Highly Selective and Fast Response/Recovery Cataluminescence Sensor Based on SnO2 for H2S Detection
Abstract
:1. Introduction
2. Results
2.1. Structural and Morphological Characterizations
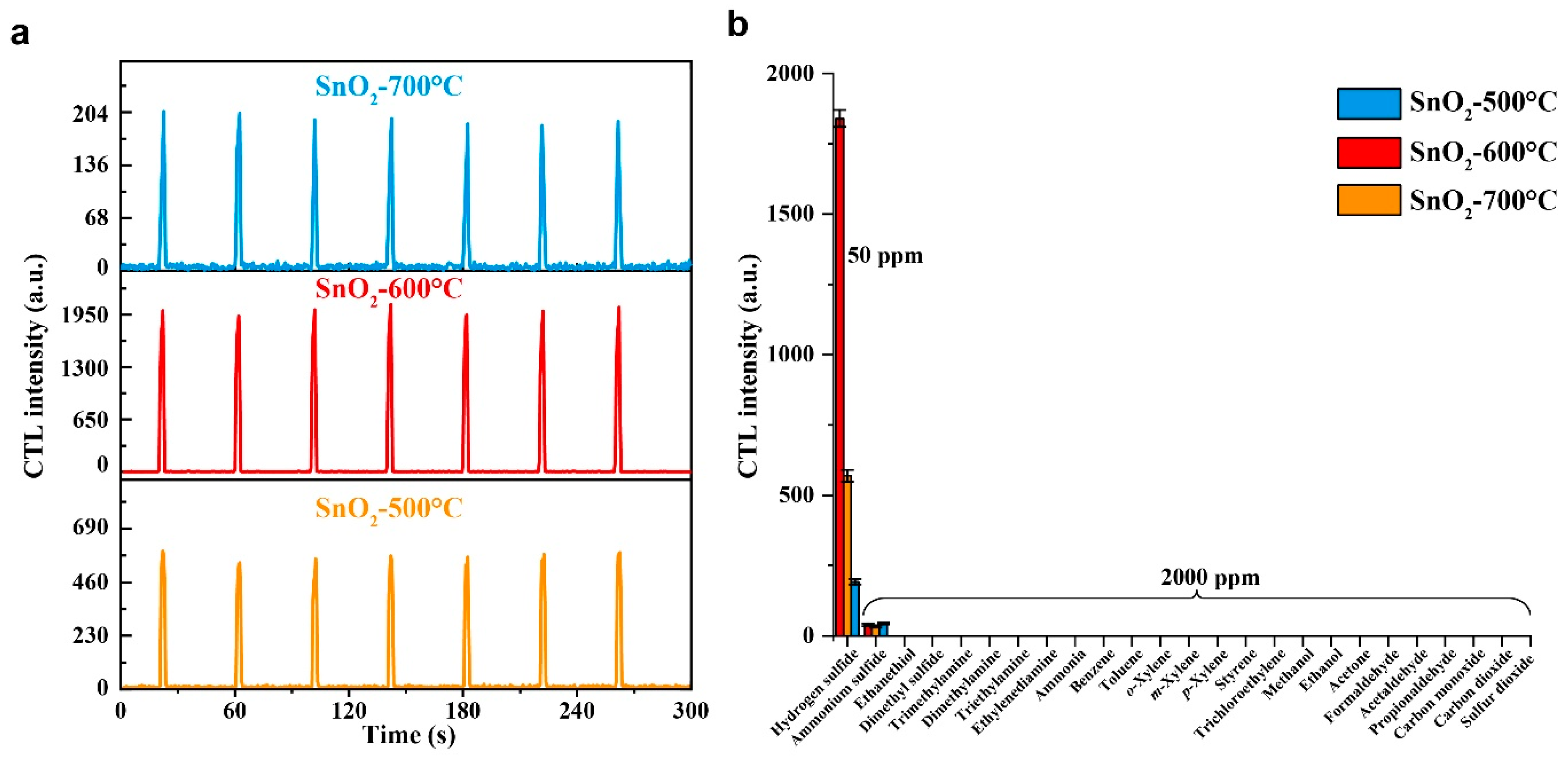
2.2. Evaluation of the Sensing Materials
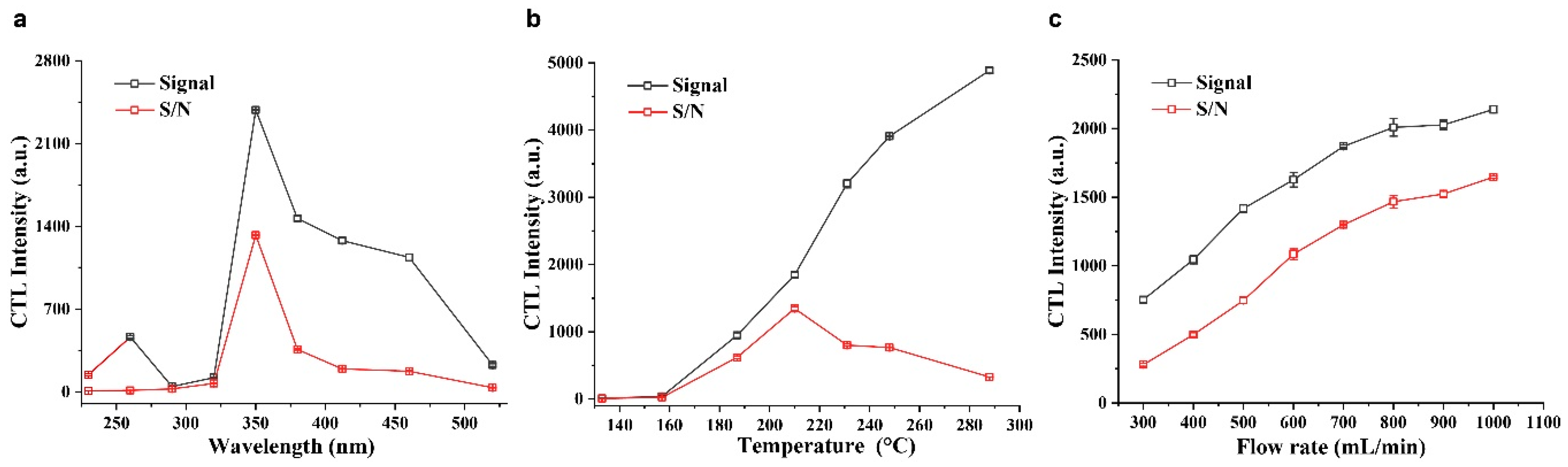
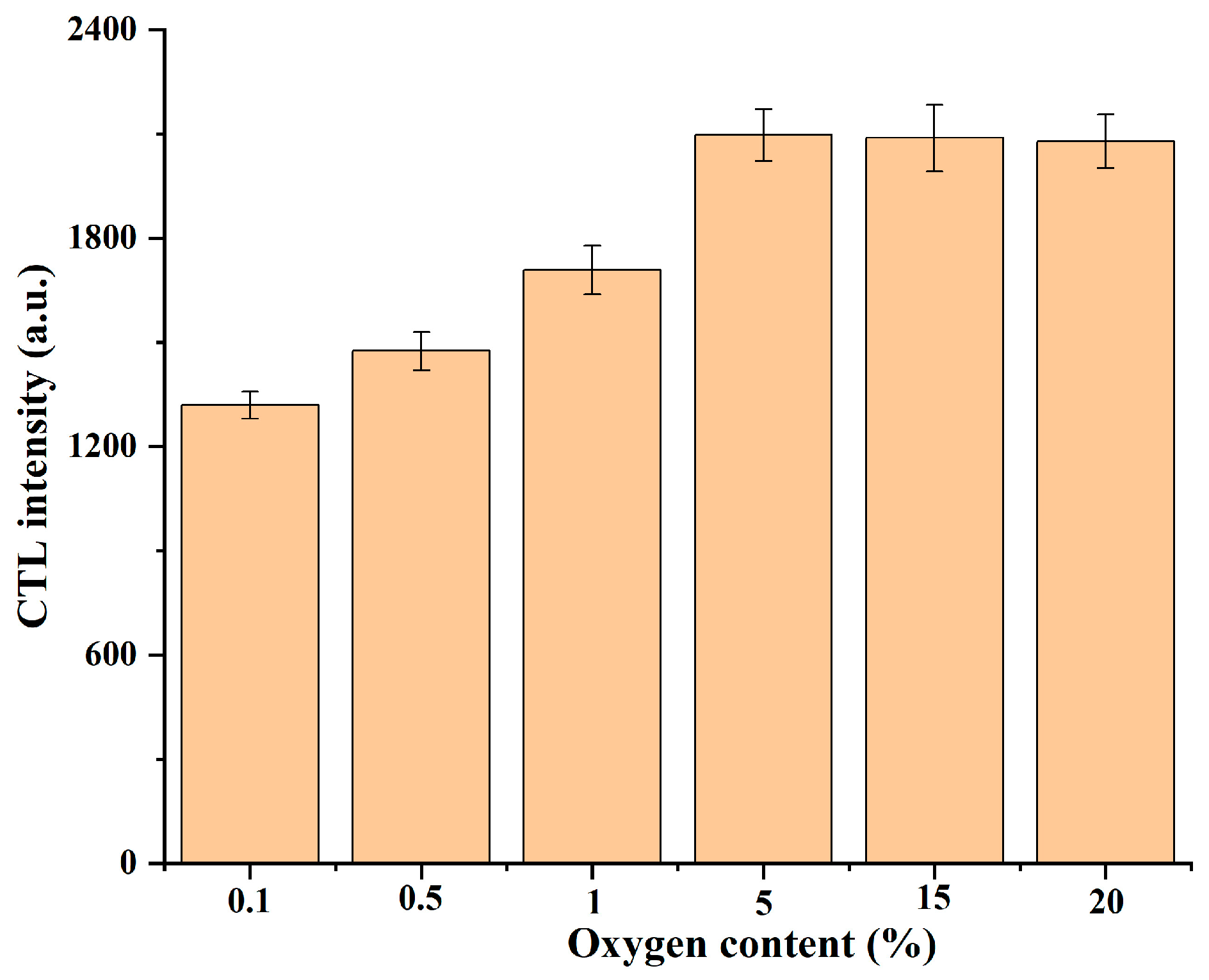
2.3. Optimization of Conditions
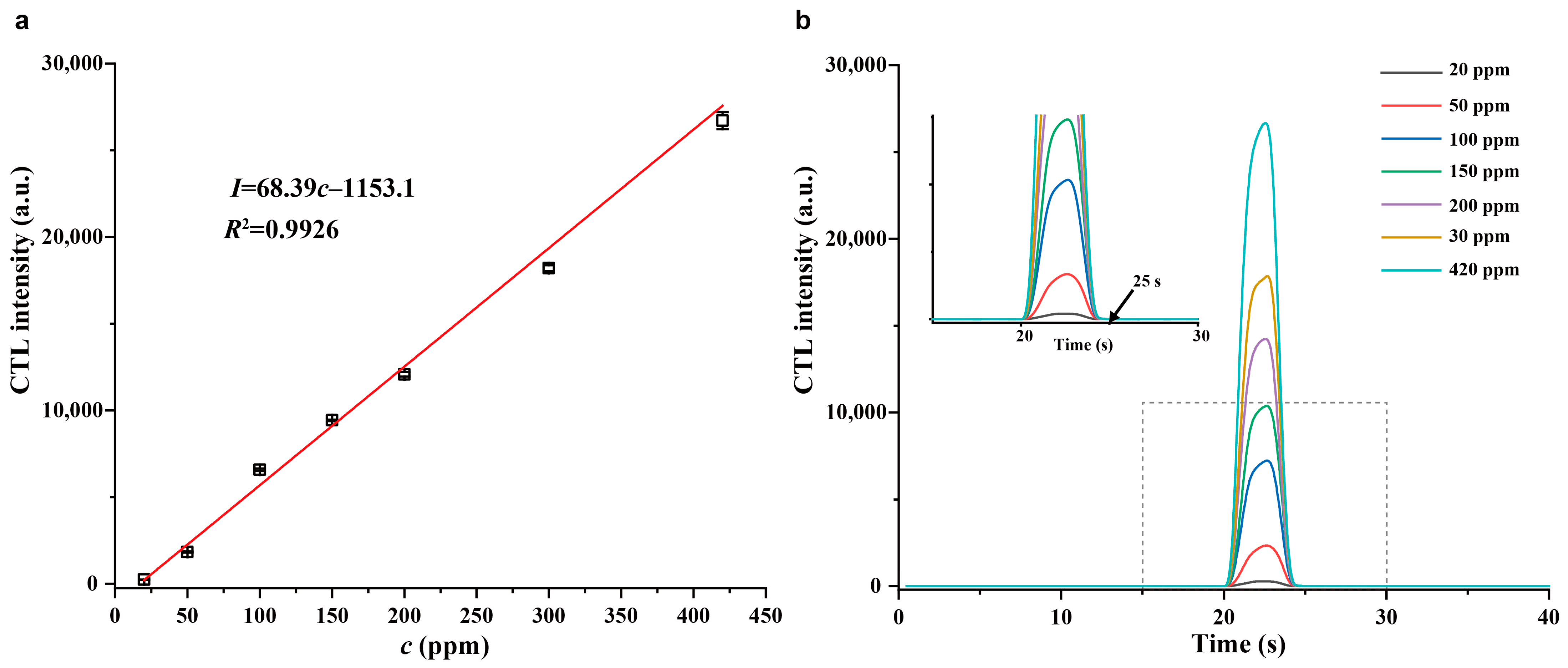
2.4. Analytical Characteristics
2.5. Mechanism Study
3. Experimental Section
3.1. Synthesis of SnO2
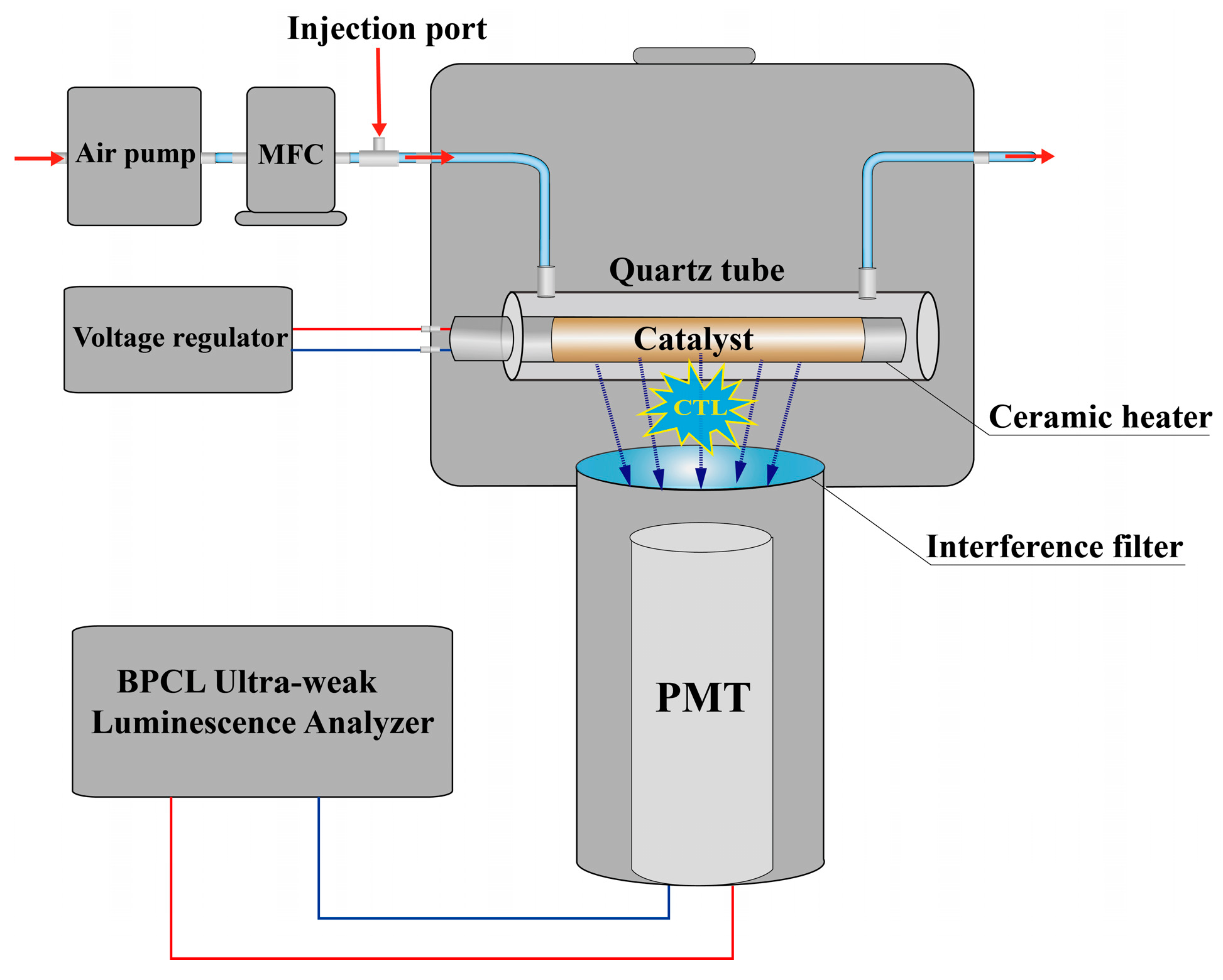
3.2. Characterization and Apparatus
