Research on the Effect of Oriental Fruit Moth Feeding on the Quality Degradation of Chestnut Rose Juice Based on Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
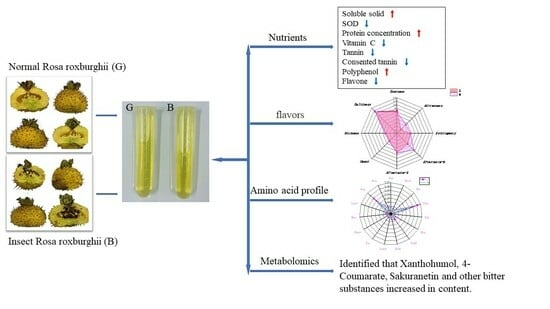
2.1. Effects of OFM Feeding on Functional Active Ingredients in Chestnut Rose Juice
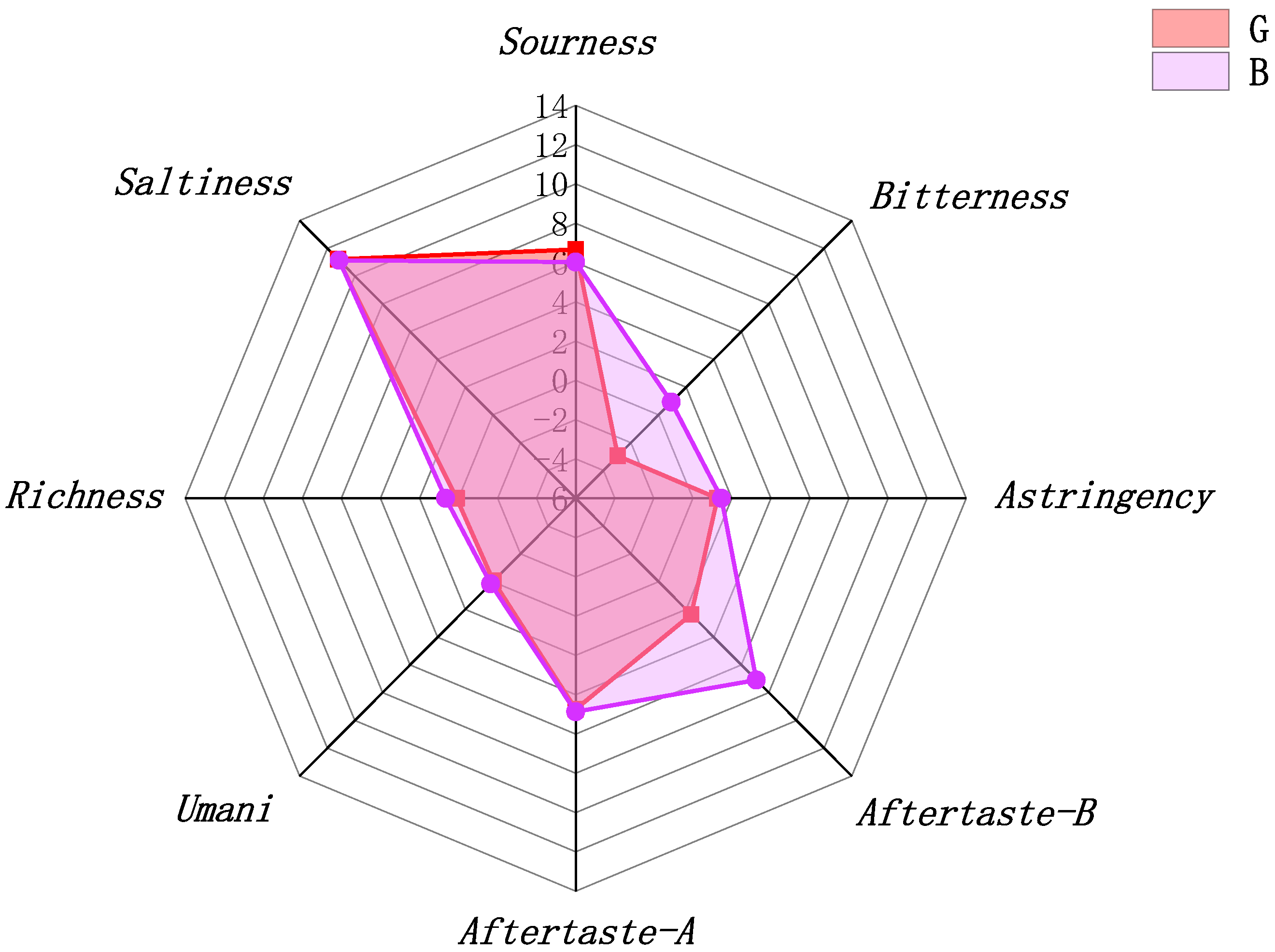
2.2. Effects of OFM Feeding on the Flavour of Chestnut Rose Juice
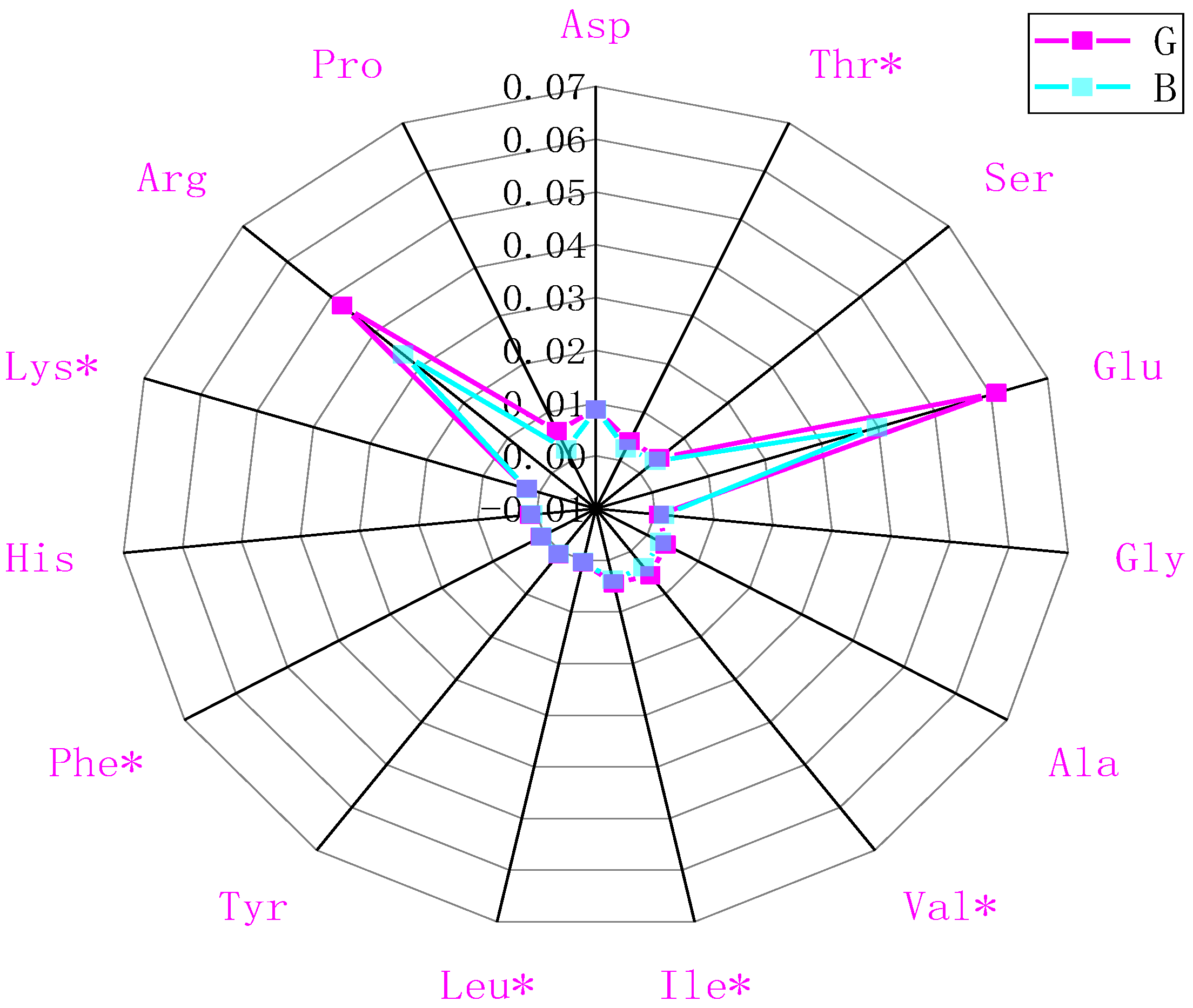
2.3. Effects of OFM Feeding on Amino Acid Profiles in Chestnut Rose Juice
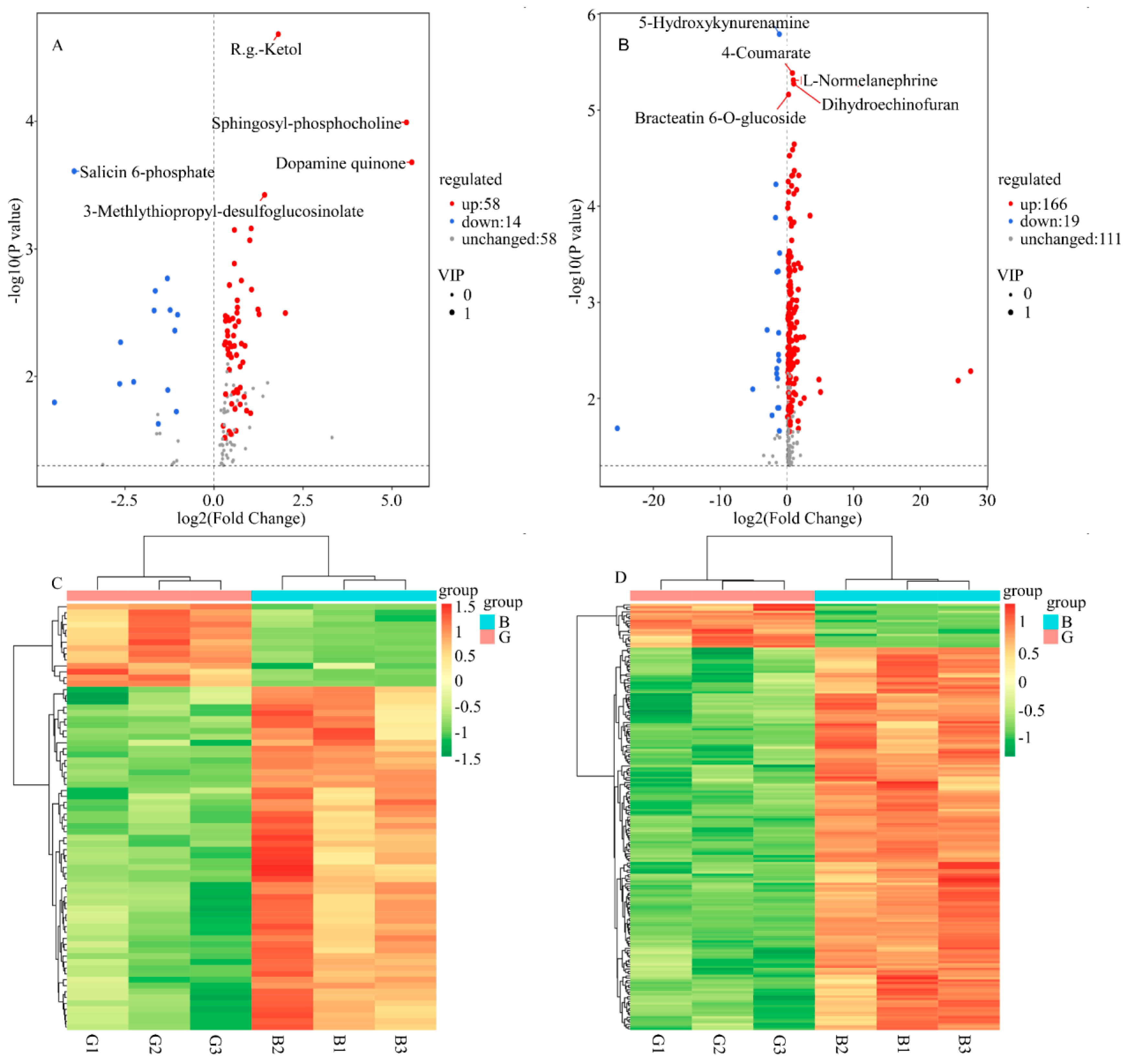
2.4. Effects of OFM Feeding on Metabolites in Chestnut Rose Juice
2.4.1. Nontargeted Metabolomics Analysis
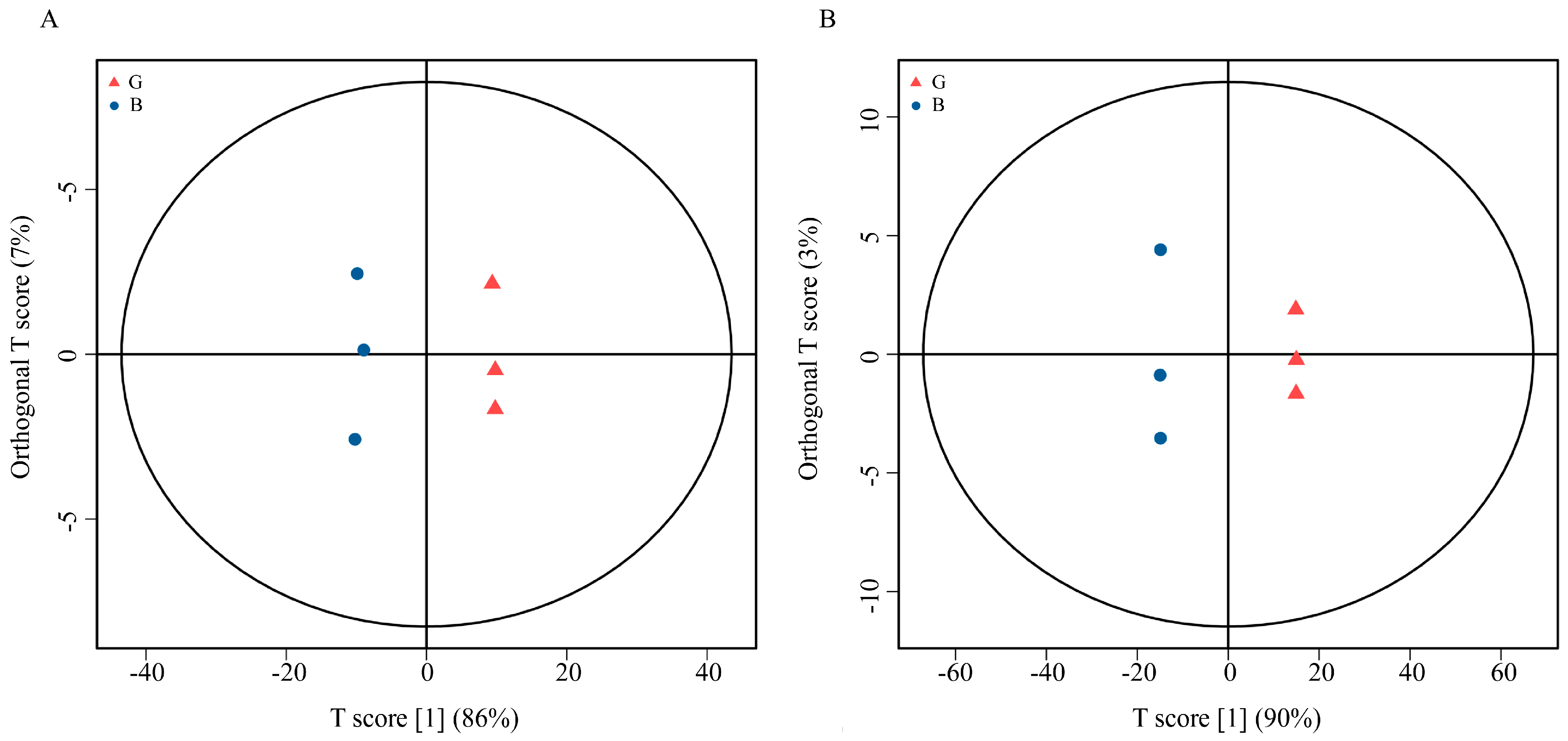
2.4.2. Principal Component Analysis (PCA)
2.4.3. Cluster Analysis of Differential Metabolites
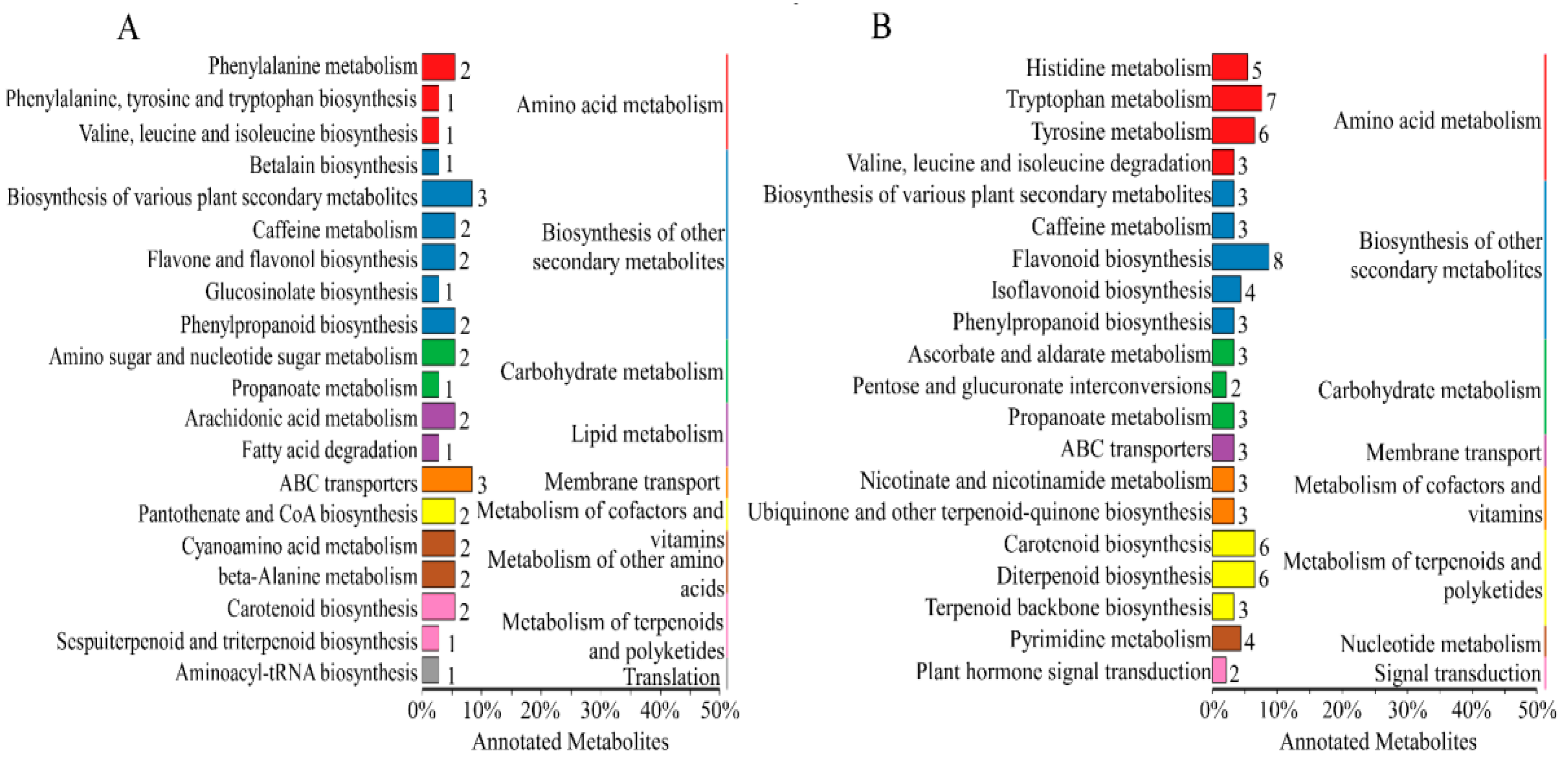
2.4.4. Enrichment Analysis of Differential Metabolites
3. Materials and Methods
3.1. Main Reagents
3.2. Collection and Juicing of Chestnut Rose Fruit
3.3. Determination of the Functional Active Ingredients in Chestnut Rose Juice
3.4. Electronic Tongue Test
3.5. Amino Acid Profile Analysis
3.6. Nontargeted Metabolomics Analysis
3.7. Data Processing and Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, Y.; Wang, J.; Cai, Z.; Huang, H.; Zhang, S.; Fu, L.; Zhao, P.; Yan, X.; Fu, Y. Improved physicochemical and functional properties of dietary fiber from Rosa roxburghii pomace fermented by bacillus natto. Food Biosci. 2022, 50, 102030. [Google Scholar] [CrossRef]
- Li, J.; Luo, Y.; Lu, M.; Wu, X.; An, H. The pathogen of top rot disease in Rosa roxburghii and its effective control fungicides. Horticulturae 2022, 8, 1036. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, H.; An, H. Chloroplast dna-based genetic variation of Rosa roxburghii in southwest china: Phylogeography and conservation implications. Hortic. Plant J. 2021, 7, 286–294. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, C.; Li, C.; Chen, J. Physicochemical, functional and antioxidant properties of dietary fiber from Rosa roxburghii tratt fruit modified by physical, chemical and biological-enzyme treatments. J. Food Process. Preserv. 2020, 44, e14858. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Design 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Wei, W.; Fan, J.; Zhenhui, Y.; Ming, L.I. Effects of different fermentation methods on the quality of Rosa roxburghii wine. Food Sci. Technol. 2020, 45, 105–113. [Google Scholar] [CrossRef]
