Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM)
Abstract
:1. Introduction
- It reduces the solid residues, of which disposal methods and costs constitute a major problem and;
- It gives a new life to these wastes by converting them into useful and inexpensive decontaminants for water purification.
2. Results and Discussion
2.1. Characterization of Biosorbents
2.1.1. Scanning Electron Microscopy (SEM)
2.1.2. X-ray Diffraction (XRD)
2.1.3. Infrared Absorption Spectroscopy (IRTF)
2.2. Adsorbent Performance Study toward Cr (VI) Adsorption Experiments
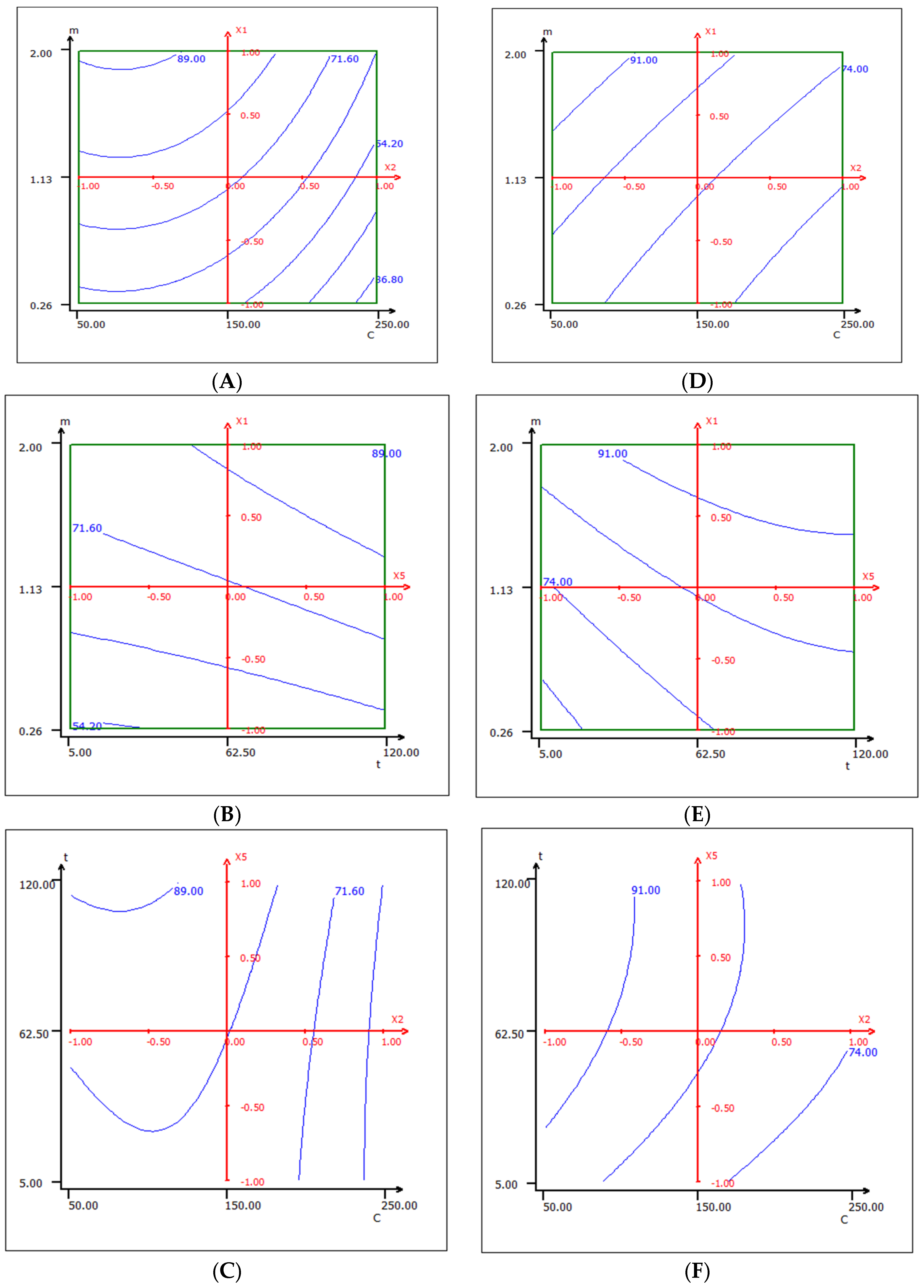
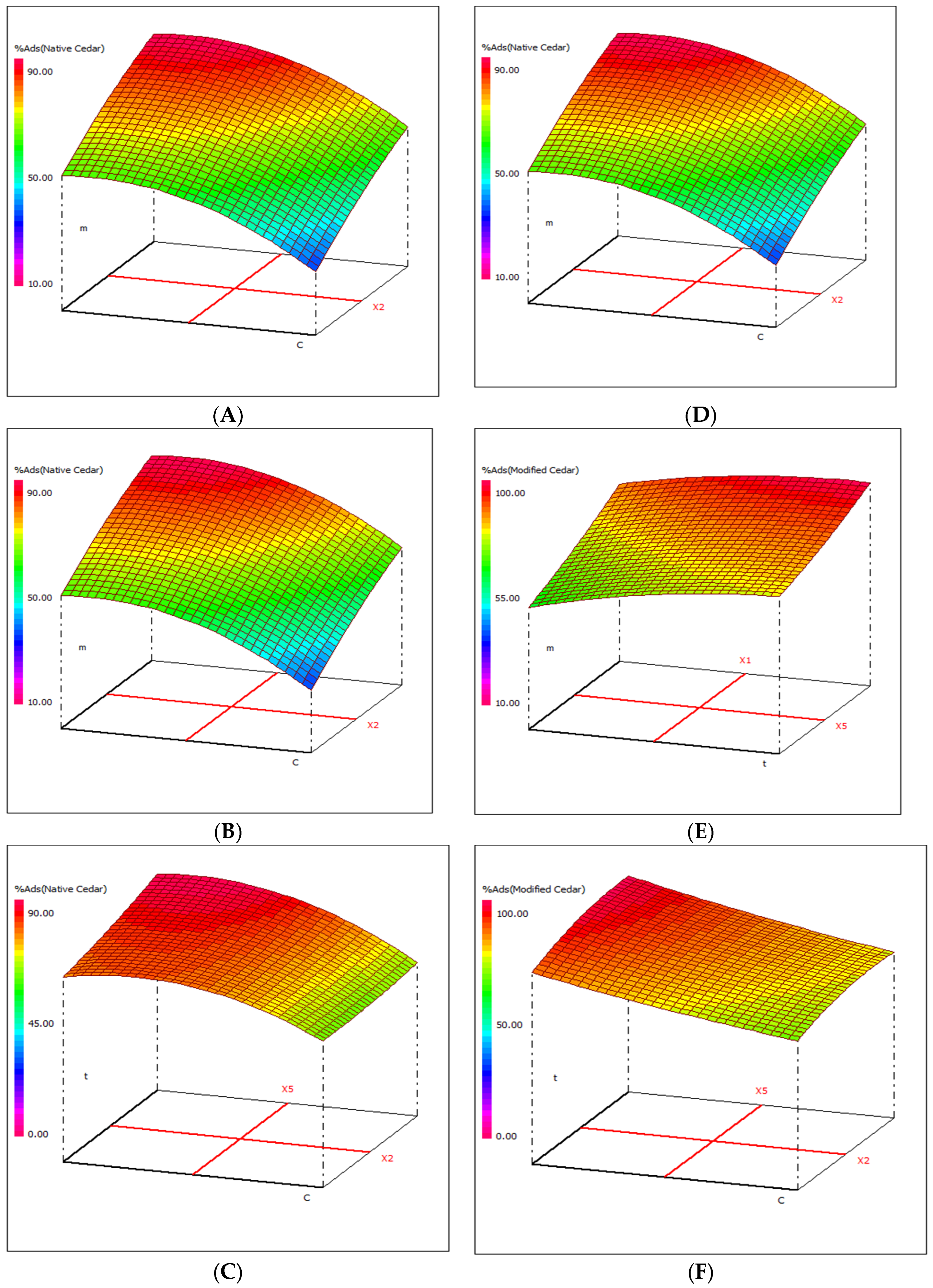
2.2.1. Experimental Design and Data Analysis via RSM
- In the case of native cedar: pH, temperature, contact time, adsorbent mass, and initial concentration of Cr (VI), and the interaction of two variables—initial concentration and contact time—are the most important.
