Homogeneous Electrochemical Aptasensor for Sensitive Detection of Zearalenone Using Nanocomposite Probe and Silica Nanochannel Film
Abstract
:1. Introduction
2. Results and Discussion
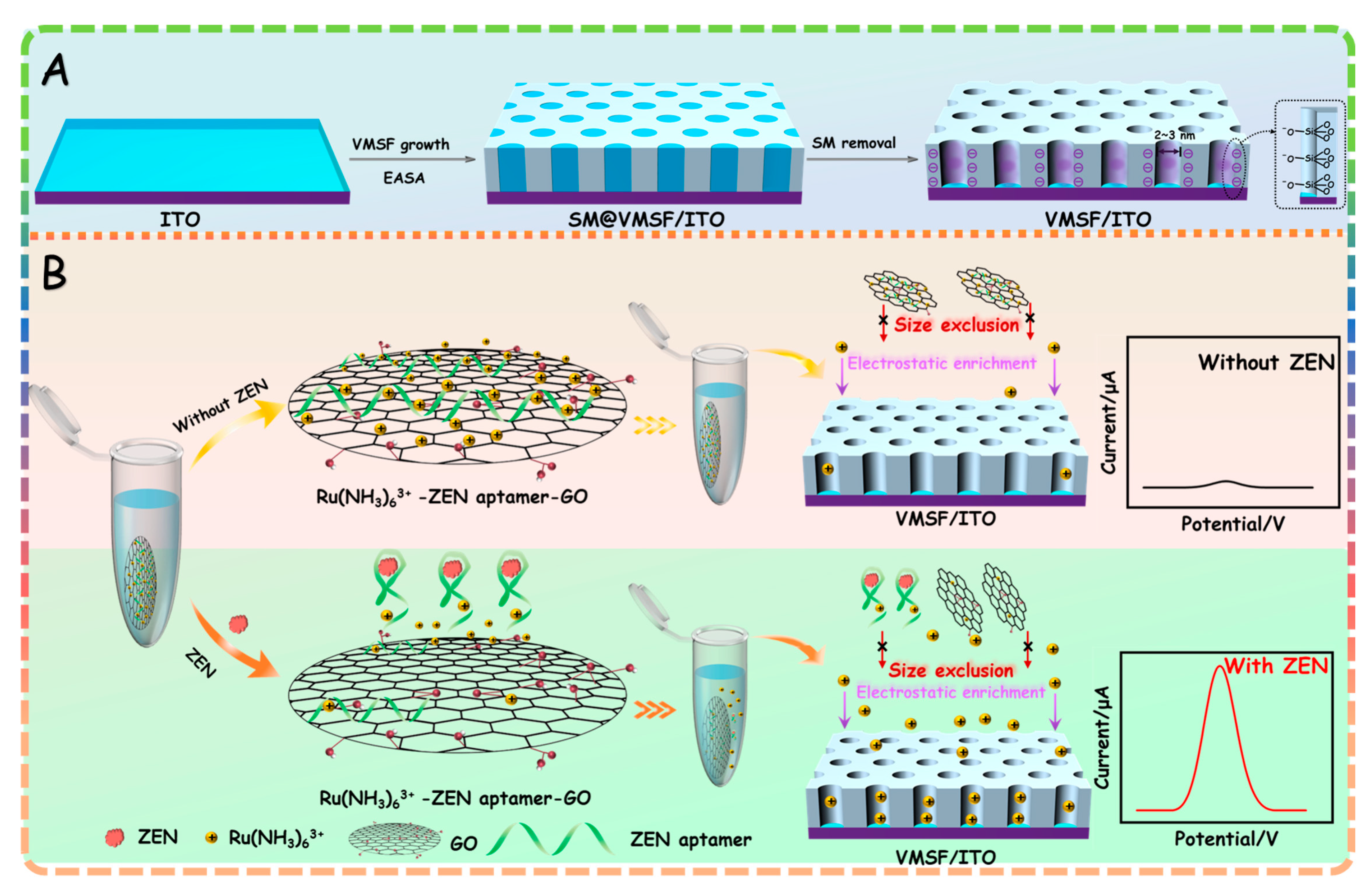
2.1. Principle of the Designed Electrochemical Aptasensor for ZEN Determination
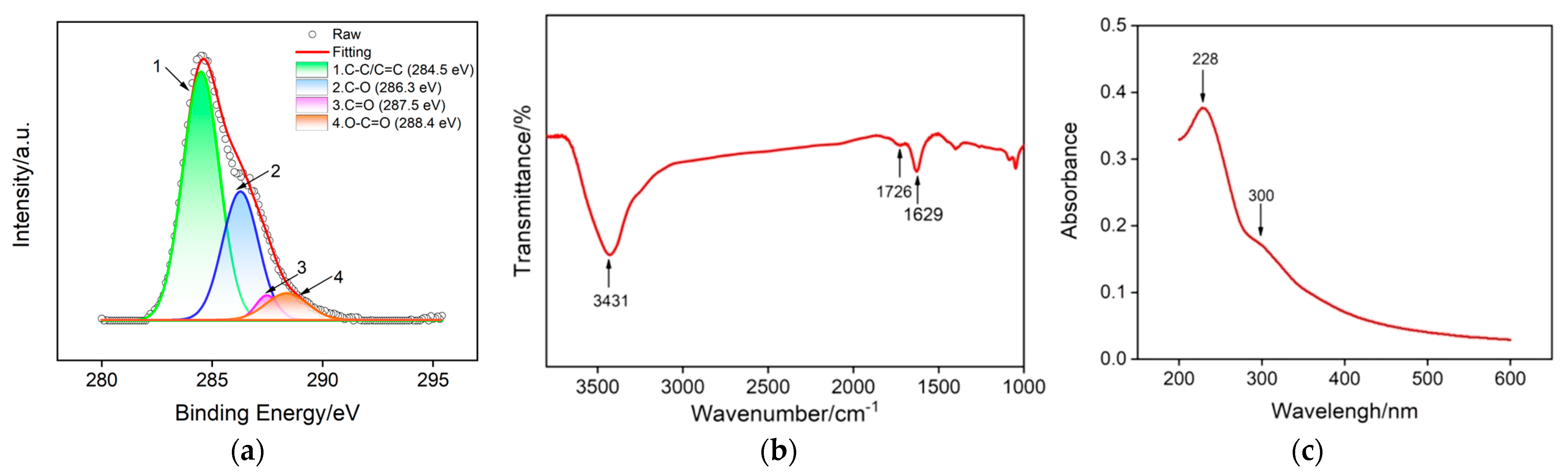
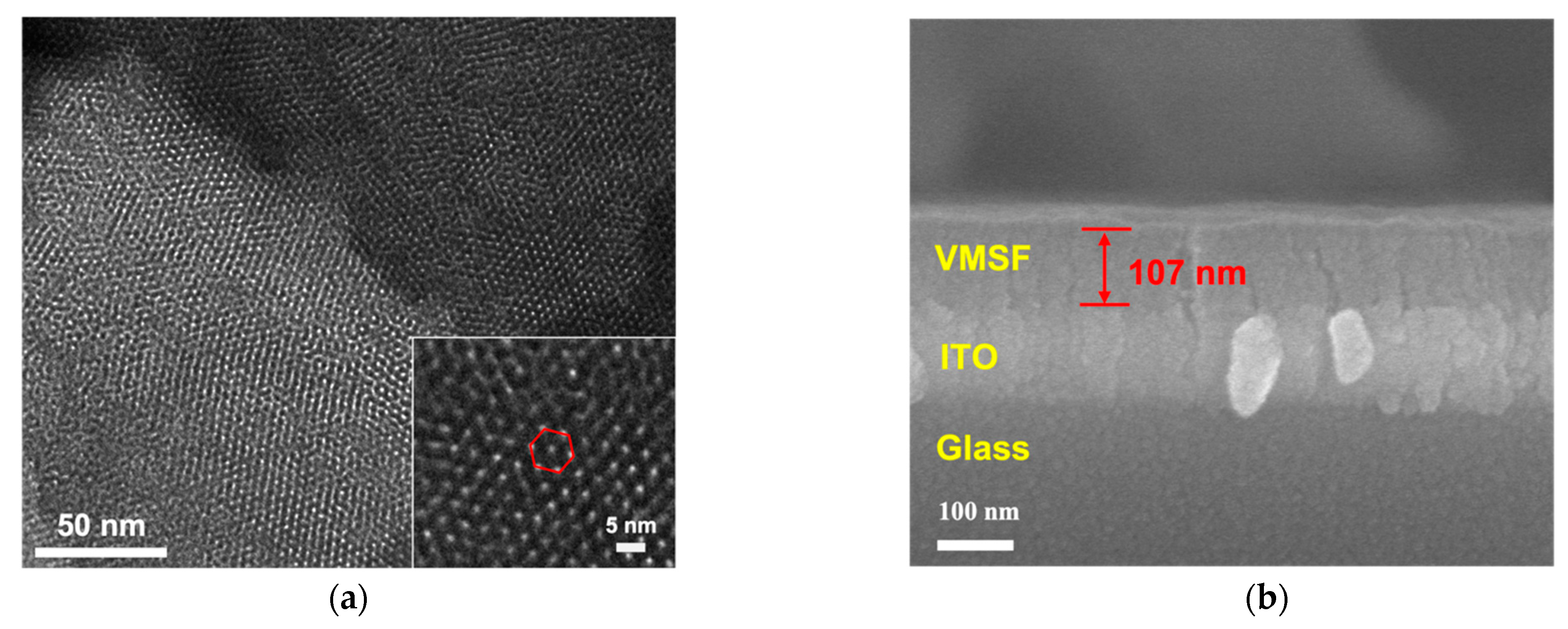
2.2. Characterizations of GO and VMSF/ITO Electrode
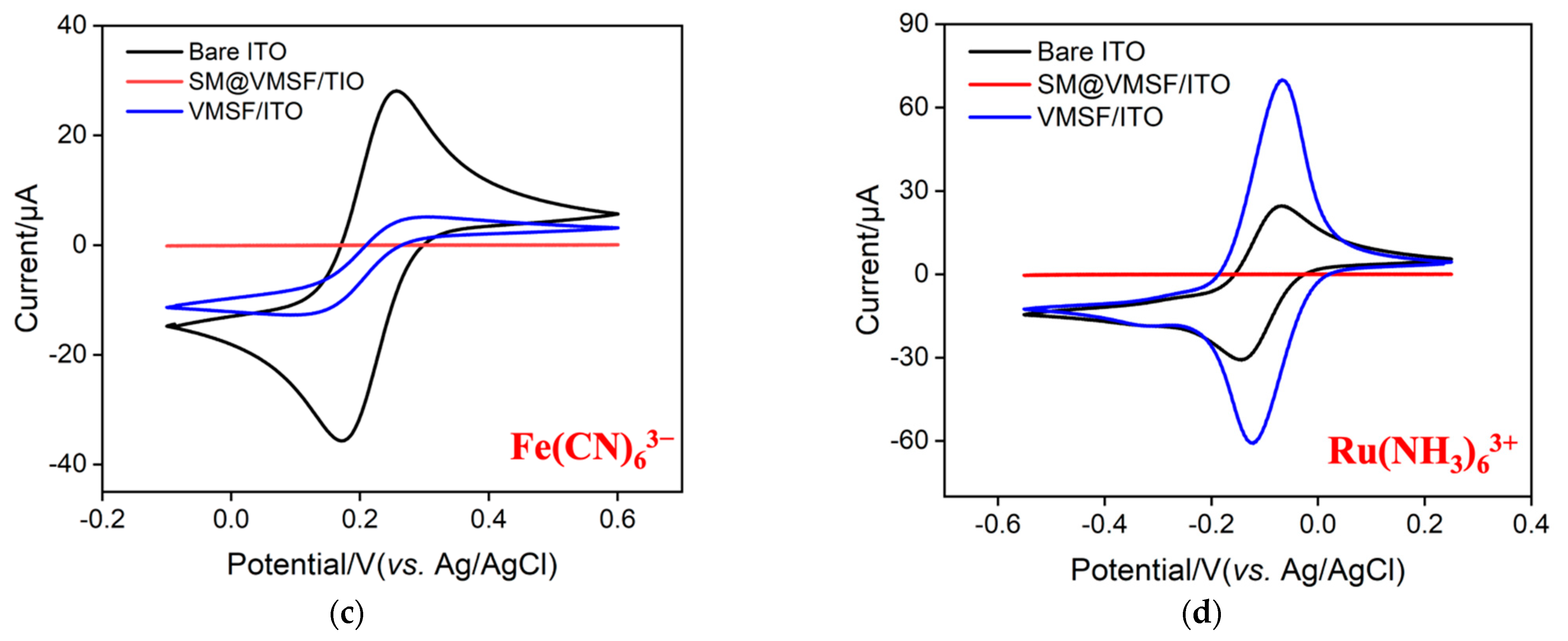
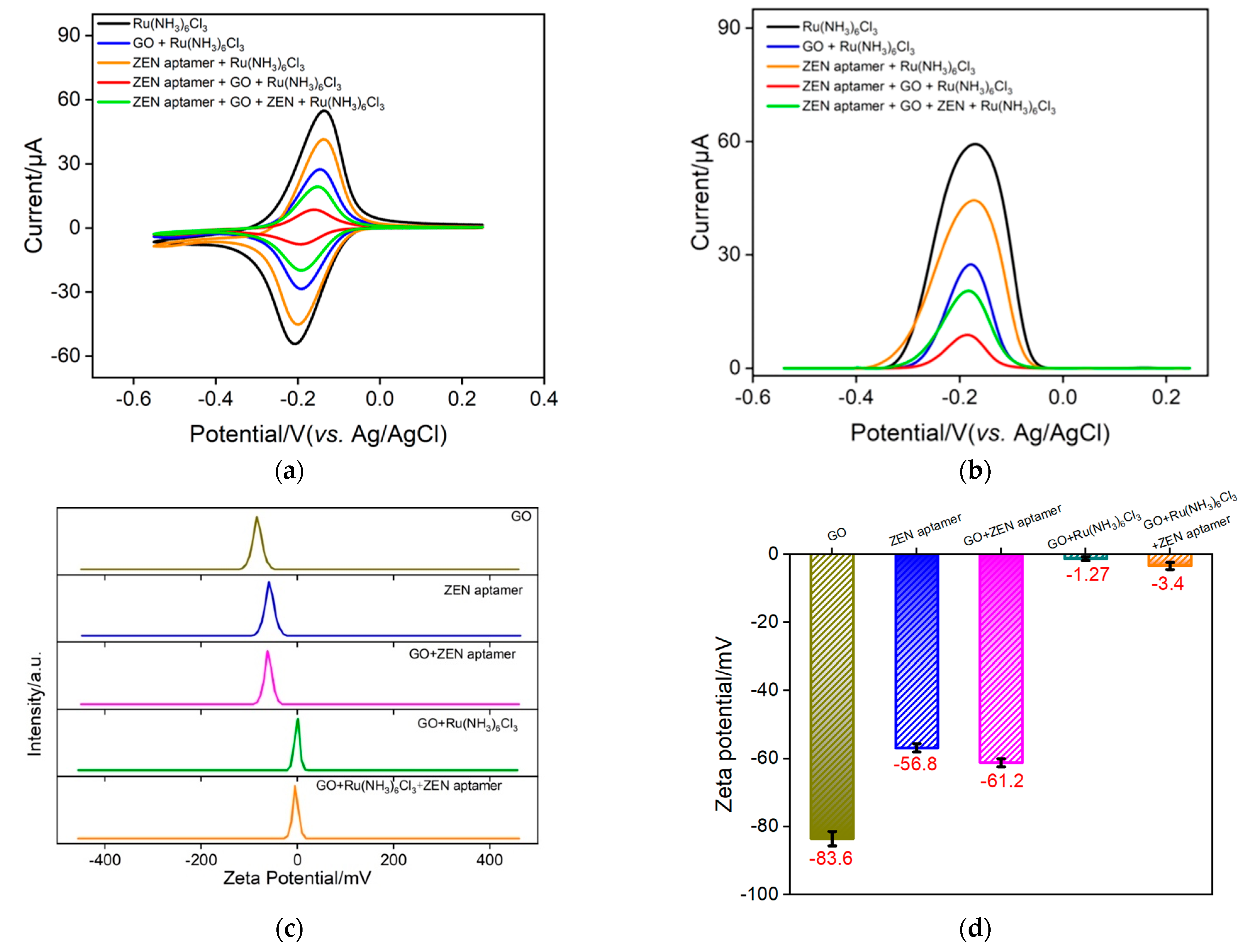
2.3. Feasibility of the Homogeneous Electrochemical Aptasensor
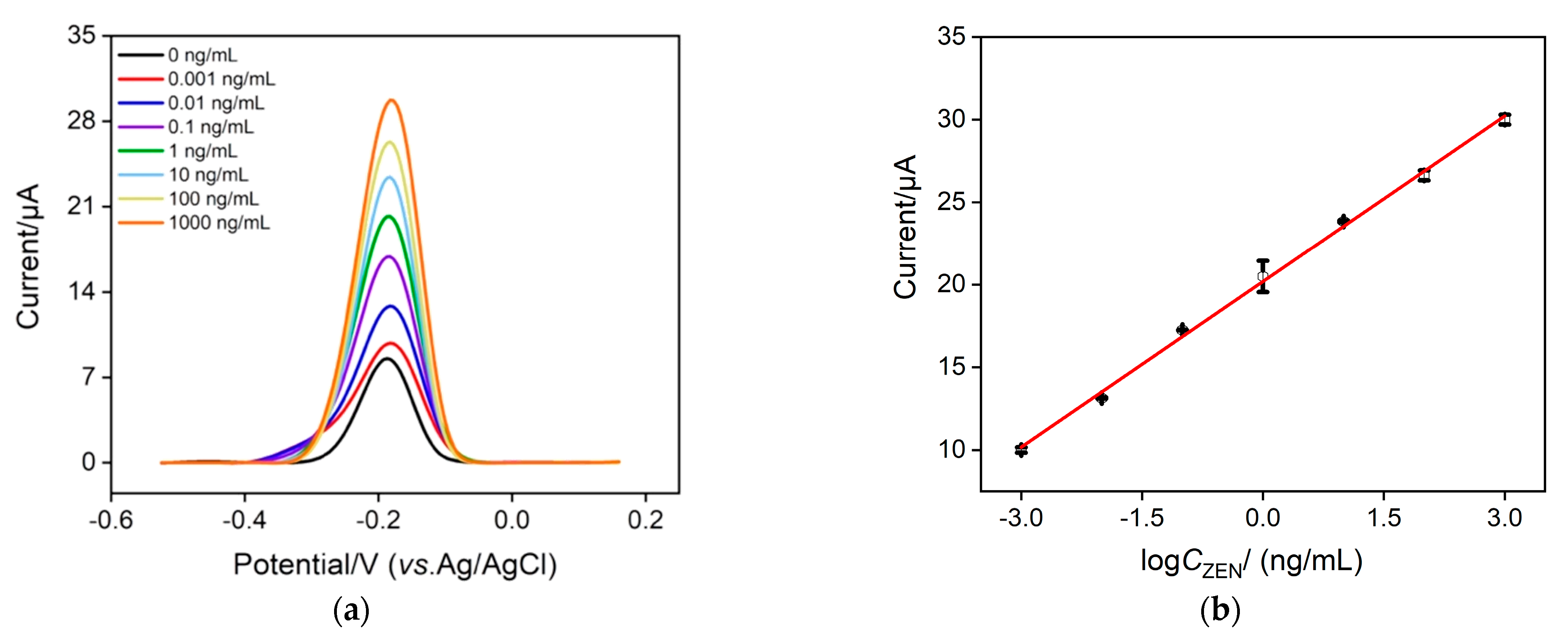
2.4. Detection of ZEN
2.5. Analytical Application in Food Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Apparatus
3.3. Preparation of the VMSF/ITO
3.4. Preparation of the Ru(NH3)63+–ZEN Aptamer–GO Nanocomposite Probe and Detection of ZEN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Viter, R.; Savchuk, M.; Iatsunskyi, I.; Pietralik, Z.; Starodub, N.; Shpyrka, N.; Ramanaviciene, A.; Ramanavicius, A. Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens. Bioelectron. 2018, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Eremin, S.A.; Wen, K.; Yu, X.; Li, C.; Ke, Y.; Jiang, H.; Shen, J.; Wang, Z. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the zearalenone class of mycotoxins in maize. J. Agric. Food Chem. 2017, 65, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.R.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2023, 403, 134384. [Google Scholar] [CrossRef]