3.3. Fabrication of the Gas Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, Y.; Sun, B.; Jiang, L.; Li, X.; Ma, Y.; Wang, K.; Han, P.; Jin, S. Effect of ag doping on SnO2 sensing for detecting H2S: A first-principles study. Vacuum 2021, 194, 110587. [Google Scholar] [CrossRef]
- Han, C.H.; Li, X.W.; Shao, C.L.; Li, X.H.; Ma, J.G.; Zhang, X.T.; Liu, Y.C. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B Chem. 2019, 285, 495–503. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, S.Y.; Zheng, H.P.; Han, Z.Z.; Lin, B.; Wang, Y.Y.; Guo, X.J.; Zhou, T.G.; Zhang, H.B.; Wu, J.J. A visual color response test paper for the detection of hydrogen sulfide gas in the air. Molecules 2023, 28, 5044. [Google Scholar] [CrossRef]
- Maldonado, C.M.S.; Kim, D.; Purnell, B.; Li, R.; Buchanan, G.F.; Smith, J.; Thedens, D.R.; Gauger, P.; Rumbeiha, W.K. Acute hydrogen sulfide-induced neurochemical and morphological changes in the brainstem. Toxicology 2023, 485, 153424. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Akhtar, A. Ppb-level hydrogen sulfide gas sensor based on the nanocomposite of MoS2 octahedron/ZnO-Zn2SnO4 nanoparticles. Molecules 2023, 28, 3230. [Google Scholar] [CrossRef] [PubMed]
- Health, A.S.E. EH40/2005 Workplace Exposure Limits for Use with the Control of Substances; TSO: London, UK, 2020.