- In the case of modified cedar: pH, temperature, contact time, initial concentration of Cr (VI), and adsorbent mass, and the interaction of two variables—adsorbent mass and initial concentration—are the most important.
2.2.2. Statistical Analysis and Validation of the Model
2.2.3. Optimization of Studied Parameters via the CCD of RSM
- The first stage is characterized by very rapid adsorption during the first 30 min in the case of native sawdust with an average adsorption rate of 55%, and during the first 25 min for activated sawdust with an average adsorption rate of 70%.
- In the second stage, the adsorption becomes increasingly slow for both sawdusts.
- The third stage is characterized by the establishment of a level that illustrates the adsorption equilibrium resulting from the saturation of the active adsorption sites, at 90 min for native cedar sawdust with an average adsorption rate of 71% and at 60 min for activated sawdust with an average adsorption rate of 97%.
2.2.4. Experimental Validity Test: Test Point
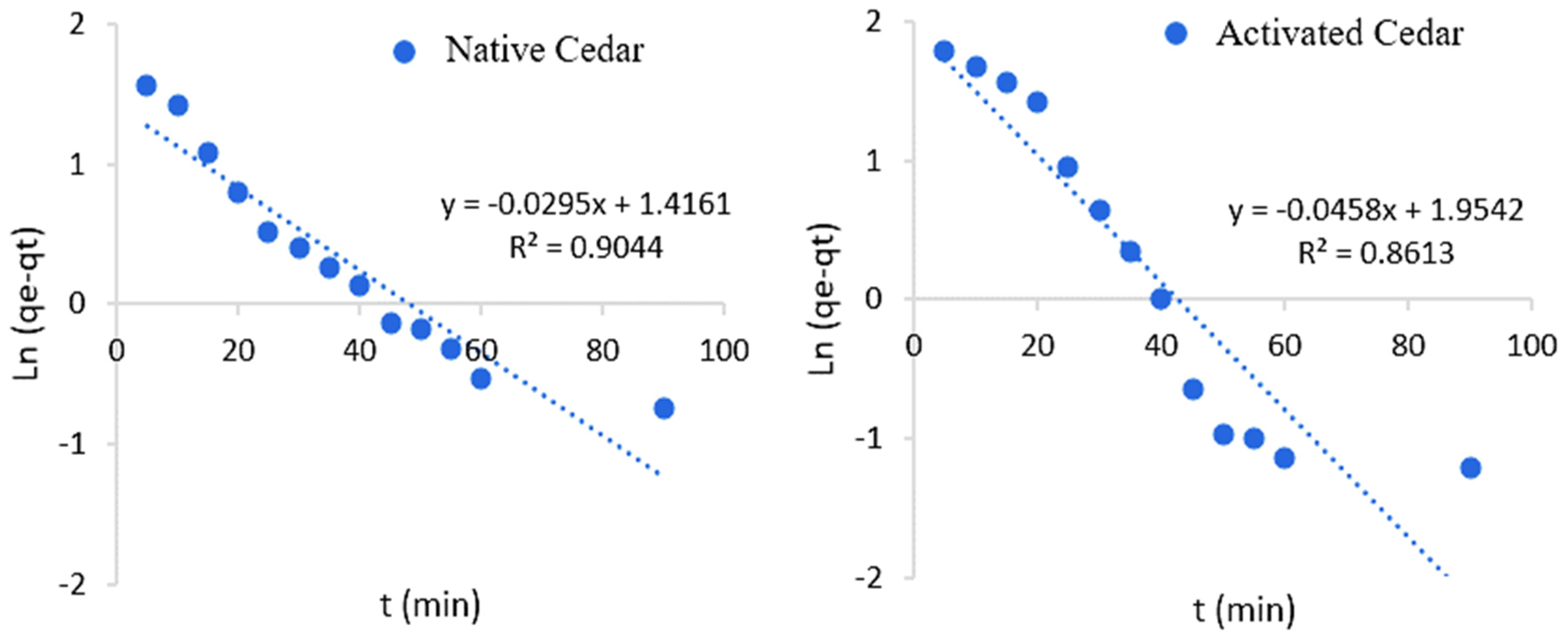
2.2.5. Kinetics of Cr (VI) Adsorption
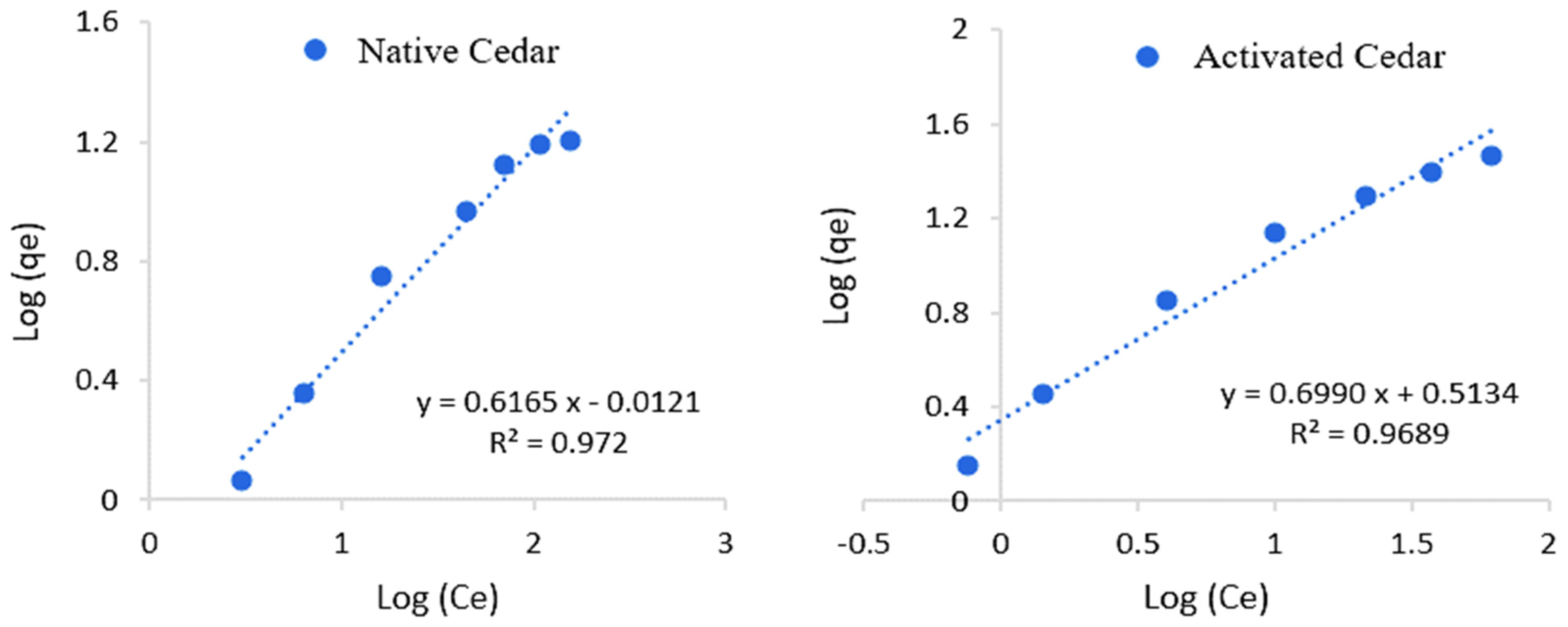
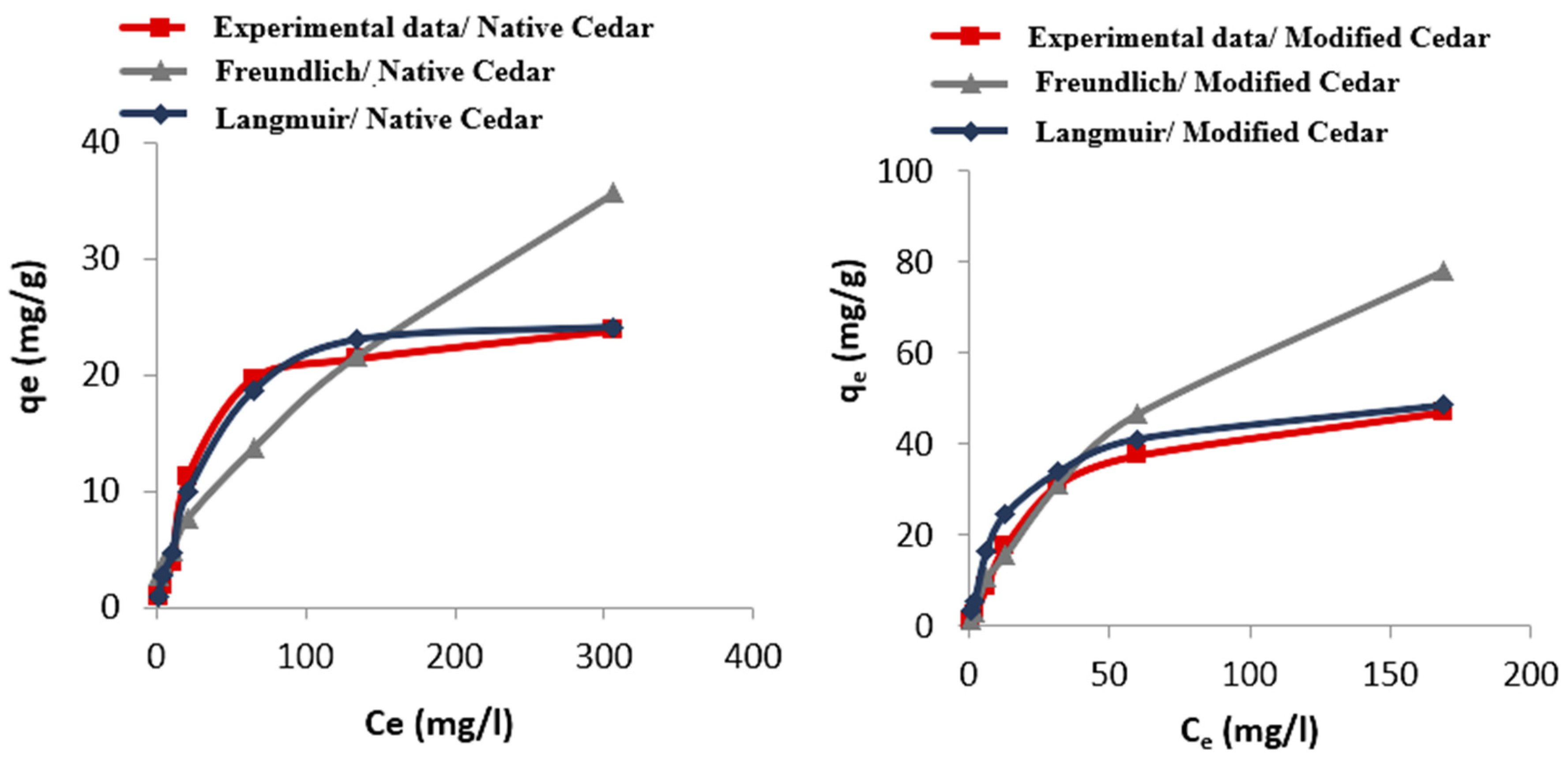
2.2.6. Isotherms of Cr (VI) Adsorption
2.3. Possible Mechanisms of Cr (VI) Adsorption onto Native and Modified Sawdust
- Chemisorption: Cr (VI) ions can undergo chemisorption onto the surface of sawdust through covalent bonding. The oxygen-containing functional groups on sawdust, such as hydroxyl and carboxyl groups, can form strong bonds with Cr (VI) ions, leading to their immobilization on the surface.