- Hao, K.; Suryoprabowo, S.; Song, S.; Liu, L.; Kuang, H. Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food Agric. Immunol. 2017, 29, 498–510. [Google Scholar] [CrossRef]
- Habler, K.; Gotthardt, M.; Schuler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ren, Y.; Zhou, H.; Luan, L.; Cai, Z.; Wu, Y. A rapid method for simultaneous determination of zearalenone, alpha-zearalenol, beta-zearalenol, zearalanone, alpha-zearalanol and beta-zearalanol in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Mudili, V.; Kamasani, J.R.; Siddaiah, C. Discrete and combined effects of Ylang-Ylang (Cananga odorata) essential oil and gamma irradiation on growth and mycotoxins production by Fusarium graminearum in maize. Food Control 2018, 94, 276–283. [Google Scholar] [CrossRef]
- Shkembi, X.; Svobodova, M.; Skouridou, V.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Aptasensors for mycotoxin detection: A review. Anal. Biochem. 2022, 644, 114156. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.; Pike, A.; Tan, L.L. Recent advances in conventional methods and electrochemical aptasensors for mycotoxin detection. Foods 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernandez, S.; Bertolin, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.E.; Gobi, K.V.; Kim, K.H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, S.; Lin, X.; Liu, J.; Wang, K. Label-free electrochemical aptasensor based on the vertically-aligned mesoporous silica films for determination of aflatoxin B1. Biosensors 2023, 13, 661. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, S.; Liu, J.; Xing, J. Homogeneous electrochemical aptamer sensor based on two-dimensional nanocomposite probe and nanochannel modified electrode for sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 5186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Zhang, C.; Ji, H.; Pang, Q.; Li, X.; Luo, Z.; Wu, Q.; Zhang, L. Development of Aptamer-Based Molecular Tools for Rapid Intraoperative Diagnosis and In Vivo Imaging of Serous Ovarian Cancer. ACS Appl. Mater. Interfaces 2021, 13, 16118–16126. [Google Scholar] [CrossRef] [PubMed]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-based rapid diagnosis for point-of-care application. Microfluid. Nanofluid. 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, G.; Ni, J.; Wang, Q.; Lin, Z. From signal amplification to restrained background: Magnetic graphene oxide assisted homogeneous electrochemiluminescence aptasensor for highly sensitive detection of okadaic acid. Sens. Actuators B Chem. 2021, 327, 128872. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Design 2022, 215, 110506. [Google Scholar] [CrossRef]