- Pu, S.R.; Pan, Y.; Zhang, L.C.; Lv, Y. Recent advances in chemiluminescence and cataluminescence for the detection of volatile sulfur compounds. Appl. Spectrosc. Rev. 2023, 58, 401–427. [Google Scholar] [CrossRef]
- Zhang, R.K.; Wang, J.X.; Cao, H. High-performance cataluminescence sensor based on nanosized V2O5 for 2-butanone detection. Molecules 2020, 25, 3552. [Google Scholar] [CrossRef]
- Zhong, Y.H.; Li, M.; Tan, R.X.; Xiao, X.H.; Hu, Y.F.; Li, G.K. Co (Ⅲ) doped-CoFe layered double hydroxide growth with graphene oxide as cataluminescence catalyst for detection of carbon monoxide. Sens. Actuators B Chem. 2021, 347, 130600. [Google Scholar] [CrossRef]
- Zhang, R.K.; Wang, D.; Wu, Y.J.; Hu, Y.H.; Chen, J.Y.; He, J.C.; Wang, J.X. A cataluminescence sensor based on NiO nanoparticles for sensitive detection of acetaldehyde. Molecules 2020, 25, 1097. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Q.; Yuan, Z.Q.; Lin, Y.J.; Ding, C.F.; Lu, C. Propanol-triggered luminescence for rapid screening of crystal facets in noble metal. Anal. Chem. 2019, 91, 4513–4519. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhang, L.C.; Song, H.J.; Lv, Y. Evaluating the band gaps of semiconductors by cataluminescence. Anal. Chem. 2021, 93, 14454–14461. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lu, X.; Xia, L.; Zhong, Y.; Li, G.; Hu, Y. Cobalt doped nitrogenous porous carbon derived from covalent organic framework as cataluminescence catalyst for rapid determination of n-hexane in edible oil. Talanta 2021, 232, 122428. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Hu, J.; Song, H.; Zhang, L.; Lv, Y. Ratiometric cataluminescence sensor of amine vapors for discriminating meat spoilage. Anal. Chem. 2021, 93, 6692–6697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Na, N.; Zhang, S.C.; Wu, Y.Y.; Zhang, X.R. Rapid screening of gold catalysts by chemiluminescence-based array imaging. J. Am. Chem. Soc. 2007, 129, 6062–6063. [Google Scholar] [CrossRef] [PubMed]
- Na, N.; Zhang, S.C.; Wang, X.; Zhang, X.R. Cataluminescence-based array imaging for high-throughput screening of heterogeneous catalysts. Anal. Chem. 2009, 81, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.X.; Li, G.K.; Hu, Y.F. Cataluminescence sensor based on Pt/NU-901 nanocomposite for rapid capture, catalysis and detection of acetone in exhaled breath. Anal. Chim. Acta 2022, 1206, 339787. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Yan, W.L.; Jiang, L.; Zheng, Y.G.; Wang, J.X.; Zhang, R.K. Synthesis of nano-praseodymium oxide for cataluminescence sensing of acetophenone in exhaled breath. Molecules 2019, 24, 4275. [Google Scholar] [CrossRef]
