A Review of Foods of Plant Origin as Sources of Vitamins with Proven Activity in Oxidative Stress Prevention according to EFSA Scientific Evidence
Abstract
1. Introduction
2. Results and Discussion
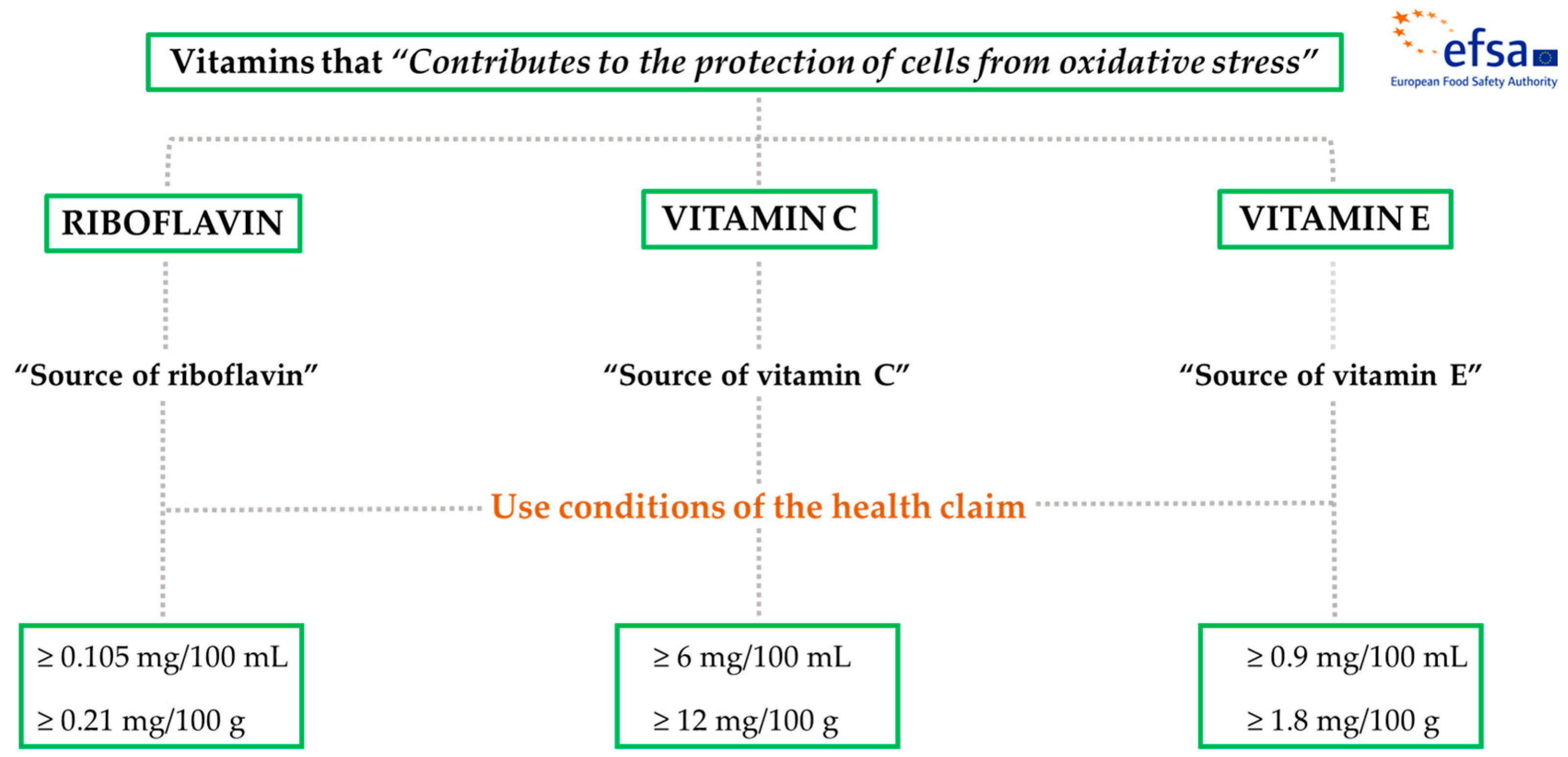
2.1. Vitamins with EFSA Scientific Evidence Related to the Oxidative Stress Prevention
2.2. Foods of Plant Origin as Sources of Vitamins with Scientific Evidence Related to Oxidative Stress Prevention
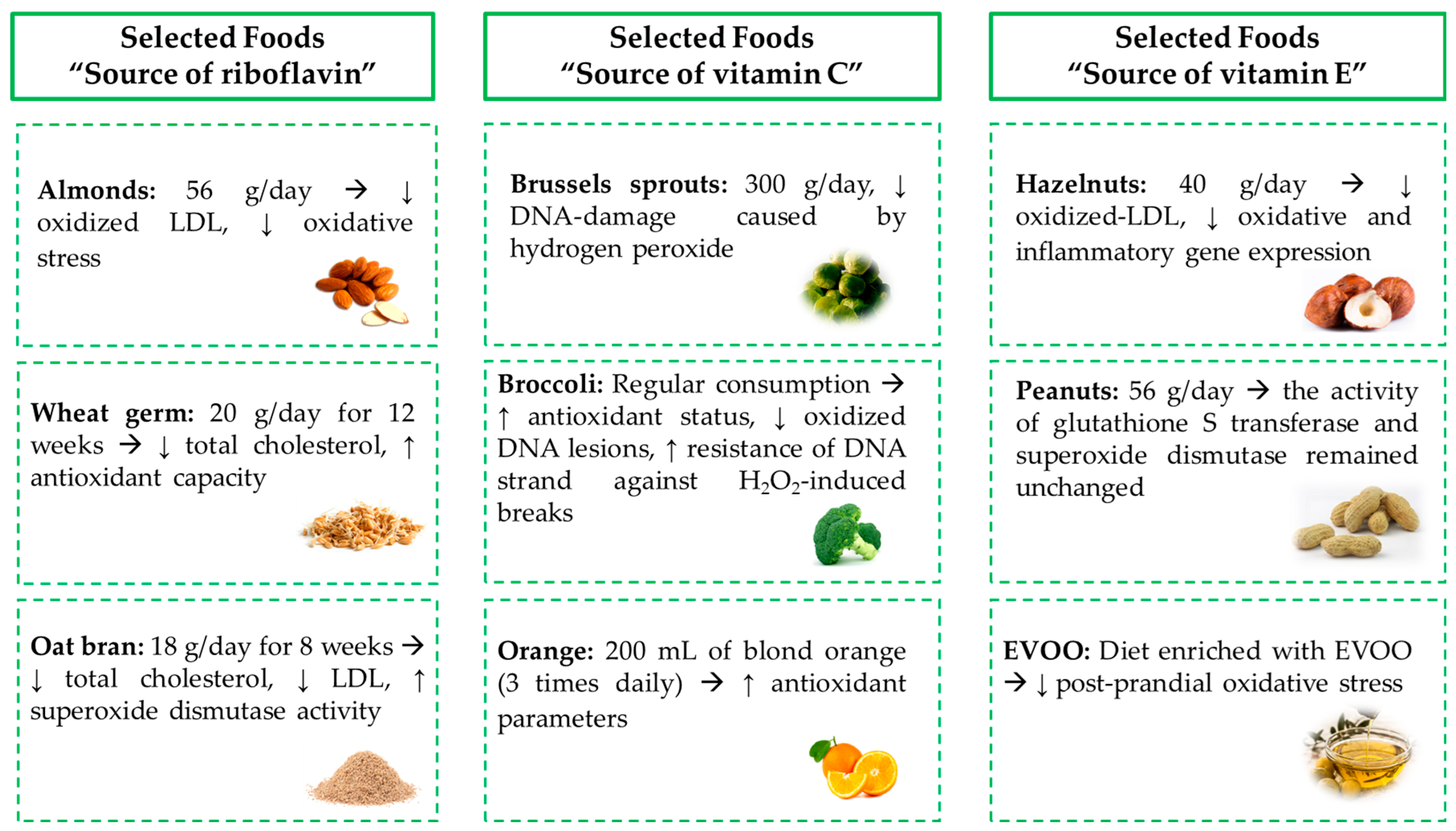
2.2.1. Riboflavin
2.2.2. Vitamin C
2.2.3. Vitamin E
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences, 1st ed.; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Fathollahipour, S.; Patil, P.S.; Leipzig, N.D. Oxygen regulation in development: Lessons from embryogenesis towards tissue engineering. Cells Tissues Organs 2018, 205, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Weiler-Sagie, M.; Kuppusamy, P.; Neufeld, G.; Neeman, M.; Blank, A. Electron spin resonance microscopic imaging of oxygen concentration in cancer spheroids. J. Magn. Reson. 2015, 256, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant fortification of the diet for anti-ageing effects: A review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Reactive oxygen species modulators in pulmonary medicine. Curr. Opin. Pharmacol. 2021, 57, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ifeanyi, O.E. A Review on Free Radicals and Antioxidants. Int. J. Curr. Res. Med. Sci. 2018, 4, 123–133. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Matallana-González, M.C.; Cámara, M.; Fernández-Ruiz, V.; Morales, P. Antioxidant Phytochemicals in Pulses and their Relation to Human Health: A Review. Curr. Pharm. Des. 2020, 26, 1880–1897. [Google Scholar] [CrossRef]
- Reis, F.S.; Ferreira, I.C.F.R.; Barros, L.; Martins, A. A comparative study of tocopherols composition and antioxidant properties of in vivo and in vitro ectomycorrhizal fungi. LWT Food Sci. Technol. 2011, 44, 820–824. [Google Scholar] [CrossRef][Green Version]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, R.M.; Domínguez, L.; Sesso, H.D. Scientific Evidence of the Beneficial Effects of Tomato Products on Cardiovascular Disease and Platelet Aggregation. Front. Nutr. 2022, 9, 849841. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative stress: The lowest common denominator of multiple diseases. Neural Regen. Res. 2019, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Pantelimon, I.; Gales, L.N.; Zgura, A.; Serbanescu, G.L.; Georgescu, D.E.; Nita, I.; Manolescu, L.S.C.; Stancu, A.M.; Gruia, M.I.; Anghel, R.M.; et al. Analysis of Oxidative Stress in Patients with Breast Cancer and Obesity. Ann. Med. Health Sci. Res. 2021, 11, 1578–1585. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Marcadenti, A.; Coelho, R.C.L.A. Dietary antioxidant and oxidative stress: Interaction between vitamins and genetics. J. Nutr. Health Food Sci. 2015, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction, and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Sánchez-Mata, M.C.; Fernández-Ruiz, V.; Cámara, R.M.; Cebadera, E.; Domínguez, L. A review of the role of micronutrients and bioactive compounds on immune system improvement to fight against the COVID-19 disease. Foods 2021, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Giner, R.M.; González-Fandos, E.; López-García, E.; Mañes, J.; Portillo, M.P.; Rafecas, M.; Domínguez, L.; Martínez, J.A. Food-Based Dietary Guidelines around the World: A Comparative Analysis to Update AESAN Scientific Committee Dietary Recommendations. Nutrients 2021, 13, 3131. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos. Guía de Prácticas, 19th ed.; Pirámide: Madrid, Spain, 2018. [Google Scholar]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Domínguez Díaz, L.; Kardinaal, A.; van Lieshout, M. Evidence of antiplatelet aggregation effects from the consumption of tomato products, according to EFSA health claim requirements. Crit. Rev. Food Sci. Nutr. 2019, 60, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- European Parliament; Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. OJEU 2006, 304, 18–63. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage, and cardiovascular health. EFSA J. 2011, 9, 2474. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies; Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; et al. Scientific opinion on vitamin E and protection of DNA, proteins and lipids from oxidative damage: Evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2016, 14, e04588. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies; Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar] [CrossRef]
- European Commission. EU Register of Nutrition and Health Claims Made on Food. Available online: http://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home (accessed on 13 June 2023).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin C and protection of DNA, proteins and lipids from oxidative damage (ID 129, 138, 143, 148), antioxidant function of lutein (ID 146), maintenance of vision (ID 141, 142), collagen formation (ID 130, 131, 136, 137, 149), function of the nervous system (ID 133), function of the immune system (ID 134), function of the immune system during and after extreme physical exercise (ID 144), non-haem iron absorption (ID 132, 147), energy yielding metabolism (ID 135), and relief in case of irritation in the upper respiratory tract (ID 1714, 1715) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA J. 2009, 7, 1226. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage (ID 160, 162, 1947), maintenance of the normal function of the immune system (ID 161, 163), maintenance of normal bone (ID 164), maintenance of normal teeth (ID 164), maintenance of normal hair (ID 164), maintenance of normal skin (ID 164), maintenance of normal nails (ID 164), maintenance of normal cardiac function (ID 166), maintenance of normal vision by protection of the lens of the eye (ID 167), contribution to normal cognitive function (ID 182, 183), regeneration of the reduced form of vitamin C (ID 203), maintenance of normal blood circulation (ID 216) and maintenance of a normal scalp (ID 2873) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1816. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EC) No 1925/2006 of the European Parliament and ofthe Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJEU 2006, 404, 26–38. [Google Scholar]
- European Parliament; Council of the European Union. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJEU 2002, L183, 51. [Google Scholar]
- European Parliament; Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. OJEU 2011, 50, 18–63. [Google Scholar]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May, establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. OJEU 2012, 136, 1–40. [Google Scholar]
- BEDCA. Base de Datos Española de Composición de Alimentos. Available online: https://www.bedca.net (accessed on 6 June 2023).
- FRIDA. DTU Foods Public Food Database. Available online: https://frida.fooddata.dk/?lang=en (accessed on 6 June 2023).
- USDA. United States Department of Agriculture. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 6 June 2023).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to riboflavin (vitamin B2) and contribution to normal energy-yielding metabolism (ID 29, 35, 36, 42), contribution to normal metabolism of iron (ID 30, 37), maintenance of normal skin and mucous membranes (ID 31,33), contribution to normal psychological functions (ID 32), maintenance of normal bone (ID 33), maintenance of normal teeth (ID 33), maintenance of normal hair (ID 33), maintenance of normal nails (ID 33), maintenance of normal vision (ID 39), maintenance of normal red blood cells (ID 40), reduction of tiredness and fatigue (ID 41), protection of DNA, proteins and lipids from oxidative damage (ID 207), and maintenance of the normal function of the nervous system (ID 213) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1814. [Google Scholar] [CrossRef]
- Hinnouho, G.M.; Hampel, D.; Shahab-Ferdows, S.; Barffour, M.A.; McAnena, L.; Arnold, C.D.; Wessells, K.R.; Kounnavong, S.; Allen, L.H.; McNulty, H.; et al. Daily supplementation of a multiple micronutrient powder improves folate but not thiamine, riboflavin, or vitamin B12 status among young Laotian children: A randomized controlled trial. Eur. J. Nutr. 2022, 61, 3423–3435. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, S.; Jiang, Y.; Chen, L.; Liao, Y.; Chen, K.; Huang, K. Association Between Riboflavin Intake and Telomere Length: A Cross-Sectional Study From National Health and Nutrition Examination Survey 1999–2002. Front. Nutr. 2022, 9, 744397. [Google Scholar] [CrossRef]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency—Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022, 128, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Karimifar, M.; Heidari, Z.; Zare, M.; Amani, R. The effects of wheat germ supplementation on metabolic profile in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2020, 34, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Blumberg, J. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J. Nutr. 2008, 138, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Liu, Y.H.; Chen, C.M.; Chang, W.H.; Chen, C.Y.O. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Rakic, J.M.; Tanprasertsuk, J.; Scott, T.M.; Rasmussen, H.M.; Mohn, E.S.; Chen, C.Y.O.; Johnson, E.J. Effects of daily almond consumption for six months on cognitive measures in healthy middle-aged to older adults: A randomized control trial. Nutr. Neurosci. 2022, 25, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.H.; Ames, M.; Hsin-Jen, C.; Feeney, M.J.; Wang, Y.; Cheskin, L.J. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite 2013, 71, 379–387. [Google Scholar] [CrossRef]
- Sahrir, N.A.; Ooi, F.K.; Chen, C.K.; Kyi, W.M.; Meor-Osman, J. Effects of oat bran and jogging on aerobic capacity, lipid profile and antioxidant parameters in young sedentary males. J. Phys. Educ. Sport 2017, 17, 48. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, A. Reported dietary intake and food sources of zinc, selenium, and vitamins A, E and C in the Spanish population: Findings from the ANIBES study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Tveden-Nyborg, P.; Lykkesfeldt, J. Does vitamin C deficiency promote fatty liver disease development? Nutrients 2014, 6, 5473–5499. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sheehy, T.; Kolonel, L. Sources of vegetables, fruits and vitamins A, C and E among five ethnic groups: Results from a multiethnic cohort study. Eur. J. Clin. Nutr. 2014, 68, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Rahmat, A.; Bakar, M.F.; Faezah, N.; Hambali, Z. The effects of consumption of guava (Psidium guajava) or papaya (Carica papaya) on total antioxidant and lipid profile in normal male youth. Asia Pac. J. Clin. Nutr. 2004, 13, S106. [Google Scholar]
- Rahmat, A.; Bakar, M.F.A.; Hambali, Z. The effects of guava (Psidium guajava) consumption on total antioxidant and lipid profile in normal male youth. Afr. J. Food Agric. Nutr. Dev. 2006, 6, 1–12. [Google Scholar] [CrossRef]
- Lee, H.J.; Han, J.H.; Park, Y.K.; Kang, M.H. Effects of glutathione s-transferase (GST) M1 and T1 polymorphisms on antioxidant vitamins and oxidative stress-related parameters in Korean subclinical hypertensive subjects after kale juice (Brassica oleracea acephala) supplementation. Nutr. Res. Pract. 2018, 12, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant-and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef]
