The Adsorption Mechanisms of SF6-Decomposed Species on Tc- and Ru-Embedded Phthalocyanine Surfaces: A Density Functional Theory Study
Abstract
:1. Introduction
2. Results and Discussion
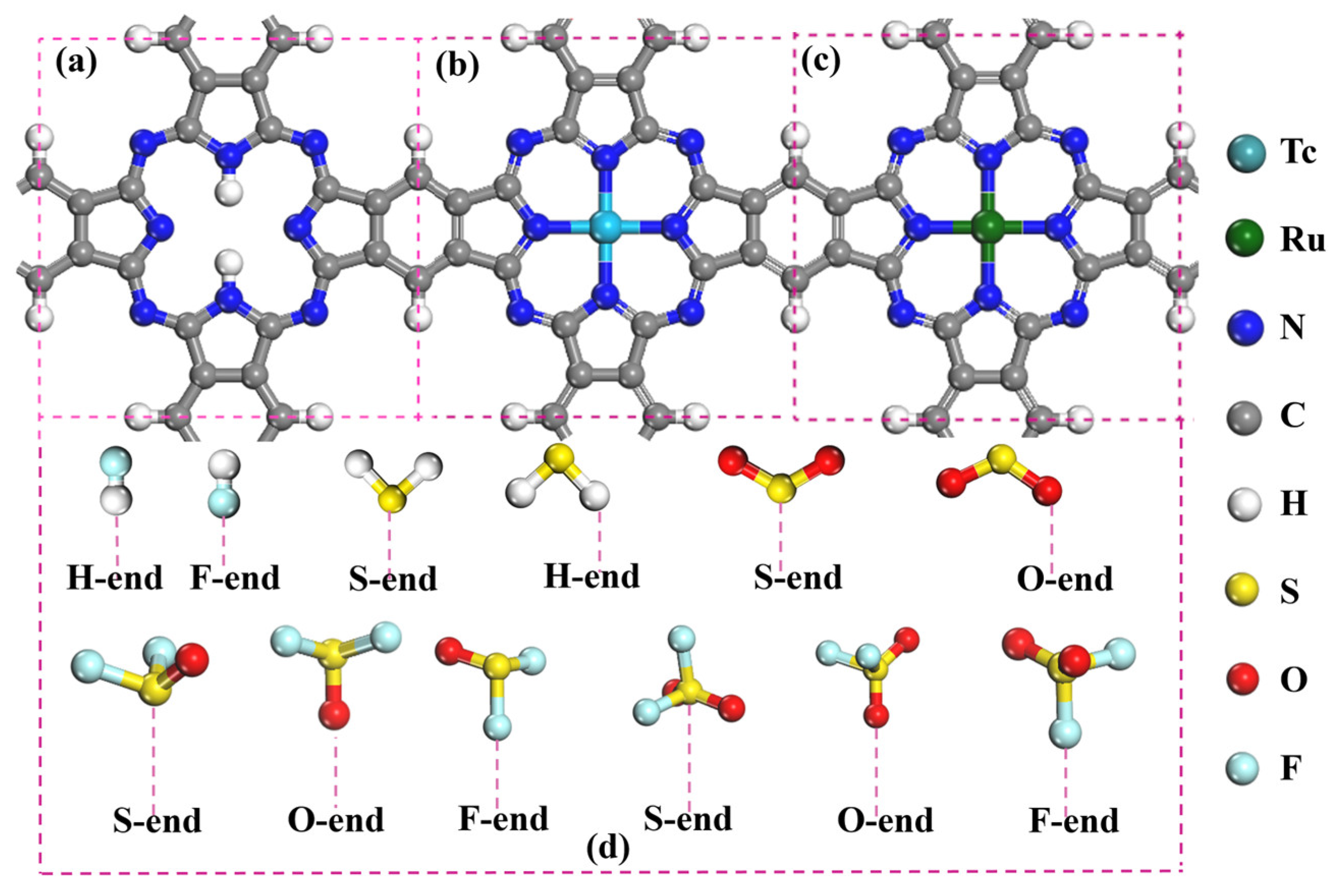
