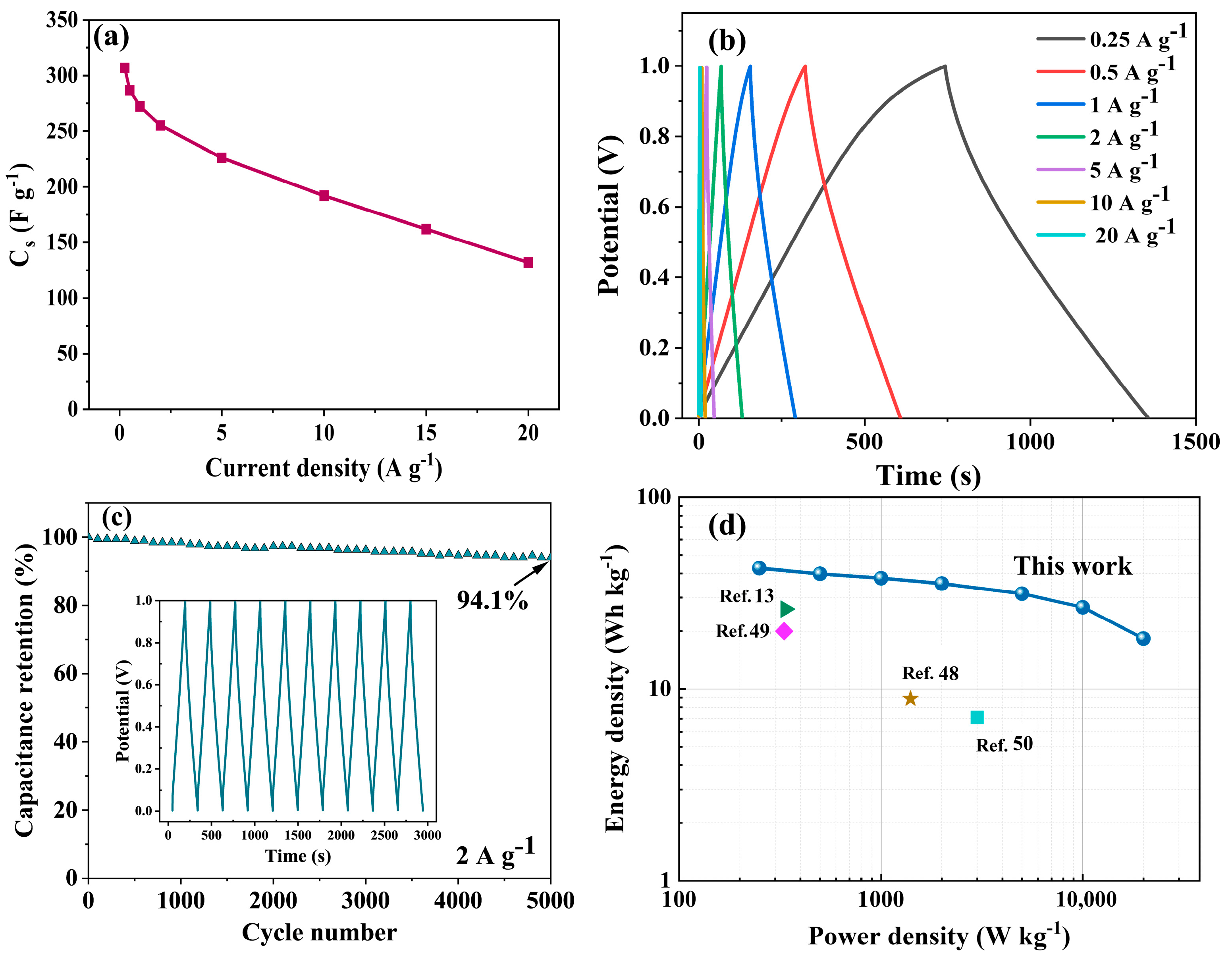
2.4.2. Electrochemical Performance of the Materials in the Two-Electrode System
3. Materials and Methods
3.1. Microalgae and Lipid Extraction
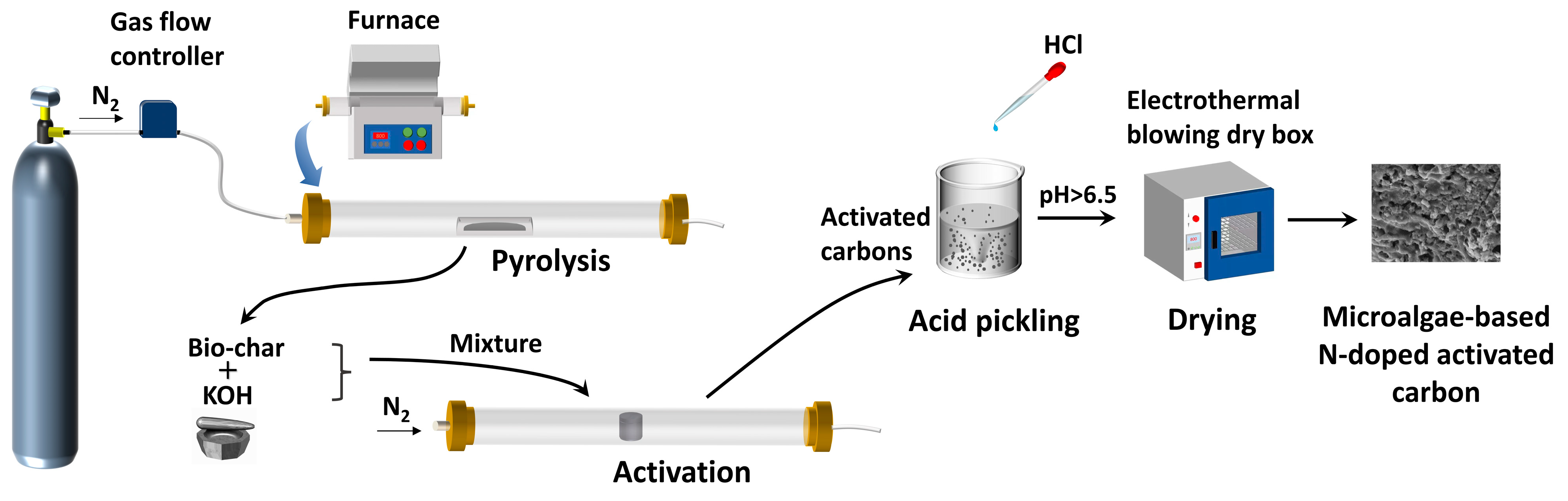
3.2. Pyrolysis of Microalgae and Activation of Biochar
3.3. Characterization of Biochar and Microalgae-Based N-Doped Activated Carbon
3.4. Electrochemical Measurements of Microalgae-Based N-Doped Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, J.; Norouzi, O.; Mašek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Shi, S.; Ochedi, F.O.; Yu, J.; Liu, Y. Porous Biochars Derived from Microalgae Pyrolysis for CO2 Adsorption. Energy Fuels 2021, 35, 7646–7656. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, S.; Wang, L.; Li, C.; Meng, F.; Wang, N.; Zhou, S.; Xiong, Y.; Wang, Z.; Wu, Y.; et al. Recent Advances in Porous Carbon Materials as Electrodes for Supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Fang, B.; van Heuveln, F.; Dias, F.; Plomp, L. Electric double-layer capacitor based on activated carbon material. Rare Metals 2000, 19, 1–10. [Google Scholar]
- Fang, B.; Wei, Y.-Z.; Suzuki, K.; Kumagai, M. Surface modification of carbonaceous materials for EDLCs application. Electrochim. Acta 2005, 50, 3616–3621. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Jia, C.; Mu, K.; Yu, M.; Lv, Y.; Shao, Z. Nitrogen and oxygen-codoped carbon nanospheres for excellent specific capacitance and cyclic stability supercapacitor electrodes. Chem. Eng. J. 2017, 330, 1166–1173. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, T.; Kim, J.H.; Ko, J.M. Nanosheets based mesoporous NiO microspherical structures via facile and template-free method for high performance supercapacitors. Electrochim. Acta 2011, 56, 4849–4857. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Catanach, J.; Gomez, J.; Richins, S.; Deng, S. Effects of nanoporous carbon derived from microalgae and its CoO composite on capacitance. ACS Appl. Mater. Interfaces 2017, 9, 4362–4373. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Gu, W.; Falco, C.; Titirici, M.M.; Fuertes, A.B.; Yushin, G. Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J. Power Sources 2014, 267, 26–32. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef]
- Yang, D.-S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.-S. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef]
- Jurewicz, K.; Babeł, K.; Źiółkowski, A.; Wachowska, H. Ammoxidation of active carbons for improvement of supercapacitor characteristics. Electrochim. Acta 2003, 48, 1491–1498. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Tian, Z.; Xiang, M.; Zhou, J.; Hu, L.; Cai, J. Nitrogen and oxygen-doped hierarchical porous carbons from algae biomass: Direct carbonization and excellent electrochemical properties. Electrochim. Acta 2016, 211, 225–233. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of synthetic strategies and properties of heteroatoms-doped (N, P, S, O) carbon materials for supercapacitors. J. Energy Storage 2022, 56, 105995. [Google Scholar] [CrossRef]
- Kim, N.D.; Kim, W.; Joo, J.B.; Oh, S.; Kim, P.; Kim, Y.; Yi, J. Electrochemical capacitor performance of N-doped mesoporous carbons prepared by ammoxidation. J. Power Sources 2008, 180, 671–675. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Zhai, D.D.; Chen, X.Y.; Zhang, Z.J. Design and theoretical study of carbon-based supercapacitors especially exhibiting superior rate capability by the synergistic effect of nitrogen and phosphor dopants. Carbon 2019, 155, 223–232. [Google Scholar] [CrossRef]