- Na, N.; Liu, H.Y.; Han, J.Y.; Han, F.F.; Liu, H.L.; Ouyang, J. Plasma-assisted cataluminescence sensor array for gaseous hydrocarbons discrimination. Anal. Chem. 2012, 84, 4830–4836. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jiang, H.J.; Xing, Z.; Zhang, X.R. A highly selective chemiluminescent H2S sensor. Sens. Actuators B Chem. 2004, 102, 155–161. [Google Scholar] [CrossRef]
- Cai, P.Y.; Bai, W.; Zhang, L.C.; Song, H.J.; Su, Y.Y.; Lv, Y. Hierarchical hollow microsphere and flower-like indium oxide: Controllable synthesis and application as H2S cataluminescence sensing materials. Mater. Res. Bull. 2012, 47, 2212–2218. [Google Scholar] [CrossRef]
- Zeng, B.R.; Zhang, L.C.; Wan, X.Y.; Song, H.J.; Lv, Y. Fabrication of α-Fe2O3/g-C3N4 composites for cataluminescence sensing of H2S. Sens. Actuators B Chem. 2015, 211, 370–376. [Google Scholar] [CrossRef]
- Wu, L.Q.; Zhang, L.C.; Sun, M.X.; Liu, R.; Yu, L.Z.; Lv, Y. Metal-free cataluminescence gas sensor for hydrogen sulfide based on its catalytic oxidation on silicon carbide nanocages. Anal. Chem. 2017, 89, 13666–13672. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.L.; Hu, J.X.; Li, X.H.; Zhang, L.C.; Lv, Y. Camellia-like NiO: A novel cataluminescence sensing material for H2S. Sens. Actuators B Chem. 2019, 288, 243–250. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.M.; Chen, Q.F.; Zhou, H.Z.; Wu, J.M. Low power consumption gas sensor created from silicon nanowires/TiO2 core-shell heterojunctions. ACS Sens. 2017, 2, 1491–1497. [Google Scholar] [CrossRef]
- Ji, H.C.; Zeng, W.; Li, Y.Q. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Kang, X.Y.; Deng, N.P.; Yan, Z.R.; Pan, Y.W.; Sun, W.; Zhang, Y.F. Resistive-type VOCs and pollution gases sensor based on SnO2: A review. Mater. Sci. Semicond. Process 2022, 138, 106246. [Google Scholar] [CrossRef]
- Kaur, J.; Roy, S.C.; Bhatnagar, M.C. Highly sensitive SnO2 thin film NO2 gas sensor operating at low temperature. Sens. Actuators B Chem. 2007, 123, 1090–1095. [Google Scholar] [CrossRef]
- Sukunta, J.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Highly-sensitive H2S sensors based on flame-made v-substituted SnO2 sensing films. Sens. Actuators B Chem. 2017, 242, 1095–1107. [Google Scholar] [CrossRef]
- Choi, M.S.; Ahn, J.; Kim, M.Y.; Mirzaei, A.; Choi, S.; Chun, D.W.; Jin, C.; Lee, K.H. Changes in the crystal structure of SnO2 nanoparticles and improved h2s gas-sensing characteristics by Al doping. Appl. Surf. Sci. 2021, 565, 150493. [Google Scholar] [CrossRef]