- Marko, B.; Sonja, B.; Andreas, S.; Annick, S. Jasmonate-dependent induction of polyphenol oxidase activity in tomato foliage is important for defense against spodoptera exigua but not against manduca sexta. BMC Plant Biol. 2014, 14, 257. [Google Scholar] [CrossRef]
- Mitra, S.; Mobarak, S.H.; Karmakar, A.; Barik, A. Activities of antioxidant enzymes in three species of Ludwigia weeds on feeding by Altica cyanea. J. King Saud Univ.—Sci. 2019, 31, 1522–1527. [Google Scholar] [CrossRef]
- Ming, L.C.; Supannee, P.; Wanida, A.; Shigeru, M.; Yooichi, K. Oviposition preferences of the oriental fruit moth Grapholita molesta (Lepidoptera: Tortricidae) to pear hipvs. Arthropod-Plant Interact. 2022, 16, 517–523. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Han, L.; Liu, X.; An, H.; Wu, X. Research progress on occurrence and control of diseases and pests of Rosa roxburghii. China Plant Prot. 2020, 40, 24–30. [Google Scholar] [CrossRef]
- Jiaohong, L.; Zhenxiang, G.; Yue, L.; Xiaomao, W.; Huaming, A. Chitosan can induce Rosa roxburghii tratt. against Sphaerotheca sp. and enhance its resistance, photosynthesis, yield, and quality. Horticulturae 2021, 7, 289. [Google Scholar] [CrossRef]
- Akiyuki, T.; Kengo, N.; Tsukasa, K.; Zhongming, M.; Osamu, N.; Kevin, F.J. Taste transduction and channel synapses in taste buds. Pflug. Arch. Eur. J. Physiol. 2021, 473, 3–13. [Google Scholar] [CrossRef]
- Bloxham, C.J.; Foster, S.R.; Thomas, W.G. A bitter taste in your heart. Front. Physiol. 2020, 11, 431. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C.; Finger, T.E. Recent advances in taste transduction and signaling. F1000Research 2019, 8, F1000–F2117. [Google Scholar] [CrossRef]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef]
- Jiao, D.-Z.; Zhao, Z.-L. Research progress on influences of saline-alkali stress on plant morphology, physiology and biochemisttry and response of plants to saline-alkali stress. Jiangsu Agric. Sci. 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Yokoi, S.; Bressan, R.A.; Hasegawa, P.M. Salt stress tolerance of plants. In JIRCAS Working Report; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 2002; pp. 25–33. Available online: https://www.researchgate.net/publication/225040559 (accessed on 6 January 2023).
- Staley, J.T.; Stewart-Jones, A.; Pope, T.W.; Wright, D.J.; Leather, S.R.; Hadley, P.; Rossiter, J.T.; Emden, H.; Poppy, G.M. Varying responses of insect herbivores to altered plant chemistry under organic and conventional treatments. Proc. R. Soc. B Biol. Sci. 2009, 1682, 779–786. [Google Scholar] [CrossRef]