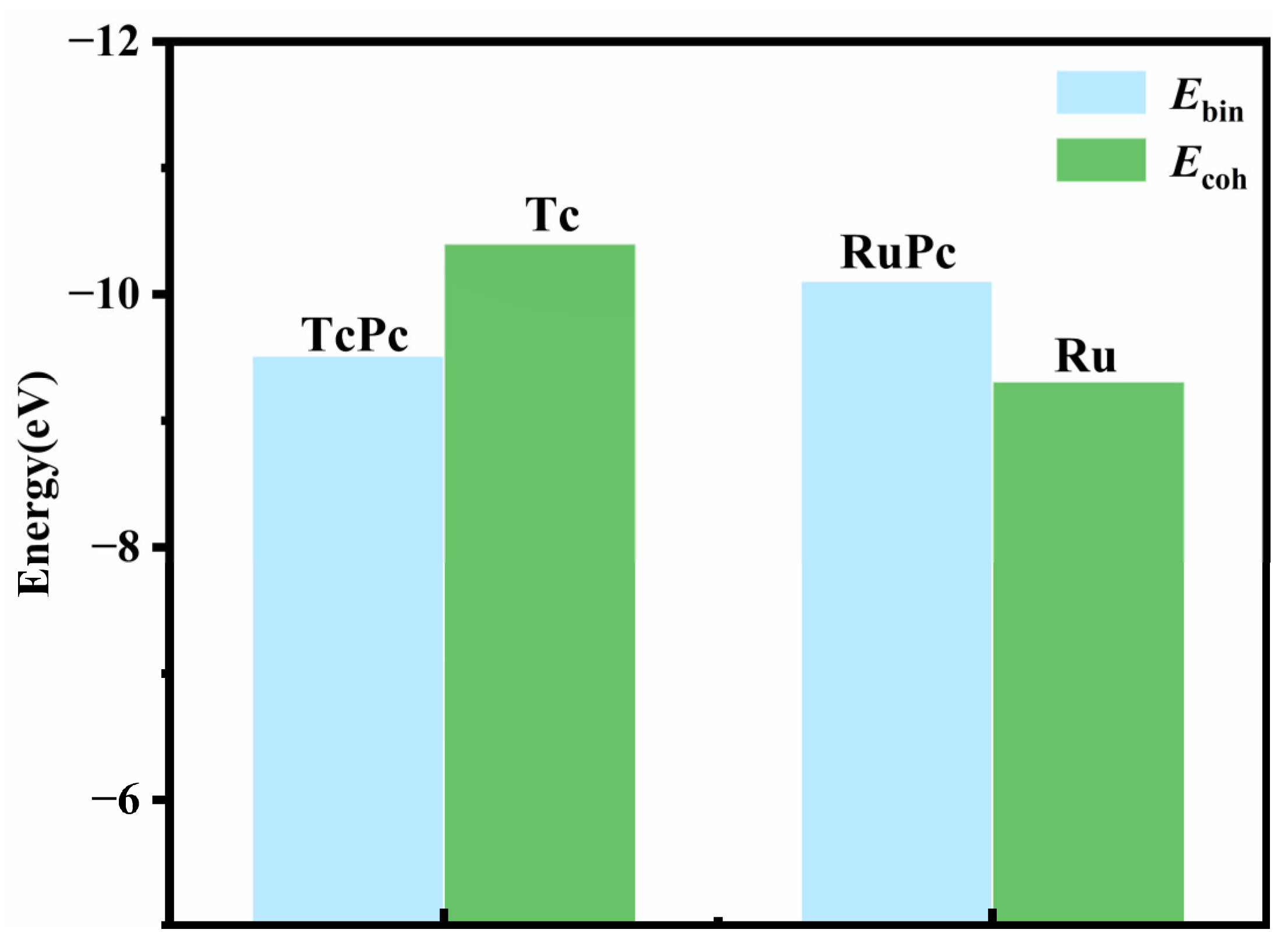
2.1. Structure and Stability of H2Pc, TcPc, RuPc, and SF6