- Nakla, W.; Wisitsora-At, A.; Tuantranont, A.; Singjai, P.; Phanichphant, S.; Liewhiran, C. H2S sensor based on SnO2 nanostructured film prepared by high current heating. Sens. Actuators B Chem. 2014, 203, 565–578. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.Y.; Dai, Y.B.; Li, G.H.; Wang, S.Q.; Li, T.; Zhang, T.; Qin, S.J. In situ growth of NiO@SnO2 hierarchical nanostructures for high performance H2S sensing. Acs Appl. Mater. Interfaces 2019, 11, 44829–44836. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.F.; Lu, Z.Y.; Zhang, R.K.; Li, G.K. Cataluminescence sensor for highly sensitive and selective detection of iso-butanol. Talanta 2019, 194, 910–918. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Sinha, T. A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Reddy, I.N.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Mousavi, H.; Mortazavi, Y.; Khodadadi, A.A.; Saberi, M.H.; Alirezaei, S. Enormous enhancement of pt/sno2 sensors response and selectivity by their reduction, to CO in automotive exhaust gas pollutants including CO, NOx and C3H8. Appl. Surf. Sci. 2021, 546, 149120. [Google Scholar] [CrossRef]
- Vojisavljević, K.; Savić, S.M.; Počuča-Nešić, M.; Hodžić, A.; Kriechbaum, M.; Ribić, V.; Rečnik, A.; Vukašinović, J.; Branković, G.; Djokić, V. Kit-5-assisted synthesis of mesoporous SnO2 for high-performance humidity sensors with a swift response/recovery speed. Molecules 2023, 28, 1754. [Google Scholar] [CrossRef]
- Yan, L.; Pan, J.S.; Ong, C.K. XPS studies of room temperature magnetic Co-doped SnO2 deposited on Si. Mater. Sci. Eng. B 2006, 128, 34–36. [Google Scholar] [CrossRef]
- Pu, S.R.; Pan, Y.; Zhang, L.C.; Lv, Y. Online evaluation of the catalytic performance of MnO2 and its application in H2S cataluminscence sensing. Anal. Chim. Acta 2021, 1180, 338883. [Google Scholar] [CrossRef] [PubMed]
- Modaberi, M.R.; Rooydell, R.; Brahma, S.; Akande, A.A.; Mwakikunga, B.W.; Liu, C.P. Enhanced response and selectivity of H2S sensing through controlled Ni doping into ZnO nanorods by using single metal organic precursors. Sens. Actuators B Chem. 2018, 273, 1278–1290. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Z.; Zheng, Y. H2S sensor based on two-dimensional MoO3 nanoflakes: Transition between sulfidation and oxidation. Sens. Actuators B Chem. 2021, 345, 130408. [Google Scholar] [CrossRef]
- Leangtanom, P.; Wisitsoraat, A.; Jaruwongrangsee, K.; Chanlek, N.; Tuantranont, A.; Phanichphant, S.; Kruefu, V. Highly sensitive and selective sensing of H2S gas using precipitation and impregnation-made CuO/SnO2 thick films. Nanoscale Res. Lett. 2021, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gong, H.; Liu, H.J. Fluorine and oxygen-functionalized silicon nitride with abundant oxygen vacancies for cataluminescence sensing of H2S. Microchem. J. 2023, 193, 109096. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, H.; Sun, T.; Zhang, L.; Lv, Y. Cataluminescence on 2D WS2 nanosheets surface for H2S sensing. Sens. Actuators B Chem. 2022, 353, 131111. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the active oxygen species in SnO2 based gas sensing materials: An operando IR spectrsocopy study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Li, H.J.; Wan, K.C.; Yu, X.; Wang, P.; Chen, A.Y.; Wang, X.Y.; Yang, J.H. Mesoporous ultrathin SnO2 nanosheets in situ modified by graphene oxide for extraordinary formaldehyde detection at low temperatures. ACS Appl. Mater. Interfaces 2019, 11, 12808–12818. [Google Scholar] [CrossRef]