- Bo, L. Study on the chilling requirment and some physiological and biochemiscal changes of tree peony during dormancy release. Master’s Thesis, Shandong Agricultural University, Taian, China, 2004. [Google Scholar]
- Pedrol, N.; Ramos, P.; Reigosa, M.J. Phenotypic plasticity and acclimation to water deficits in velvet-grass: A long-term greenhouse experiment. Changes in leaf morphology, photosynthesis and stress-induced metabolites. J. Plant Physiol. 2000, 157, 383–393. [Google Scholar] [CrossRef]
- Howe, G.A.; Koo, A.J.; Major, I.T. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef]
- Liechti, R.; Farmer, E.E. The jasmonate pathway. Science 2002, 296, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, L.; Hou, X.; Liao, Y.; Yang, Z. Low temperature synergistically promotes wounding-induced indole accumulation by inducer of cbf expression-mediated alterations of jasmonic acid signaling in Camellia sinensis. J. Exp. Bot. 2020, 71, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Siemann, E.; Xiao, L.; Yang, X.; Ding, J. Species-specific defence responses facilitate conspecifics and inhibit heterospecifics in above-belowground herbivore interactions. Nat. Commun. 2014, 5, 4851. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.-J.; Gao, X.-W. Plant defense responses induced by insect herbivory. Acta Entomol. Sin. 2005, 58, 125–134. [Google Scholar] [CrossRef]
- Asakawa, Y.; Kenmoku, H. Dietary diterpenoids. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 733–927. [Google Scholar]
- Sibel, K. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in alcoholic beverages: The profiles of perception, constituents, and contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Sonntag, T.; Kunert, C.; Dunkel, A.; Hofmann, T. Sensory-guided identification of n-(1-methyl-4-oxoimidazolidin-2-ylidene)-α-amino acids as contributors to the thick-sour and mouth-drying orosensation of stewed beef juice. J. Agric. Food Chem. 2010, 58, 6341–6350. [Google Scholar] [CrossRef]
- Zhou, C.; Le, Y.; Zheng, Y.; Wang, J.; Li, G.; Bai, Y.; Li, C.; Xu, X.; Zhou, G.; Cao, J. Characterizing the effect of free amino acids and volatile compounds on excessive bitterness and sourness in defective dry-cured ham. LWT 2020, 123, 109071. [Google Scholar] [CrossRef]
- Yu, H.; Li, Q.; Xie, J.; Chen, C.; Lou, X.; Ai, L.; Tian, H. Characterization of bitter compounds in Shaoxing Huangjiu by quantitative measurements, taste recombination, and omission experiments. J. Agric. Food Chem. 2022, 70, 12907–12915. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, X.; Wu, T.; Jiang, J.; Jiang, Y.; Zhang, S.; Ruan, Y.; Zhang, H.; Zhang, C.; Chen, P.; et al. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. J. Transl. Med. 2022, 20, 123. [Google Scholar] [CrossRef]