- Electrostatic interaction: Cr (VI) ions are anionic species in aqueous solutions. The positively charged functional groups on the sawdust surface, such as protonated amino groups or other positively charged sites, can electrostatically attract and adsorb the negatively charged Cr (VI) ions.
- Ion exchange: Sawdust contains various cations, which can undergo ion exchange with Cr (VI) ions in the solution. Cr (VI) ions can replace these cations on the sawdust surface through ion exchange mechanisms, leading to the adsorption of Cr (VI) ions. The ion exchange capacity of sawdust is influenced by the pH of the solution. At lower pH values, more H+ ions are available for exchange, while at higher pH values, competition with other anions may reduce ion exchange efficiency.
- Reduction: Sawdust may contain reducing agents or compounds that can facilitate the reduction of Cr (VI) to Cr (III). Cr (VI) reduction to Cr (III) can take place on the surface of sawdust, promoting the adsorption of Cr (III) ions, which are less toxic and less soluble than Cr (VI) ions.
- Complexation: Functional groups on sawdust, such as phenolic groups, can form complexes with Cr (VI) ions. Complexation involves the formation of stable coordination compounds between the functional groups on the sawdust surface and Cr (VI) ions, leading to their adsorption.
- Physical adsorption: Apart from chemical interactions, physical adsorption also plays a role. Van der Waals forces and other weak interactions can attract Cr (VI) ions onto the surface of sawdust, contributing to the overall adsorption process.
3. Materials and Methods
3.1. Preparation and Modification of the Biosorbent
3.2. Characterization of the Biosorbent
3.3. Adsorption Process Based on a Batch System
3.4. Experimental Design Approach and Optimization
3.5. Adsorption Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead (Pb2+) Adsorption by Monodispersed Magnetite Nanoparticles: Surface Analysis and Effects of Solution Chemistry. J. Environ. Chem. Eng. 2016, 4, 4237–4247. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. J. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, H.; Salarian, A.A.; Sid Kalal, H. Study of Pb (II) Adsorption from Aqueous Solutions by TiO2 Functionalized with Hydroxide Ethyl Aniline (PHEA/n-TiO2). J. Mol. Liq. 2018, 263, 294–302. [Google Scholar] [CrossRef]
- Al-Shahrani, S.S. Treatment of Wastewater Contaminated with Cobalt Using Saudi Activated Bentonite. Alex. Eng. J. 2014, 53, 205–211. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Removal of Lead and Chromium from Wastewater Using Bagasse Fly Ash—A Sugar Industry Waste. J. Colloid. Interfaces Sci. 2004, 271, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Sharma, G.D.; Dwivedi, B.S.; Khatik, S.K. Chromium: As a Pollutant. J. Ind. Pollut. Control 2007, 23, 209–215. [Google Scholar]
- Du, J.; Shang, X.; Shi, J.; Guan, Y. Removal of Chromium from Industrial Wastewater by Magnetic Flocculation Treatment: Experimental Studies and PSO-BP Modelling. JWPE 2022, 47, 102822. [Google Scholar] [CrossRef]
- Karegar, S.; Bhargavi, M.; Divekqr, S.V. Treatment of Wastewater from Chrome Plating Industry by Ion Exchange Method. IJRET 2015, 04, 393–401. [Google Scholar]
- Mella, B.; Glanert, A.C.C.; Gutterres, M. Removal of Chromium from Tanning Wastewater by Chemical Precipitation and Electrocoagulation. In Proceedings of the XXXII. Congress of IULTCS, Istqnbul, Turkey, 29–31 May 2013; p. 13. [Google Scholar]
- Wen, J.; Sun, Y.; Ning, P.; Xu, G.; Sun, S.; Sun, Z.; Cao, H. Deep Understanding of Sustainable Vanadium Recovery from Chrome Vanadium Slag: Promotive Action of Competitive Chromium Species for Vanadium Solvent Extraction. J. Hazard. Mater. 2022, 422, 126791. [Google Scholar] [CrossRef]
- Sowmya, C.; Purnima, D. Chrome Removal from Bulk Drug Industry Effluent Using Fly Ash Waste Generated in Industrial Process. Mater. Today Proc. 2022, 57, 1666–1670. [Google Scholar] [CrossRef]
- Ramakul, P.; Yanachawakul, Y.; Leepipatpiboon, N.; Sunsandee, N. Biosorption of Palladium(II) and Platinum(IV) from Aqueous Solution Using Tannin from Indian Almond (Terminalia catappa L.) Leaf Biomass: Kinetic and Equilibrium Studies. J. Chem. Eng. 2012, 193–194, 102–111. [Google Scholar] [CrossRef]