- Wang, S.M.; Shi, W.Y.; Lu, C. Chemisorbed oxygen on the surface of catalyst-improved cataluminescence selectivity. Anal. Chem. 2016, 88, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.; Al Shoaibi, A.; Gupta, A. Examination of emission spectra from hydrogen sulfide flames. In Proceedings of the 49th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011; p. 440. [Google Scholar]
- Benner, R.L.; Stedman, D.H. Universal sulfur detection by chemiluminescence. Anal. Chem. 1989, 61, 1268–1271. [Google Scholar] [CrossRef]
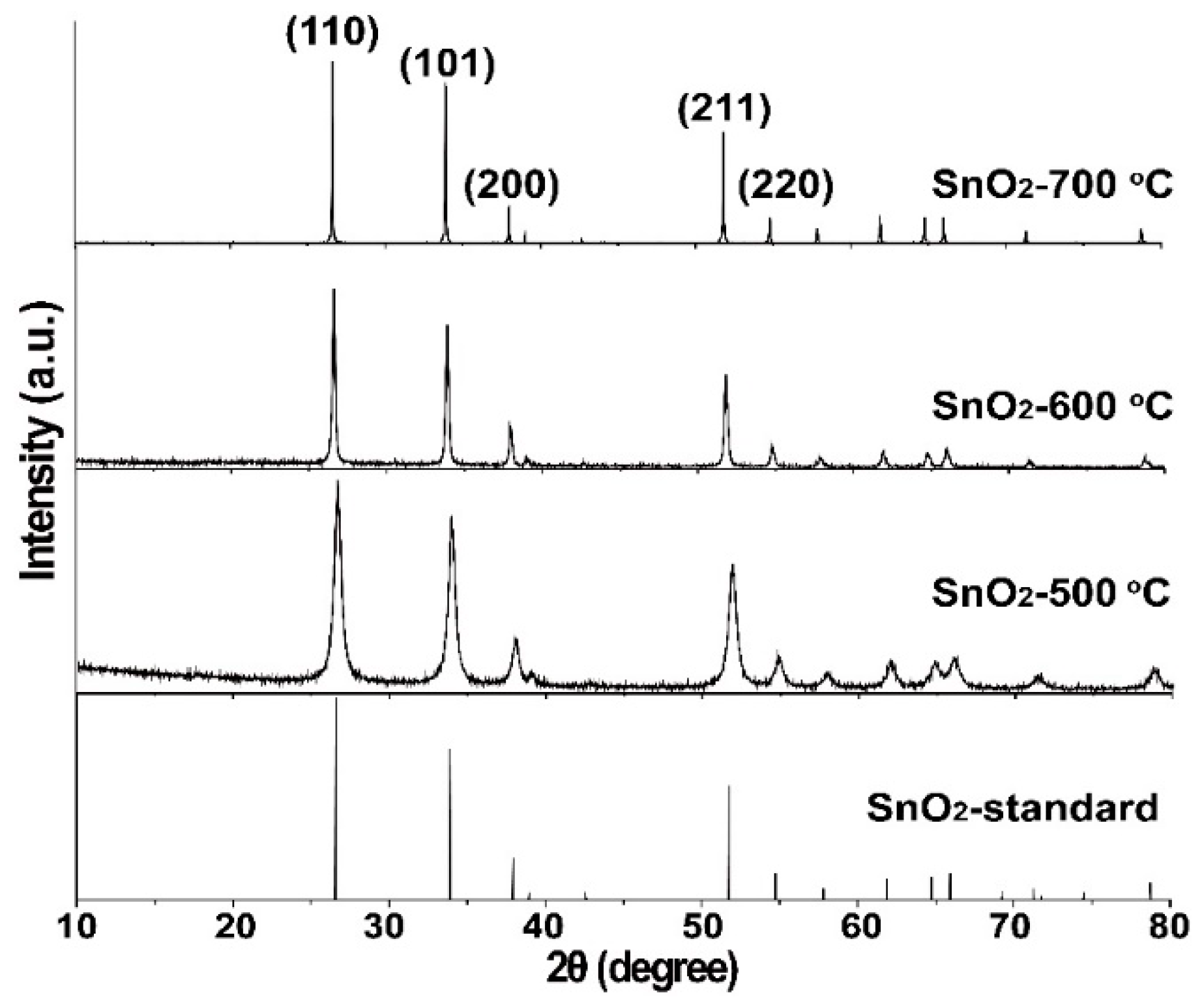
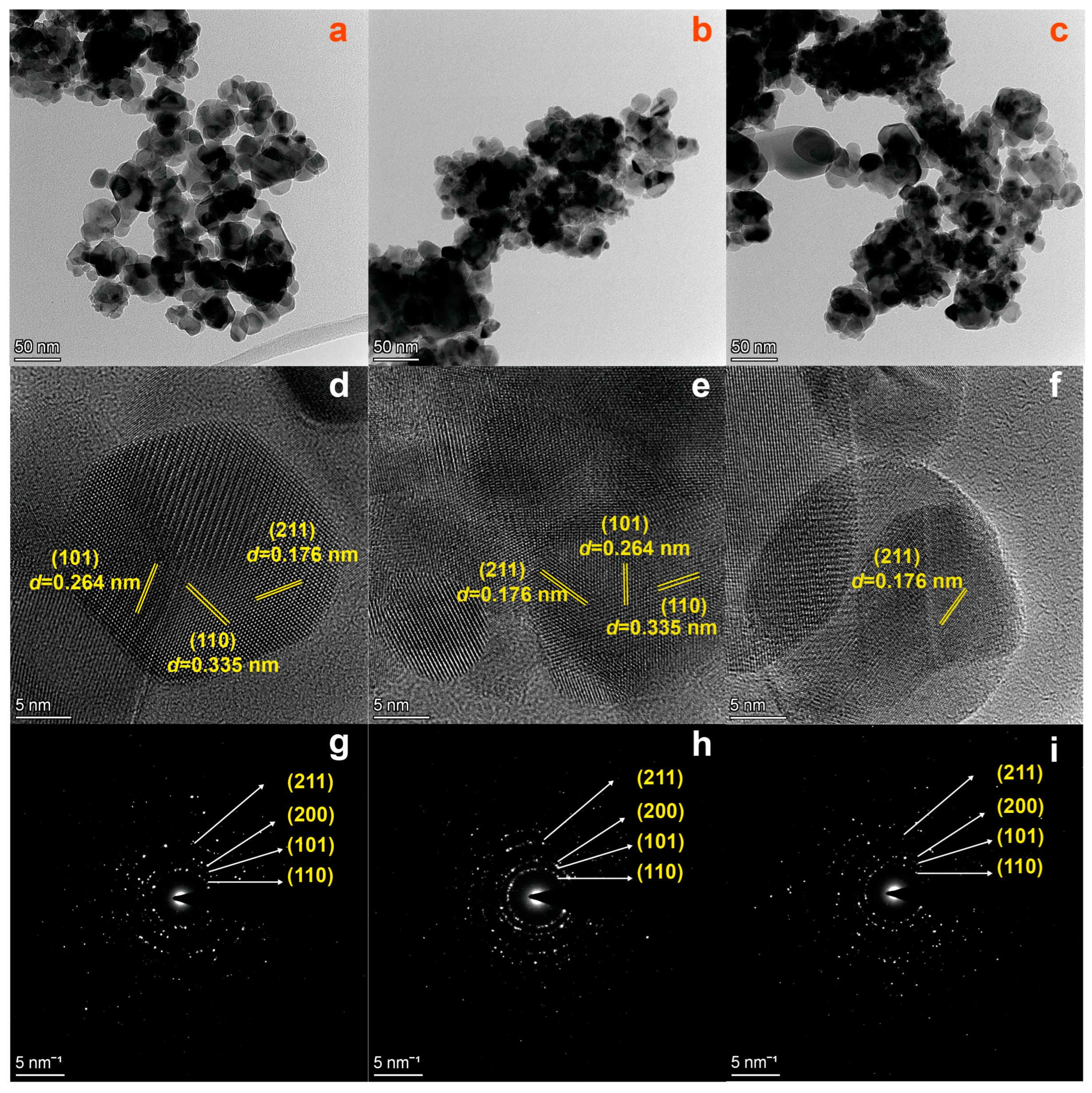

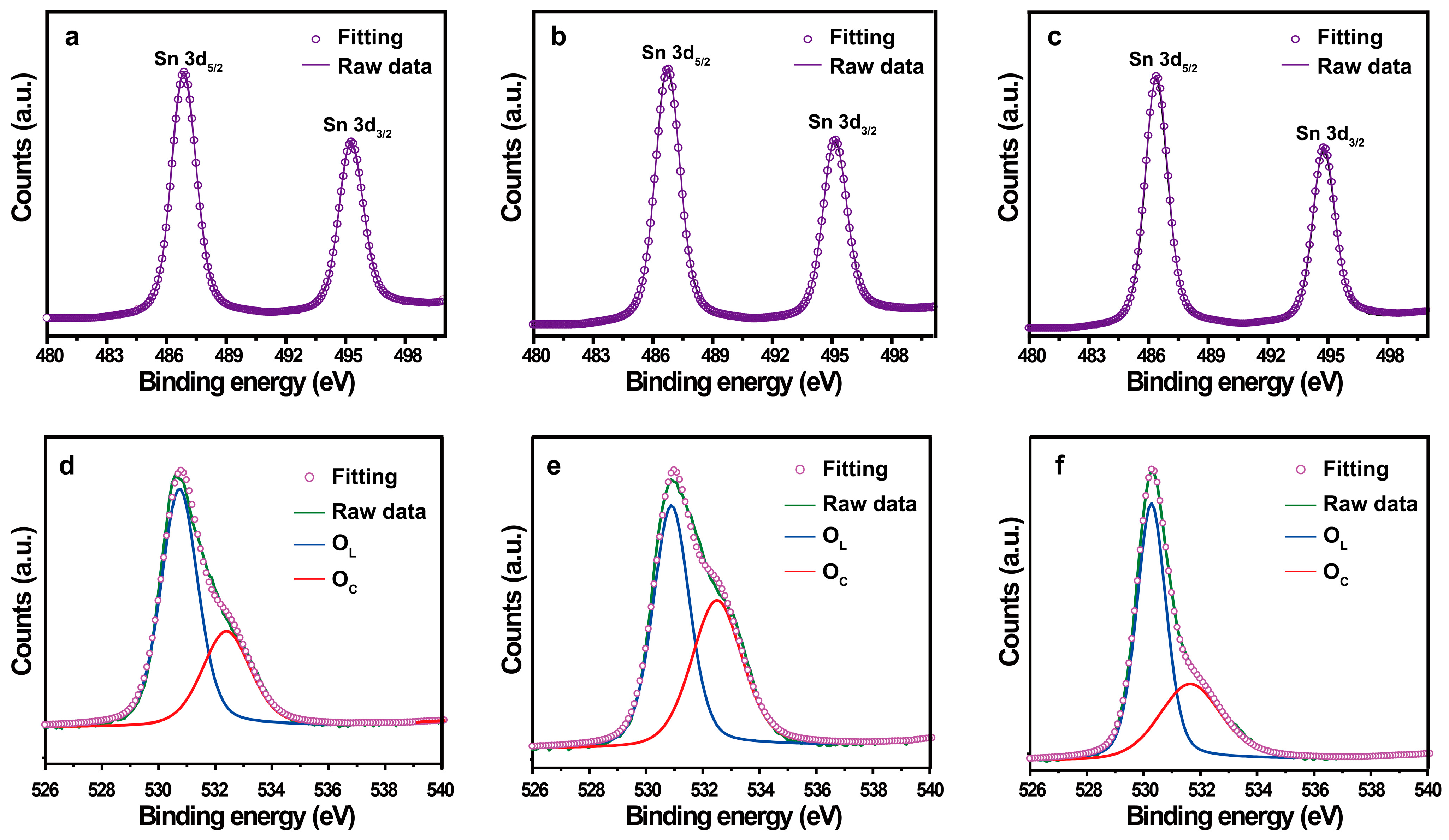
| Sample | BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | BJH (nm) |
|---|---|---|---|
| SnO2−500 °C | 19.70 | 289.07 | 17.41 |
| SnO2−600 °C | 19.73 | 295.61 | 17.02 |
| SnO2−700 °C | 2.31 | 14.00 | - |
| Sample | Binding Energy (eV) | Percentage (%) | ||
|---|---|---|---|---|
| OL | OC | OL | OC | |
| SnO2−500 °C | 530.74 | 532.39 | 65.88 | 34.12 |
| SnO2−600 °C | 530.89 | 532.50 | 54.70 | 45.30 |
| SnO2−700 °C | 530.32 | 532.11 | 73.03 | 26.97 |
| Principle | Sensing Material | Operating Temperature (°C) | Selectivity | Res./Rec. Time (s) | Linear Range (ppm) | LOD (ppm) | Ref. |
|---|---|---|---|---|---|---|---|
| Electricity | 0.1 wt% V-doped SnO2 | 350 | Little response to NO (5 ppm), NO2 (5 ppm), SO2 (500 ppm), and ethanol (50 ppm). | 2 s/few min | 0.25–10 | 0.08 | [29] |
| Electricity | SnO2-Al (1: 0.33) | 350 | Moderate response to 10 ppm of ethanol, ammonia, and toluene. | 35/ND | ND | ND | [30] |
| Electricity | SnO2 nanowires | 250 | Moderate response to 2000 ppm of ethanol and CO and strong response to NO2 (3 ppm). | 2.3/ND | 0.2–10 | ND | [31] |
| Electricity | NiO@SnO2 | 240 | Weak response to 0.5 ppm of NH3, toluene, formaldehyde, and NO2. | 37/50 | 0.1–50 | 0.0015 (in theory) | [32] |
| Resistance | Ni-doped ZnO nanorod | 200 | Moderate response to 100 ppm of methane, toluene, methanol, and ethanol. | 48/60 | 5–40 | ND | [40] |
| Electricity | MoO3 nanoflakes | 300 | No response to NH3, formaldehyde, benzene, and CO. Weak response to SO2 and ethanol. | ND | 0.5–30 | ND | [41] |
| Electricity | CuO/SnO2 | 200 | Weak response to 200 ppm of SO2 (200 ppm) and 1000 ppm of methane, hydrogen, and acetylene. | ND | 0.15–10 | 0.15 | [42] |
| CTL | Flower-like In2O3 | 400 | Weak response to methyl sulfide and ethanol. No response to methanol, propanol, benzene, cyclohexane, etc. | 5/25 | 1317.6–13,176 | 329 | [21] |