- Suh, J.H.; Madden, R.T.; Sung, J.; Chambers, A.H.; Crane, J.; Wang, Y. Pathway-based metabolomics analysis reveals biosynthesis of key flavor compounds in mango. J. Agric. Food Chem. 2022, 70, 10389–10399. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 2017, 58, 1594–1600. [Google Scholar] [CrossRef]
- Hamamouch, N.; Winkel, B.S.J.; Li, C.; Davis, E.L. Modulation of arabidopsis flavonol biosynthesis genes by cyst and root-knot nematodes. Plants 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, C.; Cluzet, S.; Fournier, J.L.; Huguet, T.; Gamas, P.; Prospéri, J.; Esquerré-Tugayé, M.; Dumas, B.; Jacquet, C. Cytological, genetic, and molecular analysis to characterize compatible and incompatible interactions between Medicago truncatula and Colletotrichum trifolii. Mol. Plant-Microbe Interact. 2004, 17, 909–920. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Vicente De Andrade Silva, G.; Demaman Arend, G.; Antonio Ferreira Zielinski, A.; Di Luccio, M.; Ambrosi, A. Xanthohumol properties and strategies for extraction from hops and brewery residues: A review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef]
- Dresel, M.; Dunkel, A.; Hofmann, T. Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of pilsner-type beer. J. Agric. Food Chem. 2015, 63, 3402–3418. [Google Scholar] [CrossRef] [PubMed]
- Ndebele, K.; Graham, B.; Tchounwou, P. Estrogenic activity and molecular mechanisms of coumestrol-induced biological effects. In Isoflavones: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: London, UK, 2012; pp. 518–528. [Google Scholar] [CrossRef]
- Boulebd, H. Computational analysis of peroxyl radical scavenging capacity of coumestrol: Insights into kinetics and reaction mechanisms. J. Phys. Org. Chem. 2022, 35, e4421. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhang, L.; Mao, J.; Ding, Z.Y.; Shi, G.Y. Metabolic engineering of Escherichia coli for the production of phenylpyruvate derivatives. Metab. Eng. 2015, 32, 55–65. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, S.; Shukuya, K.; Sogabe, K.; Ejima, Y.; Tokuhara, Y. Novel absorbance peak of gentisic acid following the oxidation reaction. PLoS ONE 2020, 15, e232263. [Google Scholar] [CrossRef] [PubMed]
- Poulami, G.; De, B. Influence of calcium channel modulators on the production of serotonin, gentisic acid, and a few other biosynthetically related phenolic metabolites in seedling leaves of salt tolerant rice variety nonabokra. Plant Signal. Behav. 2021, 16, 1929732. [Google Scholar] [CrossRef]