- Mutiara, T.; Setyaningsih, L.; Chafidz, A.; Panandita, B.S.; Raharjo, R. Alkali Modified Jackfruit Wood Sawdust as Bio Adsorbent for Removal of Pb(II) Ions from Wastewaters. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012094. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Q.; Wang, H.; Xia, N.; Zhu, S. Adsorption Mechanism of Cu(II) and Pb(II) from Aqueous Solutions Using Citric Acid Modified Beet Pulp Fiber (CDSBP) and Fe-Modified CDSBP. Desalin. Water Treat. 2019, 166, 321–333. [Google Scholar] [CrossRef]
- Yi, Z.J.; Yao, J.; Chen, H.L.; Wang, F.; Liu, X.; Xu, J.S. Equilibrium and Kinetic Studies on Adsorption of Pb(II) by Activated Palm Kernel Husk Carbon. Desalin. Water Treat. Water Treat. 2016, 57, 7245–7253. [Google Scholar] [CrossRef]
- Mitra, T.; Bar, N.; Das, S.K. Rice Husk: Green Adsorbent for Pb(II) and Cr(VI) Removal from Aqueous Solution—Column Study and GA–NN Modeling. SN Appl. Sci. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.H.; Bencheikh Lehocine, M. Experimental Study of the Removal of Copper from Aqueous Solutions by Adsorption Using Sawdust. Desalination 2005, 185, 483–490. [Google Scholar] [CrossRef]
- Boudy, P. Economie Forestière Nord Africaine: Monographie et Traitement Des Essences Résineuses. In Tome II Fascicule 2; Larousse: Paris, France, 1955. [Google Scholar]
- M’Hirit, O.; Blerot, P. Le Grand Livre de La Forêt Marocaine; Mardaga: Liège, Belgium, 1999; Volume 84. [Google Scholar]
- Uehara, A.; Tommis, B.; Belhassen, E.; Satrani, B.; Ghanmi, M.; Baldovini, N. Odor-Active Constituents of Cedrus Atlantica Wood Essential Oil. Phytochem. 2017, 144, 208–215. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Bhunia, P.; Ghangrekar, M.M. Statistical Modeling and Optimization of Biomass Granulation and COD Removal in UASB Reactors Treating Low Strength Wastewaters. Bioresour. Technol. 2008, 99, 4229–4238. [Google Scholar] [CrossRef]
- Ren, J.; Lin, W.T.; Shen, Y.J.; Wang, J.F.; Luo, X.C.; Xie, M.Q. Optimization of Fermentation Media for Nitrite Oxidizing Bacteria Using Sequential Statistical Design. Bioresour. Technol. 2008, 99, 7923–7927. [Google Scholar] [CrossRef]
- Öztürk, D.; Şahan, T. Design and Optimization of Cu(II) Adsorption Conditions from Aqueous Solutions by Low-Cost Adsorbent Pumice with Response Surface Methodology. Pol. J. Env. Stud. 2015, 24, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Su, S.N.; Nie, H.L.; Zhu, L.M.; Chen, T.X. Optimization of Adsorption Conditions of Papain on Dye Affinity Membrane Using Response Surface Methodology. Bioresour. Technol. 2009, 100, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Ren, N.Q.; Wang, X.J.; Xiang, W.S.; Ding, J.; You, Y.; Liu, B.F. Optimization of Culture Conditions for Hydrogen Production by Ethanoligenens Harbinense B49 Using Response Surface Methodology. Bioresour. Technol. 2009, 100, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Gupta, S.K.; Dey, A.; Jha, M.K.; Bajpai, V.; Joshi, S.; Gupta, A. Application of Central Composite Design Approach for Removal of Chromium (VI) from Aqueous Solution Using Weakly Anionic Resin: Modeling, Optimization, and Study of Interactive Variables. J. Hazard. Mater. 2012, 227–228, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gunaraj, V.; Murugan, N. Application of Response Surface Methodology for Predicting Weld Bead Quality in Submerged Arc Welding of Pipes. J. Mater. Process. Technol. 1999, 88, 266–275. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P. Response Surface Optimization of the Critical Medium Components for the Production of Alkaline Protease by a Newly Isolated Bacillus sp. J. Pharm. Pharm. Sci. 2002, 5, 272–278. [Google Scholar]
- Şahan, T.; Öztürk, D. Investigation of Pb(II) Adsorption onto Pumice Samples: Application of Optimization Method Based on Fractional Factorial Design and Response Surface Methodology. Clean. Technol. Env. Policy 2014, 16, 819–831. [Google Scholar] [CrossRef]
- El Hajam, M.; Idrissi Kandri, N.; Harrach, A.; El khomsi, A.; Zerouale, A. Physicochemical Characterization of Softwood Waste “Cedar” and Hardwood Waste “Mahogany”: Comparative Study. Mater. Today Proc. 2019, 13, 803–811. [Google Scholar] [CrossRef]