- Yu, J.; Maliutina, K.; Tahmasebi, A. A review on the production of nitrogen-containing compounds from microalgal biomass via pyrolysis. Bioresour. Technol. 2018, 270, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Maliutina, K.; Tahmasebi, A.; Yu, J. The transformation of nitrogen during pressurized entrained-flow pyrolysis of Chlorella vulgaris. Bioresour. Technol. 2018, 262, 90–97. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Yin, X.; Cao, J.; Shen, Z.; Wu, C. Step pyrolysis of N-rich industrial biowastes: Regulatory mechanism of NOx precursor formation via exploring decisive reaction pathways. Chem. Eng. J. 2018, 344, 320–331. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Chen, Y.; Yang, H.; Chen, Z.; Chen, X.; Chen, H. Influence of NH3 concentration on biomass nitrogen-enriched pyrolysis. Bioresour. Technol. 2018, 263, 350–357. [Google Scholar] [CrossRef]
- Chun, S.-E.; Whitacre, J. The evolution of electrochemical functionality of carbons derived from glucose during pyrolysis and activation. Electrochim. Acta 2012, 60, 392–400. [Google Scholar] [CrossRef]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Hu, L.; Huang, S.; Cai, J. Macroalgae-derived nitrogen-doped hierarchical porous carbons with high performance for H2 storage and supercapacitors. RSC Adv. 2017, 7, 32795–32805. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Calo, J.; Cazorla-Amorós, D.; Linares-Solano, A. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 2007, 45, 2529–2536. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.; Sevilla, M.; White, R.J.; Rothe, R.; Titirici, M.-M. Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem 2012, 5, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Charge storage mechanism in nanoporous carbons and its consequence for electrical double layer capacitors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3457–3467. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Kim, W.; Joo, J.B.; Kim, N.; Oh, S.; Kim, P.; Yi, J. Preparation of nitrogen-doped mesoporous carbon nanopipes for the electrochemical double layer capacitor. Carbon 2009, 47, 1407–1411. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.-M.; Veerakumar, P.; Liu, S.-B. Functional porous carbon/nickel oxide nanocomposites as binder-free electrodes for supercapacitors. Chem. A Eur. J. 2015, 21, 8200–8206. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Shi, L.; Liu, H.; Yang, L.; He, Y. A facile production of microporous carbon spheres and their electrochemical performance in EDLC. J. Phys. Chem. Solids 2012, 73, 385–390. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W.-P. Enhancing the pore wettability of coal-based porous carbon as electrode materials for high performance supercapacitors. Mater. Chem. Phys. 2020, 252, 123381. [Google Scholar] [CrossRef]
- Han, J.; Lee, K.; Choi, M.S.; Park, H.S.; Kim, W.; Roh, K.C. Chlorella-derived activated carbon with hierarchical pore structure for energy storage materials and adsorbents. Carbon Lett. 2019, 29, 167–175. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, B.; Qu, C.; Zhang, H.; Guo, W.; Liang, Z.; Chen, F.; Zou, R. Tailoring biomass-derived carbon for high-performance supercapacitors from controllably cultivated algae microspheres. J. Mater. Chem. A 2017, 6, 1523–1530. [Google Scholar] [CrossRef]
- Ren, M.; Jia, Z.; Tian, Z.; Lopez, D.; Cai, J.; Titirici, M.; Jorge, A.B. High Performance N-Doped Carbon Electrodes Obtained via Hydrothermal Carbonization of Macroalgae for Supercapacitor Applications. ChemElectroChem 2018, 5, 2686–2693. [Google Scholar] [CrossRef]
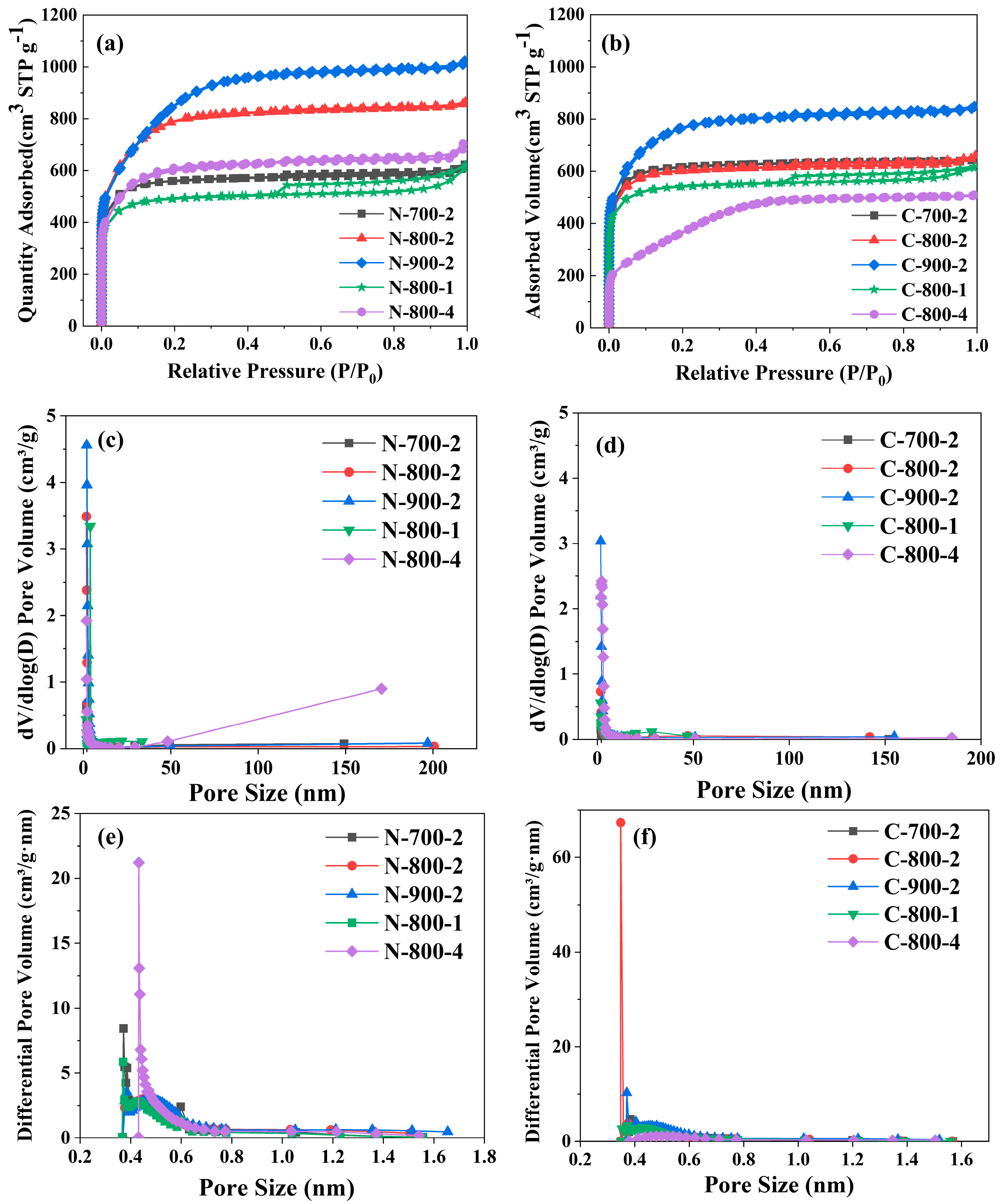