- Vannelli, T.; Qi, W.W.; Sweigard, J.; Gatenby, A.A.; Sariaslani, F.S. Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab. Eng. 2007, 9, 142–151. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, P.-C.; Zhang, S.-S.; Yang, J.; Li, G.-C.; Huang, L.-Q.; Wang, C.-Z. Functional analysis of a bitter gustatory receptor highly expressed in the larval maxillary galea of Helicoverpa armigera. PLoS Genet. 2022, 18, e1010455. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Austin, M.B.; Stewart, C.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Asakawa, Y.; Kenmoku, H. Dietary diterpenoids. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–195. [Google Scholar]
- MAlfieri, M.; Ambrosone, A.; Vaccaro, M.; De Tommasi, N.; Leone, A. Overcoming metabolic constraints in the mep-pathway enrich Salvia sclarea hairy roots in therapeutic abietane diterpenes. Appl. Sci. 2022, 12, 7116. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Tanaka, N.; Matsuoka, M.; Kitano, H.; Asano, T.; Kaku, H.; Komatsu, S. Gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (pbz1) in response to cold stress and pathogen attack. Plant Cell Environ. 2006, 29, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-H.; Su, A.-Q.; Wang, Y.-G.; Geng, G. Effects of foliar spraying gibberellin and brassicin on physiological characteristics of sugar beet. J. Eng. Heilongjiang Univ. 2022, 13, 91–96. [Google Scholar] [CrossRef]
- Li, J. Determination of reduced vitamin c by key blue colorimetry. Food Sci. 2000, 21, 42–45. [Google Scholar]
- Wu, Y.; Guo, Y. Determination of tannin in cotton plant. Chin. J. Appl. Ecol. 2000, 11, 243–245. [Google Scholar] [CrossRef]
- Li, J.; Ni, J.-Y.; Li, H.-F.; Xu, G.-F.; Wang, X.-D.; Wu, Y.-L.; Wang, Z.-X. On determination conditions for total polyphenols in fruits and its derived products by folin-phenol methods. J. Fruit Sci. 2008, 25, 126–131. [Google Scholar]
- Zhang, L.-P.; Zhou, L.; Guan, B.-F.; Wang, F.-Y.; Liu, H.-W.; Gao, H.-L.; Jia, Y.; Luo, B.-B.; Li, P.-C. Ultrasound-assisted extraction process of total flavonoids from persimmon leaves. Food Res. Dev. 2018, 39, 87–91. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Shen, X.; Liu, J.; Zhao, D.; Sun, Y.; Wang, L.; Liu, Y.; Gong, X.; Liu, Y.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by uhplc-qtof/ms. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]
| Index | G | B |
|---|---|---|
| Weight (g) | 20.34 ± 4.37 a | 21.88 ± 4.05 a |