2.2. Adsorption Characteristics of SF6 Decomposition Gases on H2Pc Monolayer
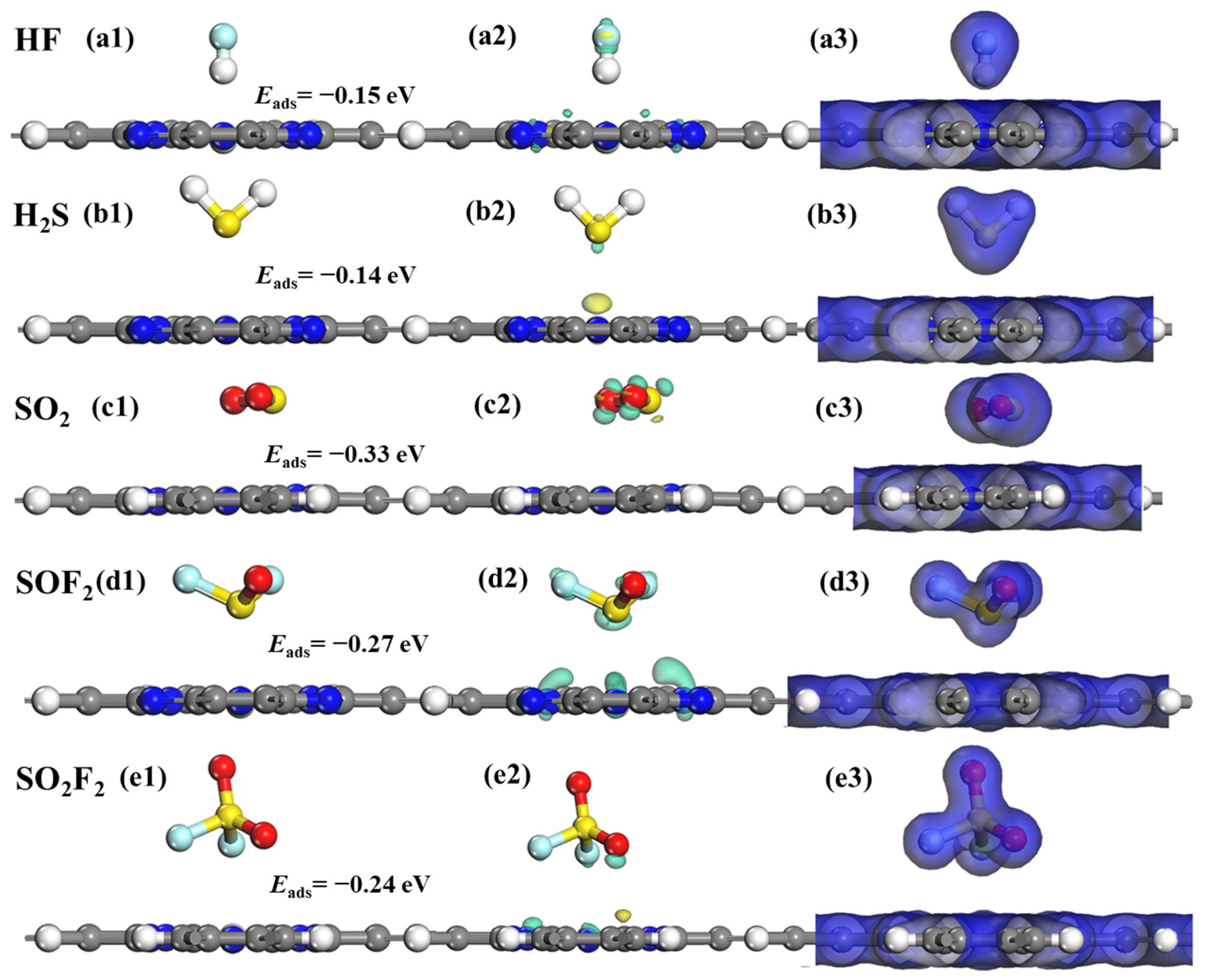