- El Hajam, M.; Kandri, N.I.; Zerouale, A.; Wang, X.; Gustafsson, J.; Wang, L.; Mäkilä, E.; Hupa, L.; Xu, C. Lignocellulosic Nanocrystals from Sawmill Waste as Biotemplates for Free-Surfactant Synthesis of Photocatalytically Active Porous Silica. ACS Appl. Mater. Interfaces 2022, 14, 19547–19560. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Regupathi, I.; Pillai, M.G.; Miranda, L.R. Modelling, Analysis and Optimization of Adsorption Parameters for H3PO4 Activated Rubber Wood Sawdust Using Response Surface Methodology (RSM). Colloids Surf. B 2009, 70, 35–45. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing Adsorption of Crystal Violet Dye from Water by Magnetic Nanocomposite Using Response Surface Modeling Approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Khezeli, T.; Daneshfar, A. Monodisperse Silica Nanoparticles Coated with Gold Nanoparticles as a Sorbent for the Extraction of Phenol and Dihydroxybenzenes from Water Samples Based on Dispersive Micro-Solid-Phase Extraction: Response Surface Methodology. J. Sep. Sci. 2015, 38, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Krishnan, S.; Ramalingam, S.; Balu, K. Optimization of Process Variables by the Application of Response Surface Methodology for Dye Removal Using a Novel Adsorbent. Dye. Pigm. 2007, 72, 66–74. [Google Scholar] [CrossRef]
- Jing, X.; Cao, Y.; Zhang, X.; Wang, D.; Wu, X.; Xu, H. Biosorption of Cr(VI) from Simulated Wastewater Using a Cationic Surfactant Modified Spent Mushroom. Desalination 2011, 269, 120–127. [Google Scholar] [CrossRef]
- Sereshti, H.; Entezari Heravi, Y.; Samadi, S. Optimized Ultrasound-Assisted Emulsification Microextraction for Simultaneous Trace Multielement Determination of Heavy Metals in Real Water Samples by ICP-OES. Talanta 2012, 97, 235–241. [Google Scholar] [CrossRef]
- Ravikumar, K.; Pakshirajan, K.; Swaminathan, T.; Balu, K. Optimization of Batch Process Parameters Using Response Surface Methodology for Dye Removal by a Novel Adsorbent. J. Chem.Eng. 2005, 105, 131–138. [Google Scholar] [CrossRef]
- Şahan, T.; Ceylan, H.; Aktaş, N. Optimization of Biosorption of Zn(II) Ions from Aqueous Solutions with Low-Cost Biomass Trametes Versicolor and the Evaluation of Kinetic and Thermodynamic Parameters. Desalin. Water Treat. 2016, 57, 12156–12167. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, S.N.; Rath, P. Hexavalent Chromium Removal from Aqueous Solution by Adsorption on Treated Sawdust. Biochem. Eng. J. 2006, 31, 216–222. [Google Scholar] [CrossRef]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T. V Biosorption of Chromium (VI) by Tamarindus Indica Pod Shells. JESRI 2008, 1, 77–81. [Google Scholar]
- Ramos, R.L.; Martinez, A.J.; Guerrero Coronado, R.M. Adsorption of Chromium (VI) from Aqueous Solutions on Activated Carbon. Water Sci. Tech. 1994, 30, 191–197. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y.; Cakici, A.; Faruk Algur, O. Biosorption of Chromium (VI) from Aqueous Solution by Cone Biomass of Pinus Sylvestris. Bioresour. Technol. 2002, 85, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.S.; Park, J.M. Use of Dead Fungal Biomass for the Detoxification of Hexavalent Chromium: Screening and Kinetics. Process Biochem. 2005, 40, 2559–2565. [Google Scholar] [CrossRef]
- Arica, M.Y.; Bayramoǧlu, G. Cr (VI) Biosorption from Aqueous Solutions Using Free and Immobilized Biomass of Lentinus Sajor-Caju: Preparation and Kinetic Characterization. Colloids Surf. A 2005, 253, 203–211. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.-H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The Role of Sawdust in the Removal of Unwanted Materials from Water. J. Hazard. Mater. 2002, B95, 137–152. [Google Scholar] [CrossRef]
- El Hajam, M.; Idrissi Kandri, N.; Harrach, A.; El khomsi, A.; Zerouale, A. Adsorption of Methylene Blue on Industrial Softwood Waste “Cedar” and Hardwood Waste “Mahogany”: Comparative Study. Mater. Today Proc. 2019, 13, 812–821. [Google Scholar] [CrossRef]
- El Hajam, M.; Idrissi Kandri, N.; Zerouale, A. Batch Adsorption of Brilliant Green Dye on Raw Beech Sawdust: Equilibrium Isotherms and Kinetic Studies. Moroc. J. Chem. 2019, 7, 431–435. [Google Scholar]
- El Hajam, M.; Kandri, N.I.; Harrach, A.; Zerouale, A. Adsorptive Removal of Brilliant Green Dye from Aqueous Solutions Using Cedar and Mahogany Sawdusts. Sci. Study Res. 2019, 20, 395–409. [Google Scholar]
- El Hajam, M.; Kandri, N.I.; Plavan, G.I.; Harrath, A.H.; Mansour, L.; Boufahja, F.; Zerouale, A. Pb2+ Ions Adsorption onto Raw and Chemically Activated Dibetou Sawdust: Application of Experimental Designs. J. King Saud. Univ. Sci. 2020, 32, 2176–2189. [Google Scholar] [CrossRef]
- Anupam, K.; Dutta, S.; Bhattacharjee, C.; Datta, S. Adsorptive Removal of Chromium (VI) from Aqueous Solution over Powdered Activated Carbon: Optimisation through Response Surface Methodology. J. Chem.Eng. 2011, 173, 135–143. [Google Scholar] [CrossRef]