- Medeiros, P.V.C.; Gueorguiev, G.K.; Stafström, S. Bonding, charge rearrangement and interface dipoles of benzene, graphene, and PAH molecules on Au(111) and Cu(111). Carbon 2015, 81, 620–628. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D structures of indium oxide of different stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 2308183. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive immunosensor for prostate-specific antigen based on enhanced electrochemiluminescence by vertically ordered mesoporous silica-nanochannel film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, C.; Ge, S.; Luo, T.; Chen, J.; Liu, J.; Xi, F.; Liu, J. Gram-scale synthesis of nitrogen doped graphene quantum dots for sensitive detection of mercury ions and L-cysteine. RSC Adv. 2019, 9, 32977–32983. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Zhao, R.; Lu, Y.; Shi, L.; Liu, J.; Dong, X.; Xi, F. Aqueous synthesis of amphiphilic graphene quantum dots and their application as surfactants for preparing of fluorescent polymer microspheres. Colloid Surface A 2019, 563, 77–83. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Pang, Y.; Chen, J.; Zhang, Z.; Xi, F.; Chen, P. Graphene quantum dots as full-color and stimulus responsive fluorescence ink for information encryption. J. Colloid Interface Sci. 2020, 579, 307–314. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, J.; Ding, Y.; Tang, H.; Xi, F. Iron and nitrogen co-doped graphene quantum dots as highly active peroxidases for the sensitive detection of l-cysteine. New J. Chem. 2021, 45, 19056–19064. [Google Scholar] [CrossRef]
- Ge, S.; He, J.; Ma, C.; Liu, J.; Xi, F.; Dong, X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019, 199, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, J.; Deng, X.; Chen, J.; Xi, F.; Wang, X. Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhao, X.; Chen, L.J.; Yang, C.; Yin, X.B.; Yan, X.P. A dual-colored persistent luminescence nanosensor for simultaneous and autofluorescence-free determination of aflatoxin B1 and zearalenone. Talanta 2021, 232, 122395. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Wang, X.; Pasha, I.; Khan, I.M.; Zhao, S.; Shoaib, M.; Wu, S.; Wang, Z. A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta 2018, 186, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, Y.; Qi, S.; Zhang, Y.; Wang, Z. Sensitive colorimetric aptasensor based on stimuli-responsive metal-organic framework nano-container and trivalent DNAzyme for zearalenone determination in food samples. Food Chem. 2022, 371, 131145. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K. Novel colorimetric aptasensor for zearalenone detection based on nontarget-induced aptamer walker, gold nanoparticles, and exonuclease-assisted recycling amplification. ACS Appl. Mater. Interfaces 2018, 10, 12504–12509. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yu, C.; Wen, Y.; Chen, J.; Yu, Y.; Zhang, C.; Gao, R.; Mu, X.; He, J. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yan, X. A “signal-on” voltammetric aptasensor fabricated by hcPt@AuNFs/PEI-rGO and Fe3O4NRs/rGO for the detection of zearalenone. Sens. Actuators B Chem. 2019, 290, 477–483. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical oxidation of glassy carbon provides similar electrochemical response as graphene oxide prepared by Tour or Hummers routes. ChemElectroChem 2015, 2, 761–767. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
| Electrode | Detection Method | Linear Range (ng/mL) | LOD (ng/mL) | References |
|---|---|---|---|---|
| - | PL | 0.001–50 | 2.2 × 10−4 | [51] |
| - | FL | 0.001–10 | 2.1 × 10−4 | [52] |
| - | FL | 0.01–100 | 3.6 × 10−4 | [53] |
| - | Colorimetry | 20–8 × 104 | 10 | [54] |
| Au-PANI-Au/GCE | i-t | 1 × 10−6–102 | 4.5 × 10−7 | [55] |
| AuNPs/Fe3O4NRs/rGO/AuE | EC | 0.5 × 10−3–50 | 1.1 × 10−4 | [56] |
| PtNi@Co-MOF/AuE | EC | 10 × 10−6–10 | 1.37 × 10−6 | [57] |