2.3. Adsorption Characteristics of SF6 Decomposition Gases on TcPc Monolayer
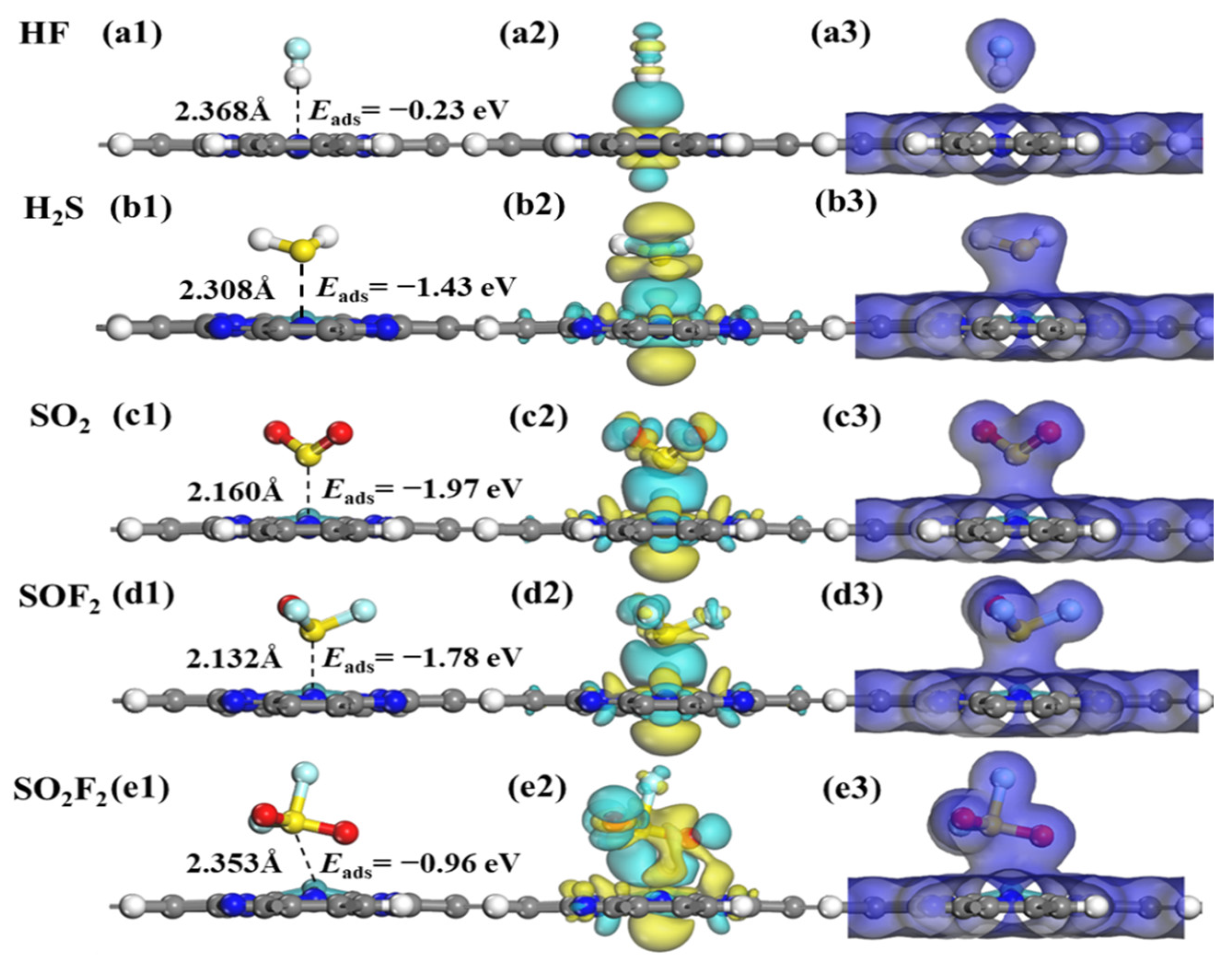