- Kütahyali, C.; Sert, Ş.; Çetinkaya, B.; Yalçintaş, E.; Acar, M.B. Biosorption of Ce(III) onto Modified Pinus Brutia Leaf Powder Using Central Composite Design. Wood Sci. Technol. 2012, 46, 721–736. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Tavakoli, H.; Torab-Mostaedi, M.; Hosseini, G.; Hemmati, A. Response Surface Methodology Based on Central Composite Design as a Chemometric Tool for Optimization of Dispersive-Solidification Liquid-Liquid Microextraction for Speciation of Inorganic Arsenic in Environmental Water Samples. Talanta 2014, 123, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yetilmezsoy, K.; Demirel, S.; Vanderbei, R.J. Response Surface Modeling of Pb(II) Removal from Aqueous Solution by Pistacia Vera L.: Box-Behnken Experimental Design. J. Chem. Eng. 2009, 171, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, M. Optimization of Process Variables for Essential Oil Components from Satureja Hortensis by Supercritical Fluid Extraction Using Box-Behnken Experimental Design. J. Supercrit. Fluids 2011, 55, 944–948. [Google Scholar] [CrossRef]







| Exp N° | M | C0 | T | pH | t | %Ads (Native Cedar) | %Ads (Modified Cedar) |
|---|---|---|---|---|---|---|---|
| g | mg/L | °C | -- | min | % | % | |
| 1 | 0.25 | 50.00 | 25.00 | 1.00 | 120.00 | 45.00 | 62.00 |
| 2 | 0.25 | 50.00 | 25.00 | 1.00 | 120.00 | 30.00 | 48.00 |
| 3 | 2.00 | 50.00 | 25.00 | 1.00 | 5.00 | 58.00 | 61.00 |
| 4 | 2.00 | 50.00 | 25.00 | 1.00 | 5.00 | 35.00 | 58.00 |
| 5 | 0.25 | 250.00 | 25.00 | 1.00 | 5.00 | 40.00 | 41.00 |
| 6 | 0.25 | 250.00 | 25.00 | 1.00 | 5.00 | 20.00 | 29.00 |
| 7 | 2.00 | 250.00 | 25.00 | 1.00 | 120.00 | 42.00 | 42.00 |
| 8 | 2.00 | 250.00 | 25.00 | 1.00 | 120.00 | 28.00 | 33.00 |
| 9 | 0.25 | 50.00 | 50.00 | 1.00 | 5.00 | 56.00 | 67.00 |
| 10 | 0.25 | 50.00 | 50.00 | 1.00 | 5.00 | 48.00 | 52.00 |
| 11 | 2.00 | 50.00 | 50.00 | 1.00 | 120.00 | 98.00 | 100.00 |
| 12 | 2.00 | 50.00 | 50.00 | 1.00 | 120.00 | 75.00 | 89.00 |
| 13 | 0.25 | 250.00 | 50.00 | 1.00 | 120.00 | 36.00 | 62.00 |
| 14 | 0.25 | 250.00 | 50.00 | 1.00 | 120.00 | 23.00 | 51.00 |
| 15 | 2.00 | 250.00 | 50.00 | 1.00 | 5.00 | 72.00 | 78.00 |
| 16 | 2.00 | 250.00 | 50.00 | 1.00 | 5.00 | 44.00 | 52.00 |
| 17 | 0.25 | 50.00 | 25.00 | 6.00 | 5.00 | 13.00 | 30.00 |
| 18 | 0.25 | 50.00 | 25.00 | 6.00 | 5.00 | 4.00 | 8.00 |
| 19 | 2.00 | 50.00 | 25.00 | 6.00 | 120.00 | 23.00 | 30.00 |
| 20 | 2.00 | 50.00 | 25.00 | 6.00 | 120.00 | 6.00 | 12.00 |
| 21 | 0.25 | 250.00 | 25.00 | 6.00 | 120.00 | 9.00 | 28.00 |
| 22 | 0.25 | 250.00 | 25.00 | 6.00 | 120.00 | 2.00 | 6.00 |
| 23 | 2.00 | 250.00 | 25.00 | 6.00 | 5.00 | 17.00 | 29.00 |
| 24 | 2.00 | 250.00 | 25.00 | 6.00 | 5.00 | 9.00 | 7.00 |
| 25 | 0.25 | 50.00 | 50.00 | 6.00 | 120.00 | 29.00 | 30.00 |
| 26 | 0.25 | 50.00 | 50.00 | 6.00 | 120.00 | 11.00 | 19.00 |
| 27 | 2.00 | 50.00 | 50.00 | 6.00 | 5.00 | 30.00 | 43.00 |
| 28 | 2.00 | 50.00 | 50.00 | 6.00 | 5.00 | 16.00 | 23.00 |
| 29 | 0.25 | 250.00 | 50.00 | 6.00 | 5.00 | 10.00 | 17.00 |
| 30 | 0.25 | 250.00 | 50.00 | 6.00 | 5.00 | 6.00 | 13.00 |
| 31 | 2.00 | 250.00 | 50.00 | 6.00 | 120.00 | 29.00 | 35.00 |
| 32 | 2.00 | 250.00 | 50.00 | 6.00 | 120.00 | 20.00 | 25.00 |
| 33 | 0.25 | 150.00 | 37.50 | 3.50 | 62.50 | 50.00 | 81.00 |
| 34 | 0.25 | 150.00 | 37.50 | 3.50 | 62.50 | 39.00 | 70.00 |
| 35 | 2.00 | 150.00 | 37.50 | 3.50 | 62.50 | 75.00 | 99.00 |
| 36 | 2.00 | 150.00 | 37.50 | 3.50 | 62.50 | 52.00 | 78.00 |
| 37 | 1.125 | 50.00 | 37.50 | 3.50 | 62.50 | 60.00 | 98.00 |
| 38 | 1.125 | 50.00 | 37.50 | 3.50 | 62.50 | 45.00 | 82.00 |
| 39 | 1.125 | 250.00 | 37.50 | 3.50 | 62.50 | 45.00 | 85.00 |
| 40 | 1.125 | 250.00 | 37.50 | 3.50 | 62.50 | 37.00 | 62.00 |
| 41 | 1.125 | 150.00 | 25.00 | 3.50 | 62.50 | 31.00 | 36.00 |
| 42 | 1.125 | 150.00 | 25.00 | 3.50 | 62.50 | 29.00 | 32.00 |
| 43 | 1.125 | 150.00 | 50.00 | 3.50 | 62.50 | 89.00 | 93.00 |
| 44 | 1.125 | 150.00 | 50.00 | 3.50 | 62.50 | 65.00 | 80.00 |
| 45 | 1.125 | 150.00 | 37.50 | 1.00 | 62.50 | 75.00 | 100.00 |
| 46 | 1.125 | 150.00 | 37.50 | 1.00 | 62.50 | 52.00 | 74.00 |
| 47 | 1.125 | 150.00 | 37.50 | 6.00 | 62.50 | 27.00 | 40.00 |
| 47 | 1.125 | 150.00 | 37.50 | 6.00 | 62.50 | 13.00 | 29.00 |
| 49 | 1.125 | 150.00 | 37.50 | 3.50 | 5.00 | 55.00 | 78.00 |
| 50 | 1.125 | 150.00 | 37.50 | 3.50 | 5.00 | 44.00 | 58.00 |
| 51 | 1.125 | 150.00 | 37.50 | 3.50 | 120.00 | 74.00 | 96.00 |
| 51 | 1.125 | 150.00 | 37.50 | 3.50 | 120.00 | 56.00 | 77.00 |
| 53 | 1.125 | 150.00 | 37.50 | 3.50 | 62.50 | 86.00 | 93.00 |
| 54 | 1.125 | 150.00 | 37.50 | 3.50 | 62.50 | 64.00 | 72.00 |
| Coef. | Effect | t. Experimental | Signification (p-Value) | |||