- Bogaards, J.J.; Verhagen, H.; Willems, M.I.; Poppel, G.V.; Bladeren, P.J.V. Consumption of Brussels sprouts results in elevated α-class glutathione S-transferase levels in human blood plasma. Carcinogenesis 1994, 15, 1073–1075. [Google Scholar] [CrossRef]
- Nijhoff, W.A.; Mulder, T.P.; Verhagen, H.; van Poppel, G.; Peters, W.H. Effects of consumption of Brussels sprouts on plasma and urinary glutathione S-transferase class-α and-π in humans. Carcinogenesis 1995, 16, 955–957. [Google Scholar] [CrossRef]
- Nijhoff, W.A.; Grubben, M.J.; Nagengast, F.M.; Jansen, J.B.; Verhagen, H.; van Poppel, G.; Peters, W.H. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis 1995, 16, 2125–2128. [Google Scholar] [CrossRef][Green Version]
- Hoelzl, C.; Glatt, H.; Simic, T.; Ferk, F.; Nersesyan, A.; Knasmuller, S. DNA protective effects of Brussels sprouts: Results of a human intervention study. Cancer Epidemiol. Biomark. Prev. 2007, 16, B67. [Google Scholar]
- Riso, P.; Brusamolino, A.; Moro, M.; Porrini, M. Absorption of bioactive compounds from steamed broccoli and their effect on plasma glutathione S-transferase activity. Int. J. Food Sci. Nutr. 2009, 60, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Martini, D.; Møller, P.; Loft, S.; Bonacina, G.; Moro, M.; Porrini, M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 2010, 25, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Del Bo’, C.; Vendrame, S.; Brusamolino, A.; Martini, D.; Bonacina, G.; Porrini, M. Modulation of plasma antioxidant levels, glutathione S-transferase activity and DNA damage in smokers following a single portion of broccoli: A pilot study. J. Sci. Food Agric. 2014, 94, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Cara, K.C.; Beauchesne, A.R.; Wallace, T.C.; Chung, M. Effects of 100% Orange Juice on Markers of Inflammation and Oxidation in Healthy and At-Risk Adult Populations: A Scoping Review, Systematic Review, and Meta-analysis. Adv. Nutr. 2022, 13, 116–137. [Google Scholar] [CrossRef] [PubMed]
- Constans, J.; Bennetau-Pelissero, C.; Martin, J.F.; Rock, E.; Mazur, A.; Bedel, A.; Morand, C.; Bérard, A.M. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin. Nutr. 2015, 34, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, S.; Riso, P.; Porrini, M. Orange juice vs vitamin C: Effect on hydrogen peroxide-induced DNA damage in mononuclear blood cells. Br. J. Nutr. 2007, 97, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Hale, J.C. Oxidation of ascorbic acid in stored orange juice is associated with reduced plasma vitamin C concentrations and elevated lipid peroxides. J. Am. Diet. Assoc. 2005, 105, 106–109. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Gardana, C.; Grande, S.; Brusamolino, A.; Galvano, F.; Galvano, G.; Porrini, M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J. Agric. Food Chem. 2005, 53, 941–947. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Kemnic, T.R.; Coleman, M. Vitamin E Deficiency. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Merra, G.; Botta, R.; Gualtieri, P.; Manzo, A.; Perrone, M.A.; Mazza, M.; Cascapera, S.; De Lorenzo, A. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidative status, oxidative and inflammatory gene expression of healthy subjects: A randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1610–1626. [Google Scholar] [PubMed]
- Di Renzo, L.; Cioccoloni, G.; Bernardini, S.; Abenavoli, L.; Aiello, V.; Marchetti, M.; Cammarano, A.; Alipourfard, I.; Ceravolo, I.; Gratteri, S. A hazelnut-enriched diet modulates oxidative stress and inflammation gene expression without weight gain. OMCL 2019, 1, 4683723. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.A.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds Decrease Postprandial Glycemia, Insulinemia, and Oxidative Damage in Healthy Individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Chen, C.Y.O.; Blumberg, J.B.; Kwak, H.K. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: A randomized clinical trial. Eur. J. Nutr. 2018, 57, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.; Clark, J.; Griffiths, H.R. An almond-enriched diet increases plasma α-tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radic. Res. 2014, 48, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.P.S.; Alves, R.D.M.; Hermsdorff, H.H.M.; de Oliveira, L.L.; Bressan, J. Effects of high-oleic peanuts within a hypoenergetic diet on inflammatory and oxidative status of overweight men: A randomised controlled trial. Br. J. Nutr. 2020, 123, 673–680. [Google Scholar] [CrossRef]
- Gunathilake, M.; Van, N.T.H.; Kim, J. Effects of nut consumption on blood lipid profile: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 537–549. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory, and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef]
- Canudas, S.; Hernández-Alonso, P.; Galié, S.; Muralidharan, J.; Morell-Azanza, L.; Zalba, G.; García-Gavilán, J.; Martí, A.; Salas-Salvadó, J.; Bulló, M. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: A crossover randomized clinical trial. Am. J. Clin. Nutr. 2019, 109, 1738–1745. [Google Scholar] [CrossRef]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; San Julian, B.; Sanchez-Tainta, A.; Corella, D.; Lamuela-Raventos, R.M.; Martínez, J.A.; Martinez-Gonzalez, M.A. Virgin olive oil supplementation and long-term cognition: The PREDIMED-NAVARRA randomized, trial. J. Nutr. Health Aging 2013, 17, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Chiva-Blanch, G.; Ros, E.; Martinez-Gonzalez, M.A.; Covas, M.I.