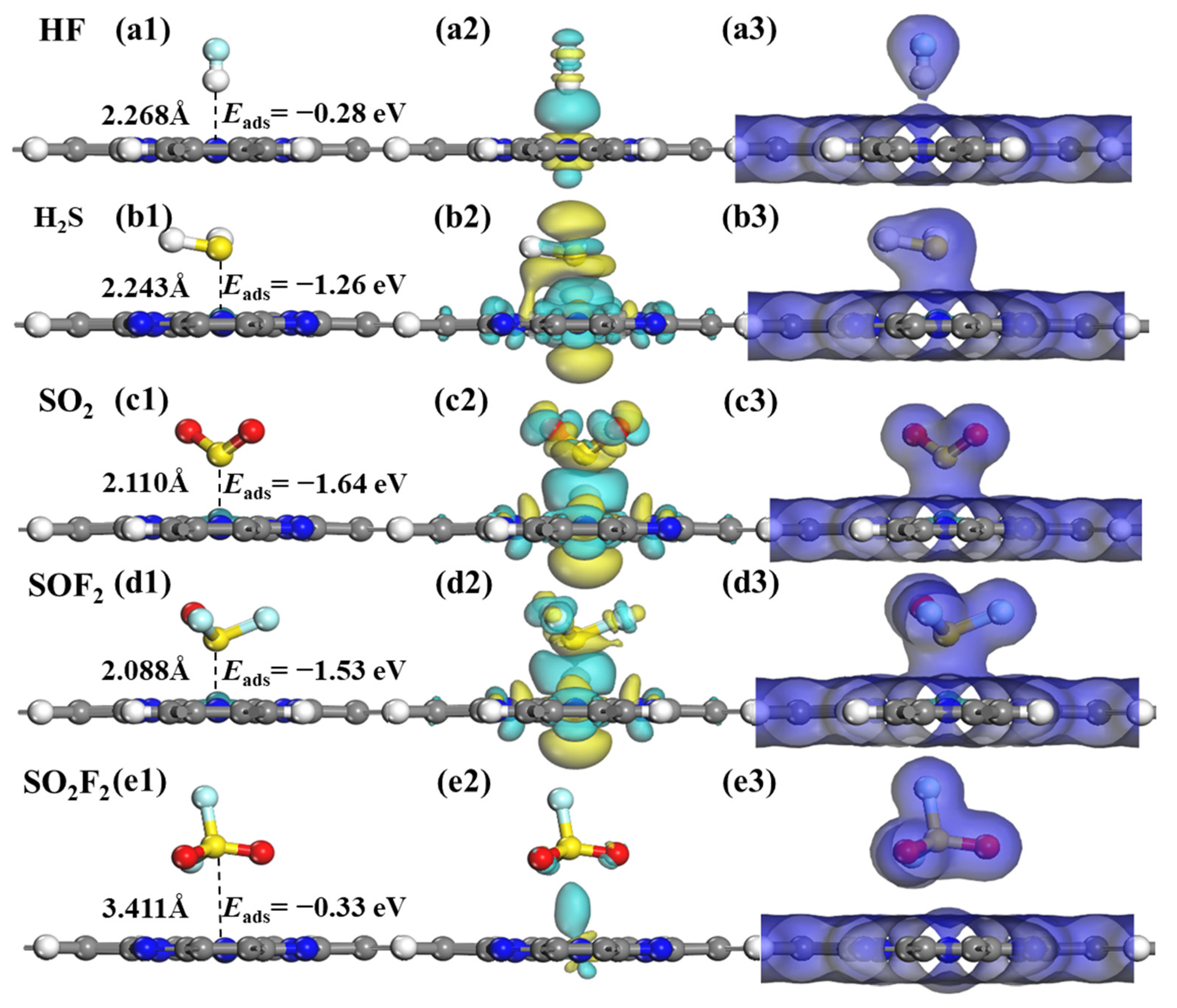
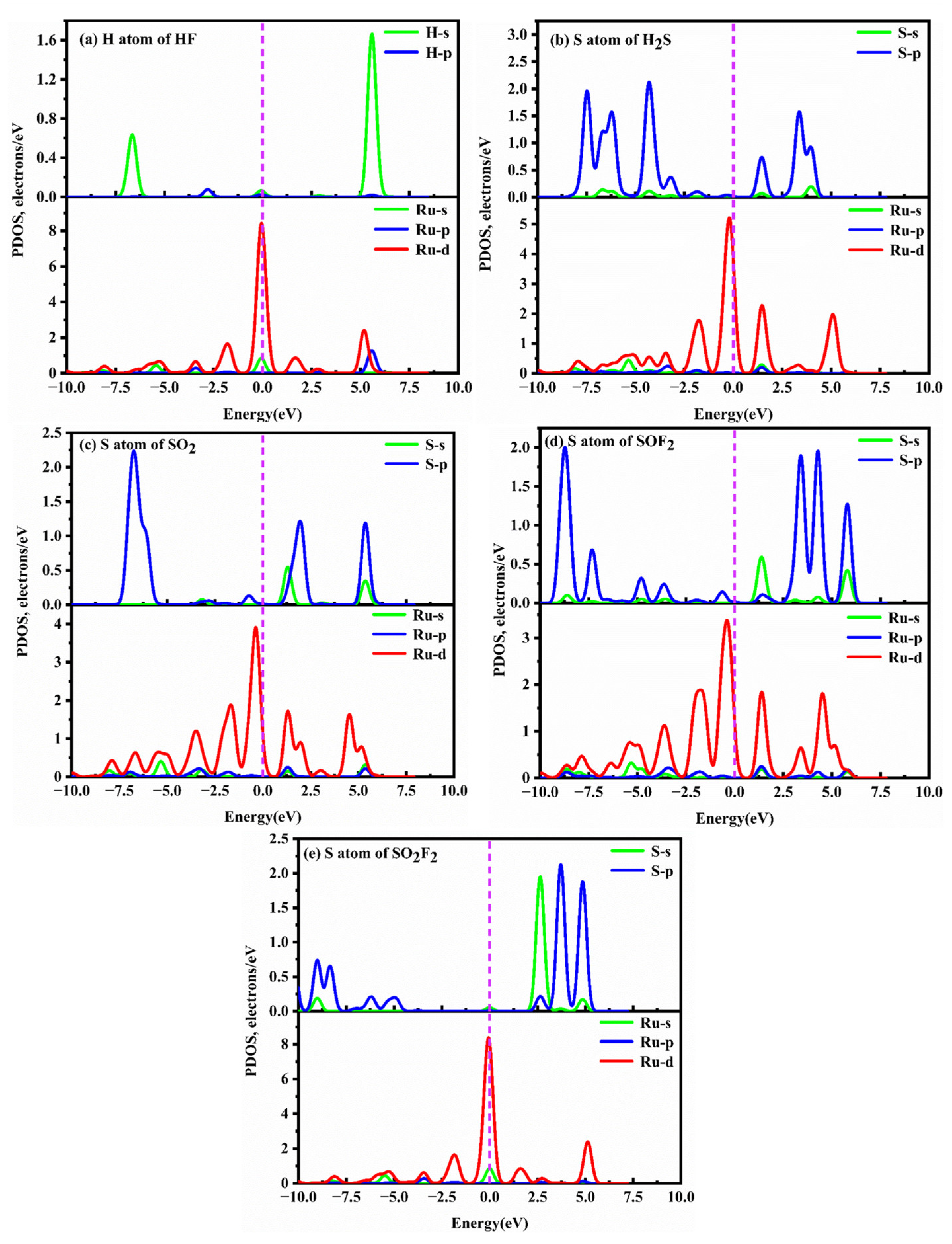