| Transverse and longitudinal ratio | 1.37 ± 0.13 a | 1.30 ± 0.12 b |
| Total color difference | 52.40 ± 2.20 a | 52.55 ± 1.77 a |
| Juice yield (%) | 67.19 ± 1.05 a | 55.36 ± 5.95 b |
| Bad fruit rate (%) | 27.68 ± 1.27 | |
| Soluble solid (%) | 9.39 ± 0.06 b | 11.07 ± 0.06 a |
| SOD (U/mL) | 118.41 ± 1.04 a | 117.23 ± 0.51 a |
| Protein concentration (mg/mL) | 20.64 ± 0.52 b | 22.81 ± 1.24 a |
| Vitamin C (mg/100 g) | 2038.93 ± 134.36 a | 1971.22 ± 139.85 a |
| Tannin (mg/mL) | 4.47 ± 0.12 a | 4.12 ± 0.21 a |
| Consented tannin (mg/mL) | 36.62 ± 0.87 a | 28.35 ± 5.64 a |
| Polyphenol (mg/mL) | 1.91 ± 0.12 a | 1.93 ± 0.02 a |
| Flavone (mg/mL) | 25.15 ± 0.97 a | 17.93 ± 0.68 b |
| Pathway | Metabolite | Formula | p | VIP | Fold Change | Regulated |
|---|---|---|---|---|---|---|
| Phenylalanine metabolism | Phenylacetyl-CoA | 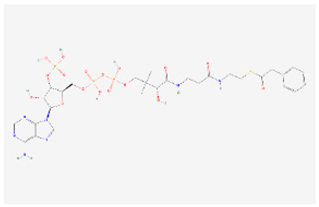 C29H42N7O17P3S | 0.003 | 1.039 | 1.572 | Up |
| 2-Hydroxyphenylacetate |  C8H8O3 | 0.005 | 1.025 | 1.462 | Up | |
| Tryptophan metabolism | 5-Hydroxyindoleacetate |  C10H9NO3 | 0.004 | 1.019 | 1.626 | Up |
| Formyl-5-hydroxykynurenamine | 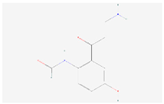 C10H12N2O3 | 0.006 | 1.020 | 1.245 | Up | |
| L-Formylkynurenine | 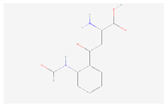 C11H12N2O4 | 0.001 | 1.024 | 1.199 | Up | |
| 3-Hydroxyanthranilic acid | 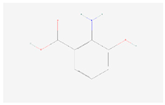 C7H7NO3 | 0.012 | 1.021 | 1.227 | Up | |
| 5-Hydroxykynurenamine |  C9H12N2O2 C9H12N2O2 | 0.000002 | 1.052 | 0.460 | Down | |
| 2-Aminophenoxazin-3-one | 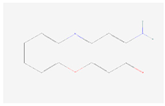 C12H8N2O2 | 0.012 | 1.002 | 1.392 | Up | |
| 3-Hydroxykynurenamine | 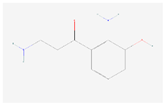 C9H12N2O2 | 0.0005 | 1.045 | 2.288 | Up | |
| Tyrosine metabolism | Gentisic acid | 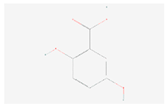 C7H6O4 | 0.002 | 1.019 | 1.169 | Up |
| 5-(L-Alanin-3-yl)-2-hydroxy-cis,cis-muconate 6-semialdehyde |  C9H11NO6 | 0.001 | 1.042 | 1.118 | Up | |
| Gentisate aldehyde |  C7H6O3 | 0.016 | 1.014 | 1.084 | Up | |
| Acetoacetate | 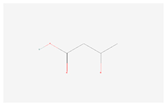 C4H6O3 | 0.001 | 1.026 | 1.446 | Up | |
| Hydroquinone | 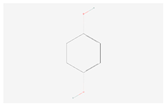 C6H6O2 | 0.007 | 1.011 | 1.449 | Up | |
| 4-Coumarate |  C9H8O3 | 0.000004 | 1.052 | 1.780 | Up | |
| Histidine metabolism | N-Formimino-L-glutamate | 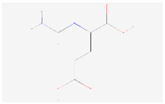 C6H10N2O4 | 0.002 | 1.031 | 1.352 | Up |
| L-Histidinol phosphate | 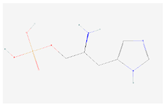 C6H12N3O4P | 0.011 | 1.006 | 1.820 | Up | |