| CTL | α-Fe2O3/g-C3N4 | 183 | No response to 64 mg/L of methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, formaldehyde, and acetaldehyde. | 0.1/0.6 | 580–4618 | 329 | [22] |
| CTL | F-doped cage-like SiC | 298 | Weak response to 64 ppm of 1-dodecanethiol and 1-thioglycerol. No response to 64 ppm of ethanol, isobutanol, tert-butyl alcohol, n-pentanol, methanol, acetone, etc. | 0.1/0.6 | 6.1–30.4 | 3.0 | [23] |
| CTL | Camellia-like NiO | 246 | Weak response to 65 ppm of formaldehyde, acetaldehyde, formic acid, acetic acid, acetone, ether, carbon disulfide, methyl mercaptan, ethyl mercaptan, etc. | 0.2/0.4 | 0.8–30.8 | 0.3 | [24] |
| CTL | β-MnO2 | 224 | Weak response to acetone, 2-propanol, propionaldehyde, isobutanol, n-propanol, ethanol, and methanol. | 0.3/0.4 | 1601–19,173 | 184 | [39] |
| CTL | F/O-Si3N4 | 230 | Weak response to 64 ppm of ether and acetone. No response to ethanol, isobutanol, tert-butyl alcohol, n-pentanol, methanol, acetone, cyclohexanone, etc. | 0.5/1 | 322–5382 | 17.8 | [43] |
| CTL | 2D WS2 nanosheets | 187 | No response to methanol, ethanol, acetone, formaldehyde, acetaldehyde, formic acid, carbon disulfide, benzene, etc. | 0.2/0.2 | 171–4203 | 40 | [44] |
| CTL | SnO2 | 210 | Weak response to 2000 ppm of ammonium sulfide. No response to 2000 ppm of ethanethiol, dimethyl sulfide, trimethylamine, dimethylamine, triethylamine, ethylenediamine, ammonia, benzene, toluene, o-xylene, m-xylene, p-xylene, styrene, trichloroethylene, methanol, ethanol, acetone, formaldehyde, acetaldehyde, propionaldehyde, CO, CO2, and SO2, and 1000 ppm of dimethyl sulfide. | 3.5/1.5 | 20–420 | 8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Zhang, J.-R.; Chen, J.-L.; Yang, Z.-T.; Li, B.; Wang, L.; Ye, M.; Zhang, L.-L. Highly Selective and Fast Response/Recovery Cataluminescence Sensor Based on SnO2 for H2S Detection. Molecules 2023, 28, 7143. https://doi.org/10.3390/molecules28207143
Fan B, Zhang J-R, Chen J-L, Yang Z-T, Li B, Wang L, Ye M, Zhang L-L. Highly Selective and Fast Response/Recovery Cataluminescence Sensor Based on SnO2 for H2S Detection. Molecules. 2023; 28(20):7143. https://doi.org/10.3390/molecules28207143
Chicago/Turabian StyleFan, Bin, Jing-Ru Zhang, Jia-Liang Chen, Ze-Tao Yang, Bin Li, Lin Wang, Mai Ye, and Lu-Lu Zhang. 2023. "Highly Selective and Fast Response/Recovery Cataluminescence Sensor Based on SnO2 for H2S Detection" Molecules 28, no. 20: 7143. https://doi.org/10.3390/molecules28207143
APA StyleFan, B., Zhang, J.-R., Chen, J.-L., Yang, Z.-T., Li, B., Wang, L., Ye, M., & Zhang, L.-L. (2023). Highly Selective and Fast Response/Recovery Cataluminescence Sensor Based on SnO2 for H2S Detection. Molecules, 28(20), 7143. https://doi.org/10.3390/molecules28207143