| Microalgae Species | Sample | SBET (m2/g) | Vt a (cm3/g) | Dd b (nm) | Smic c (m2/g) | Vmic d (cm3/g) | Micropore Surface Area (%) | Micropore Volume (%) |
|---|---|---|---|---|---|---|---|---|
| Nanochloropsis | N-700-2 | 2134.73 | 0.91 | 3.66 | 1803.75 | 0.72 | 84.50 | 78.62 |
| N-900-2 | 3186.74 | 1.54 | 2.32 | 2055.13 | 0.76 | 64.49 | 49.47 | |
| N-800-1 | 1898.70 | 0.86 | 4.42 | 1593.00 | 0.62 | 83.90 | 72.15 | |
| N-800-2 | 2946.12 | 1.31 | 2.30 | 1745.69 | 0.68 | 59.25 | 52.12 | |
| N-800-4 | 2223.03 | 1.00 | 3.00 | 1366.59 | 0.56 | 61.47 | 55.66 | |
| Chlorella | C-700-2 | 2397.06 | 0.99 | 2.80 | 2149.50 | 0.84 | 89.67 | 85.30 |
| C-900-2 | 2815.04 | 1.29 | 2.34 | 1365.55 | 0.54 | 48.51 | 41.79 | |
| C-800-1 | 2099.36 | 0.92 | 3.90 | 1790.52 | 0.70 | 85.29 | 75.91 | |
| C-800-2 | 2495.37 | 0.98 | 3.41 | 2028.98 | 0.77 | 81.03 | 78.14 | |
| C-800-4 | 1317.35 | 0.78 | 2.39 | 624.63 | 0.22 | 47.42 | 28.48 |
| Sample | Element Content (at %) | ||
|---|---|---|---|
| C | O | N | |
| N-500 | 56.36 | 14.56 | 6.23 |
| N-700-4 | 84.89 | 13.05 | 2.05 |
| N-800-1 | 85.83 | 11.55 | 2.62 |
| N-800-4 | 87.14 | 10.77 | 2.09 |
| N-800-2 | 85.04 | 13.07 | 1.90 |
| N-900-4 | 93.66 | 5.11 | 1.23 |
| C-500 | 77.69 | 12.65 | 9.38 |
| C-700-4 | 85.99 | 12.08 | 1.93 |
| C-800-1 | 92.11 | 7.64 | 0.25 |
| C-800-4 | 86.05 | 12.47 | 1.53 |
| C-800-2 | 89.90 | 9.83 | 0.27 |
| C-900-4 | 93.32 | 5.44 | 1.24 |
| Sample | Quaternary-N | Pyrrolic-N | Pyridinic-N | Pyridine-N-Oxide |
|---|---|---|---|---|
| N-500 | 57.28 | 26.21 | 14.56 | - |
| N-700-4 | 45.80 | 35.56 | 18.46 | - |
| N-800-1 | 40.00 | 10.48 | 29.85 | 19.66 |
| N-800-4 | 50.00 | 16.52 | 3.10 | 30.38 |
| N-800-2 | 82.84 | 14.88 | 2.92 | - |
| N-900-4 | 87.57 | 4.68 | 7.75 | - |
| C-500 | 28.81 | 34.91 | 36.28 | - |