| Dihydrourocanate | 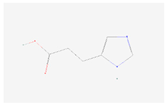 C6H8N2O2 | 0.005 | 1.017 | 1.163 | Up | |
| Hydantoin-5-propionate |  C6H8N2O4 | 0.0003 | 1.038 | 1.357 | Up | |
| D-erythro-1-(Imidazol-4-yl)glycerol 3-phosphate |  C6H11N2O6P | 0.001 | 1.046 | 3.296 | Up | |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | Quinate | 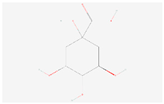 C7H12O6 | 0.013 | 1.023 | 0.440 | Down |
| Valine, leucine, and isoleucine degradation | 2-Methyl-1-hydroxypropyl-ThPP |  C16H27N4O8P2S+ | 0.006 | 1.033 | 1.450 | Up |
| (S)-3-Methyl-2-oxopentanoic acid | 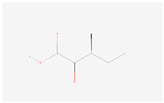 C6H10O3 | 0.006 | 1.036 | 1.225 | Up | |
| Diterpenoid biosynthesis | Gibberellin A36 | 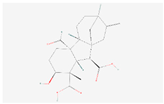 C20H26O6 | 0.0004 | 1.048 | 1.199 | Up |
| Gibberellin A8 | 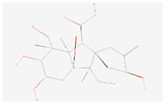 C19H24O7 | 0.001 | 1.025 | 1.404 | Up | |
| ent-Copalyl diphosphate | 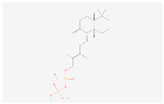 C20H36O7P2 | 0.002 | 1.035 | 1.265 | Up | |
| Gibberellin A53 | 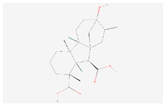 C20H28O5 | 0.00006 | 1.051 | 1.643 | Up | |
| Gibberellin A29-catabolite |  C19H22O6 | 0.017 | 1.012 | 1.433 | Up | |
| Geranylgeranyl diphosphate |  C20H36O7P2 | 0.005 | 1.012 | 1.242 | Up | |
| Sesquiterpenoid and triterpenoid biosynthesis | (−)-Germacrene D | 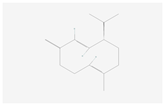 C15H24 | 0.001 | 1.050 | 2.007 | Up |
| Terpenoid backbone biosynthesis | all-trans-Hexaprenyl diphosphate | 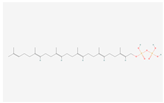 C30H52O7P2 | 0.002 | 1.016 | 2.209 | Up |
| 4-(Cytidine 5′-diphospho)-2-C-methyl-D-erythritol |  C14H25N3O14P2 | 0.006 | 1.010 | 0.377 | Down | |
| Pyrimidine metabolism | dCMP |  C9H14N3O7P | 0.013 | 1.019 | 1.184 | Up |
| Pseudouridine | 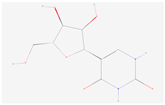 C9H12N2O6 | 0.013 | 1.008 | 1.166 | Up | |
| 3-Hydroxypropanoate | 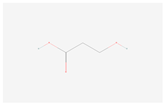 C3H6O3 | 0.0003 | 1.043 | 1.312 | Up | |
| Cytidine |  C9H13N3O5 | 0.009 | 1.015 | 1.175 | Up | |
| Phenylpropanoid biosynthesis | Caffeyl alcohol | 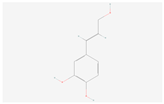 C9H10O3 | 0.014 | 1.019 | 1.462 | Up |
| 1-O-Sinapoyl-beta-D-glucose | 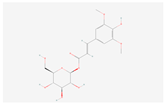 C17H22O10 | 0.001 | 1.045 | 1.331 | Up | |
| 5-Hydroxyconiferaldehyde | 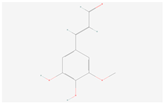 C10H10O4 | 0.004 | 1.066 | 1.252 | Up | |
| Coniferyl aldehyde | 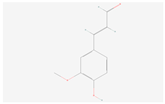 C10H10O3 | 0.003 | 1.026 | 1.249 | Up | |
| Caffeine metabolism | 7-Methylxanthine | 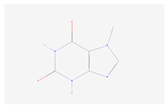 C6H6N4O2 | 0.007 | 1.008 | 1.548 | Up |