| homogeneous electrochemical aptasensor | EC | 0.001–1000 | 1.2 × 10−6 | This work |
| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Maize a | 0.0100 | 0.00971 | 97.1 | 1.2 |
| 0.1 | 0.103 | 103 | 2.1 | |
| 1000 | 992 | 99.2 | 1.2 | |
| Chestnut a | 0.0100 | 0.00960 | 96.0 | 1.9 |
| 0.100 | 0.105 | 105 | 1.9 | |
| 1000 | 991 | 99.1 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Luo, X.; Yan, F.; Zhou, B. Homogeneous Electrochemical Aptasensor for Sensitive Detection of Zearalenone Using Nanocomposite Probe and Silica Nanochannel Film. Molecules 2023, 28, 7241. https://doi.org/10.3390/molecules28217241
Huang Z, Luo X, Yan F, Zhou B. Homogeneous Electrochemical Aptasensor for Sensitive Detection of Zearalenone Using Nanocomposite Probe and Silica Nanochannel Film. Molecules. 2023; 28(21):7241. https://doi.org/10.3390/molecules28217241
Chicago/Turabian StyleHuang, Zhongnan, Xuan Luo, Fei Yan, and Bo Zhou. 2023. "Homogeneous Electrochemical Aptasensor for Sensitive Detection of Zearalenone Using Nanocomposite Probe and Silica Nanochannel Film" Molecules 28, no. 21: 7241. https://doi.org/10.3390/molecules28217241
APA StyleHuang, Z., Luo, X., Yan, F., & Zhou, B. (2023). Homogeneous Electrochemical Aptasensor for Sensitive Detection of Zearalenone Using Nanocomposite Probe and Silica Nanochannel Film. Molecules, 28(21), 7241. https://doi.org/10.3390/molecules28217241