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Fiol, M.; et al. The Effects of the Mediterranean Diet on Biomarkers of Vascular Wall Inflammation and Plaque Vulnerability in Subjects with High Risk for Cardiovascular Disease. A Randomized Trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Rosenblat, M.; Borochov-Neori, H.; Volkova, N.; Judeinstein, S.; Elias, M.; Aviram, M. Effects of date (Phoenix dactylifera L., Medjool or Hallawi Variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status: A pilot study. J. Agric. Food Chem. 2009, 57, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, P.P.; Alfthan, G.; Tikkanen, M.J.; Adlercreutz, H. Rye bread intake improves oxidation resistance of LDL in healthy humans. Atherosclerosis 2012, 221, 583–586. [Google Scholar] [CrossRef]
| Vitamins | Health Claim | Use Conditions of the Health Claim (“Source of”) | ||
|---|---|---|---|---|
| NRV | Beverages | Foods | ||
| Riboflavin (vitamin B2) | “Contributes to the protection of cells from oxidative stress” | 1.4 mg | ≥0.105 mg/100 mL | ≥0.21 mg/100 g |
| Vitamin C | 80 mg | ≥6 mg/100 mL | ≥12 mg/100 g | |
| Vitamin E | 12 mg | ≥0.9 mg/100 mL | ≥1.8 mg/100 g | |
| Food | Riboflavin Content (mg/100 g Edible Portion) |
|---|---|
| Almond | 0.78–1.14 |
| Wheat germ | 0.5–0.61 |
| Wheat bran | 0.36–0.6 |
| Mushroom | 0.35–0.41 |
| Kale | 0.29–0.35 |
| Soy flour | 0.28–1.16 |
| Rice bran | 0.28 |
| Lupin | 0.22 |
| Oat bran | 0.22 |
| Pinto bean | 0.21–0.22 |
| Food | Vitamin C Content (mg/100 g Edible Portion) |
|---|---|
| Guava | 176–228 |
| Kale | 169–93.4 |
| Black currant | 159.6–181 |
| Brussels sprouts | 140–85 |
| Red pepper | 128–163 |
| Broccoli | 89.2–117 |
| Cauliflower | 76.8–48.2 |
| Papaya | 60.9–78.5 |
| Kiwi | 59–74.7 |
| Orange | 50–54.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciudad-Mulero, M.; Domínguez, L.; Morales, P.; Fernández-Ruiz, V.; Cámara, M. A Review of Foods of Plant Origin as Sources of Vitamins with Proven Activity in Oxidative Stress Prevention according to EFSA Scientific Evidence. Molecules 2023, 28, 7269. https://doi.org/10.3390/molecules28217269
Ciudad-Mulero M, Domínguez L, Morales P, Fernández-Ruiz V, Cámara M. A Review of Foods of Plant Origin as Sources of Vitamins with Proven Activity in Oxidative Stress Prevention according to EFSA Scientific Evidence. Molecules. 2023; 28(21):7269. https://doi.org/10.3390/molecules28217269
Chicago/Turabian StyleCiudad-Mulero, María, Laura Domínguez, Patricia Morales, Virginia Fernández-Ruiz, and Montaña Cámara. 2023. "A Review of Foods of Plant Origin as Sources of Vitamins with Proven Activity in Oxidative Stress Prevention according to EFSA Scientific Evidence" Molecules 28, no. 21: 7269. https://doi.org/10.3390/molecules28217269
APA StyleCiudad-Mulero, M., Domínguez, L., Morales, P., Fernández-Ruiz, V., & Cámara, M. (2023). A Review of Foods of Plant Origin as Sources of Vitamins with Proven Activity in Oxidative Stress Prevention according to EFSA Scientific Evidence. Molecules, 28(21), 7269. https://doi.org/10.3390/molecules28217269