| C-700-4 | 35.52 | 16.17 | 36.81 | 11.51 |
| C-800-1 | 84.23 | 4.48 | 11.29 | - |
| C-800-4 | 8.22 | 57.38 | 26.88 | 7.52 |
| C-800-2 | 43.03 | 22.83 | 14.27 | 19.43 |
| C-900-4 | 21.88 | 36.27 | 25.12 | 16.74 |
| Sample | Cm (F g−1) | Sample | Cm (F g−1) |
|---|---|---|---|
| N-700-1 | 234.94 | C-700-1 | 368.46 |
| N-700-2 | 501.87 | C-700-2 | 351.87 |
| N-700-4 | 358.01 | C-700-4 | 432.33 |
| N-800-1 | 403.30 | C-800-1 | 318.95 |
| N-800-2 | 380.74 | C-800-2 | 391.20 |
| N-800-4 | 283.45 | C-800-4 | 293.78 |
| N-900-1 | 213.69 | C-900-1 | 224.04 |
| N-900-2 | 408.85 | C-900-2 | 233.84 |
| N-900-4 | 243.43 | C-900-4 | 178.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zuo, L.; Wang, Y.; Zhen, M.; Xu, L.; Kong, W.; Shen, B. Electrochemical Performance of Nitrogen Self-Doping Carbon Materials Prepared by Pyrolysis and Activation of Defatted Microalgae. Molecules 2023, 28, 7280. https://doi.org/10.3390/molecules28217280
Wang X, Zuo L, Wang Y, Zhen M, Xu L, Kong W, Shen B. Electrochemical Performance of Nitrogen Self-Doping Carbon Materials Prepared by Pyrolysis and Activation of Defatted Microalgae. Molecules. 2023; 28(21):7280. https://doi.org/10.3390/molecules28217280
Chicago/Turabian StyleWang, Xin, Lu Zuo, Yi Wang, Mengmeng Zhen, Lianfei Xu, Wenwen Kong, and Boxiong Shen. 2023. "Electrochemical Performance of Nitrogen Self-Doping Carbon Materials Prepared by Pyrolysis and Activation of Defatted Microalgae" Molecules 28, no. 21: 7280. https://doi.org/10.3390/molecules28217280
APA StyleWang, X., Zuo, L., Wang, Y., Zhen, M., Xu, L., Kong, W., & Shen, B. (2023). Electrochemical Performance of Nitrogen Self-Doping Carbon Materials Prepared by Pyrolysis and Activation of Defatted Microalgae. Molecules, 28(21), 7280. https://doi.org/10.3390/molecules28217280