|---|---|---|---|---|---|---|
| Native Cedar | Modified Cedar | Native Cedar | Modified Cedar | Native Cedar | Modified Cedar | |
| b0 | 58.30 | 80.776 | 17.17 | 23.77 | <0.01 *** | <0.01 *** |
| b1 | 7.16 | 5.000 | 3.33 | 2.32 | 0.216 ** | 2.67 * |
| b2 | −5.36 | −6.028 | −2.49 | −2.80 | 1.80 * | 0.856 ** |
| b3 | 8.78 | 9.361 | 4.07 | 4.34 | 0.0272 *** | 0.0126 *** |
| b4 | −16.75 | −18.750 | −7.78 | −8.70 | <0.01 *** | <0.01 *** |
| b5 | 1.64 | 2.806 | 0.76 | 1.30 | 0.0452 *** | 0.02 *** |
| b1-1 | −2.21 | 1.439 | −0.38 | 0.25 | 70.8 | 80.7 |
| b2-2 | −9.46 | 1.189 | −1.62 | 0.20 | 11.5 | 84.0 |
| b3-3 | −2.71 | −20.311 | −0.46 | −3.47 | 64.6 | 0.146 ** |
| b4-4 | −14.46 | −19.811 | −2.47 | −3.39 | 1.87 * | 0.184 ** |
| b5-5 | 1.04 | −3.311 | 0.18 | −0.57 | 86.0 | 0.575 * |
| b1-2 | 0.31 | 1.438 | 0.14 | −0.63 | 1.809 * | 0.05 *** |
| b1-3 | 3.44 | 3.563 | 1.50 | 1.56 | 14.2 | 12.9 |
| b2-3 | −2.37 | 0.125 | −1.04 | 0.05 | 30.6 | 95.7 |
| b1-4 | −2.75 | −1.500 | −1.20 | −0.66 | 23.7 | 51.6 |
| b2-4 | 3.44 | 3.563 | 1.50 | 1.56 | 14.2 | 12.9 |
| b3-4 | −2.69 | −3.813 | −1.18 | −1.67 | 24.8 | 10.5 |
| b1-5 | 1.62 | 1.063 | 0.71 | −0.46 | 0.482 ** | 0.0645 *** |
| b2-5 | −2.69 | −1.000 | −1.18 | −0.44 | 0.248 ** | 0.665 ** |
| b3-5 | 1.56 | 2.125 | 0.68 | 0.93 | 49.9 | 35.9 |
| b4-5 | 0.62 | −1.263 | 0.27 | −0.66 | 78.6 | 64.5 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | Rapport | Signif. p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Native Cedar | Modified Cedar | Native Cedar | Modified Cedar | Native Cedar | Modified Cedar | Native Cedar | Modified Cedar | ||
| Regression | 2.5 × 104 | 3.6 × 104 | 20 | 1.3 × 103 | 1.8 × 103 | 7.51 | 10.92 | <0.01 | <0.01 |
| Residual | 5.5 × 103 | 5.5 × 103 | 33 | 1.6 × 102 | 1.6 × 102 | ||||
| Validity | 1.9 × 103 | 1.7 × 103 | 6 | 3.2 × 102 | 2.8 × 102 | 2.42 | 2.11 | 5.3 | 8.5 |
| Error | 3.6 × 103 | 3.7 × 103 | 27 | 1.3 × 102 | 1.4 × 102 | ||||
| Total | 3.1 × 104 | 4.2 × 104 | 53 | ||||||
| R² | 0.82 (Native Cedar), 0.88 (Modified Cedar) | ||||||||
| R²Adj | 0.74 (Native Cedar), 0.79 (Modified Cedar) | ||||||||
| Parameters | Value | Code | Predicted Response (%) | Experimental Response (%) | |
|---|---|---|---|---|---|
| Native Cedar | m (g) | 2 | +1 | 83 | 84.16 |
| C (ppm) | 150 | 0 | |||
| T (°C) | 50 | +1 | |||
| pH | 1 | −1 | |||
| t (min) | 62.5 | 0 | |||
| Modified Cedar | m (g) | 1.125 | 0 | 100 | 99.04 |
| C (ppm) | 250 | +1 | |||
| T (°C) | 50 | +1 | |||
| pH | 1 | −1 | |||
| t (min) | 62.5 | 0 |
| Sawdust | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | k1 (min−1) | qe cal (mg/g) | qe exp (mg/g) | R2 | k2 (g/mg.min) | qe cal (mg/g) | qe exp (mg/g) | |
| Native Cedar | 0.90 | 0.03 | 4.12 | 10.89 | 0.99 | 0.02 | 11.10 | 10.89 |
| Modified Cedar | 0.86 | 0.05 | 7.06 | 13.24 | 0.99 | 0.01 | 14.45 | 13.24 |
| Sawdust | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| R2 | qmax (mg/g) | kl (L/mg) | R2 | kf | n | |
| Native Cedar | 0.99 | 23.64 | 0.02 | 0.97 | 0.65 | 1.47 |
| Modified Cedar | 0.99 | 48.31 | 0.04 | 0.96 | 2.22 | 1.46 |
| Designation | Notation | Low Level (−1) | Central Level (0) | High Level (+1) |
|---|---|---|---|---|
| X1 | Mass: m (g) | 0.25 | 1.125 | 2 |
| X2 | Concentration: C (mg/L) | 50 | 150 | 250 |
| X3 | Temperature: T (°C) | 25 | 37.5 | 50 |
| X4 | pH | 1 | 3.5 | 6 |
| X5 | Contact time: t (min) | 15 | 67.5 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajam, M.; Idrissi Kandri, N.; Özdemir, S.; Plavan, G.; Ben Hamadi, N.; Boufahja, F.; Zerouale, A. Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM). Molecules 2023, 28, 7271. https://doi.org/10.3390/molecules28217271
El Hajam M, Idrissi Kandri N, Özdemir S, Plavan G, Ben Hamadi N, Boufahja F, Zerouale A. Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM). Molecules. 2023; 28(21):7271. https://doi.org/10.3390/molecules28217271
Chicago/Turabian StyleEl Hajam, Maryam, Noureddine Idrissi Kandri, Sadin Özdemir, Gabriel Plavan, Naoufel Ben Hamadi, Fehmi Boufahja, and Abdelaziz Zerouale. 2023. "Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM)" Molecules 28, no. 21: 7271. https://doi.org/10.3390/molecules28217271
APA StyleEl Hajam, M., Idrissi Kandri, N., Özdemir, S., Plavan, G., Ben Hamadi, N., Boufahja, F., & Zerouale, A. (2023). Statistical Design and Optimization of Cr (VI) Adsorption onto Native and HNO3/NaOH Activated Cedar Sawdust Using AAS and a Response Surface Methodology (RSM). Molecules, 28(21), 7271. https://doi.org/10.3390/molecules28217271