| N,N’-Dimethylurea | 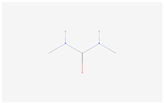 C3H8N2O | 0.006 | 1.029 | 1.487 | Up | |
| 1-Methyluric acid | 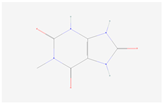 C6H6N4O3 | 0.001 | 1.031 | 1.530 | Up | |
| 5-Acetylamino-6-formylamino-3-methyluracil | 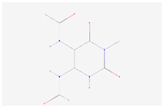 C8H10N4O4 | 0.004 | 1.043 | 2.943 | Up | |
| 7-Methylxanthosine | 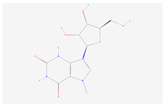 C11H15N4O6 | 0.0004 | 1.038 | 3.267 | Up | |
| Flavone and flavonol biosynthesis | Quercetin 3-sulfate | 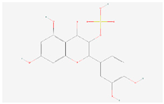 C15H10O10S | 0.004 | 1.066 | 0.466 | Down |
| Lampranthin II | 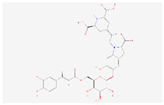 C34H34N2O16 | 0.003 | 1.049 | 0.426 | Down | |
| Flavonoid biosynthesis | Xanthohumol | 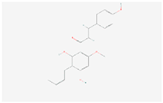 C21H22O5 | 0.0005 | 1.044 | 1.336 | Up |
| Pseudobaptigenin | 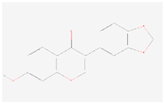 C16H10O5 | 0.007 | 1.031 | 1.512 | Up | |
| Coumestrol | 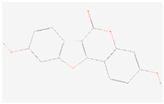 C15H8O5 | 0.00007 | 1.053 | 1.193 | Up | |
| Butin | 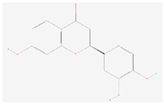 C15H12O5 | 0.004 | 1.004 | 1.143 | Up | |
| Liquiritigenin | 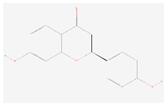 C15H12O4 | 0.006 | 1.004 | 1.282 | Up | |
| (+)-Gallocatechin | 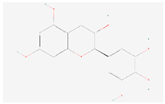 C15H14O7 | 0.004 | 1.002 | 1.195 | Up | |
| Dihydromyricetin |  C15H12O8 | 0.003 | 1.023 | 1.969 | Up | |
| Sakuranetin | 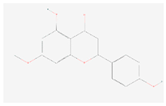 C16H14O5 | 0.00007 | 1.046 | 2.763 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, T.; Li, B.; Xu, F.; Chen, Z.; Lu, M.; Tan, S. Research on the Effect of Oriental Fruit Moth Feeding on the Quality Degradation of Chestnut Rose Juice Based on Metabolomics. Molecules 2023, 28, 7170. https://doi.org/10.3390/molecules28207170
Ren T, Li B, Xu F, Chen Z, Lu M, Tan S. Research on the Effect of Oriental Fruit Moth Feeding on the Quality Degradation of Chestnut Rose Juice Based on Metabolomics. Molecules. 2023; 28(20):7170. https://doi.org/10.3390/molecules28207170
Chicago/Turabian StyleRen, Tingyuan, Bei Li, Fangyan Xu, Zhen Chen, Mintao Lu, and Shuming Tan. 2023. "Research on the Effect of Oriental Fruit Moth Feeding on the Quality Degradation of Chestnut Rose Juice Based on Metabolomics" Molecules 28, no. 20: 7170. https://doi.org/10.3390/molecules28207170
APA StyleRen, T., Li, B., Xu, F., Chen, Z., Lu, M., & Tan, S. (2023). Research on the Effect of Oriental Fruit Moth Feeding on the Quality Degradation of Chestnut Rose Juice Based on Metabolomics. Molecules, 28(20), 7170. https://doi.org/10.3390/molecules28207170