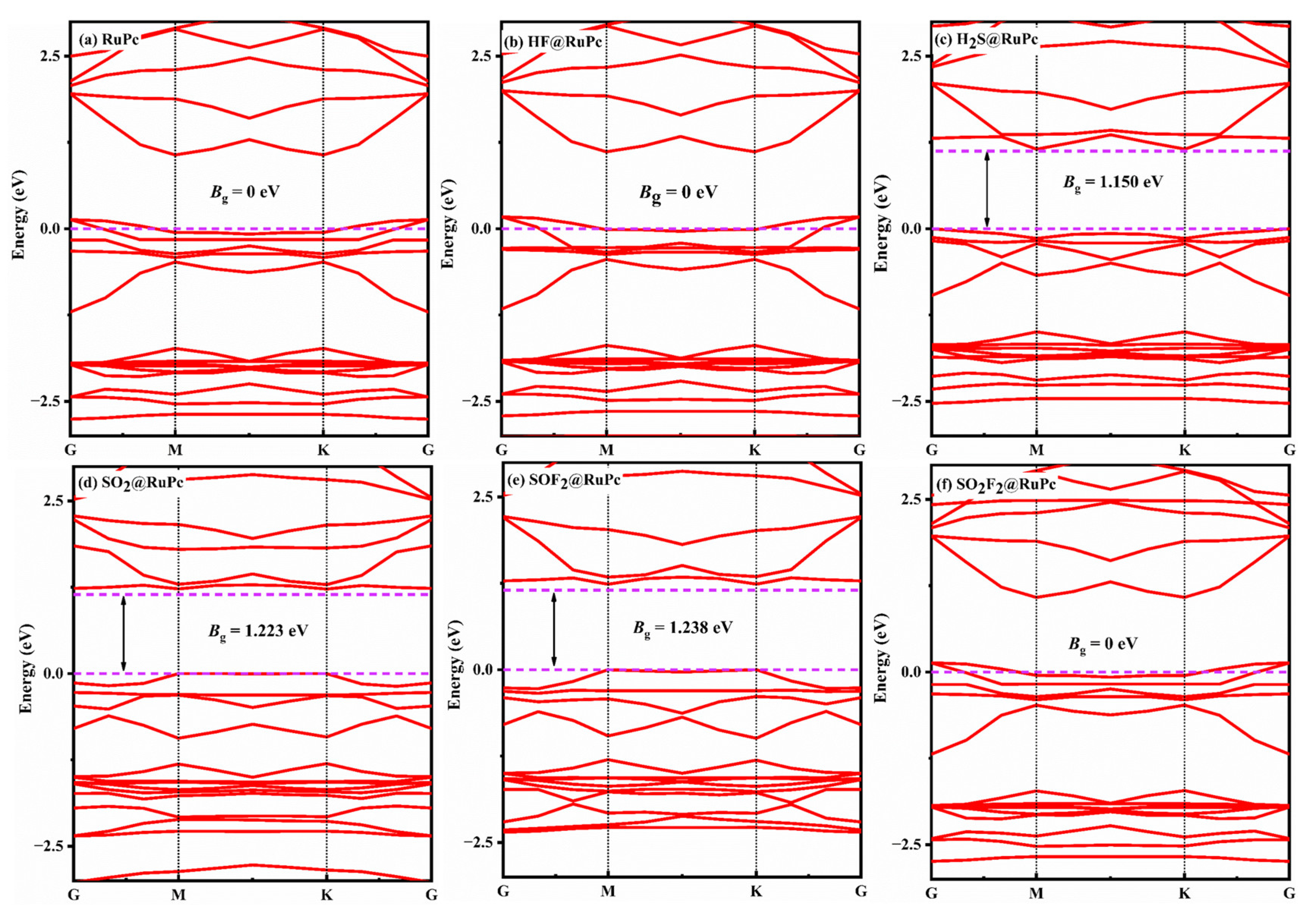
2.4. Adsorption Characteristics of SF6 Decomposition Gases on RuPc Monolayer
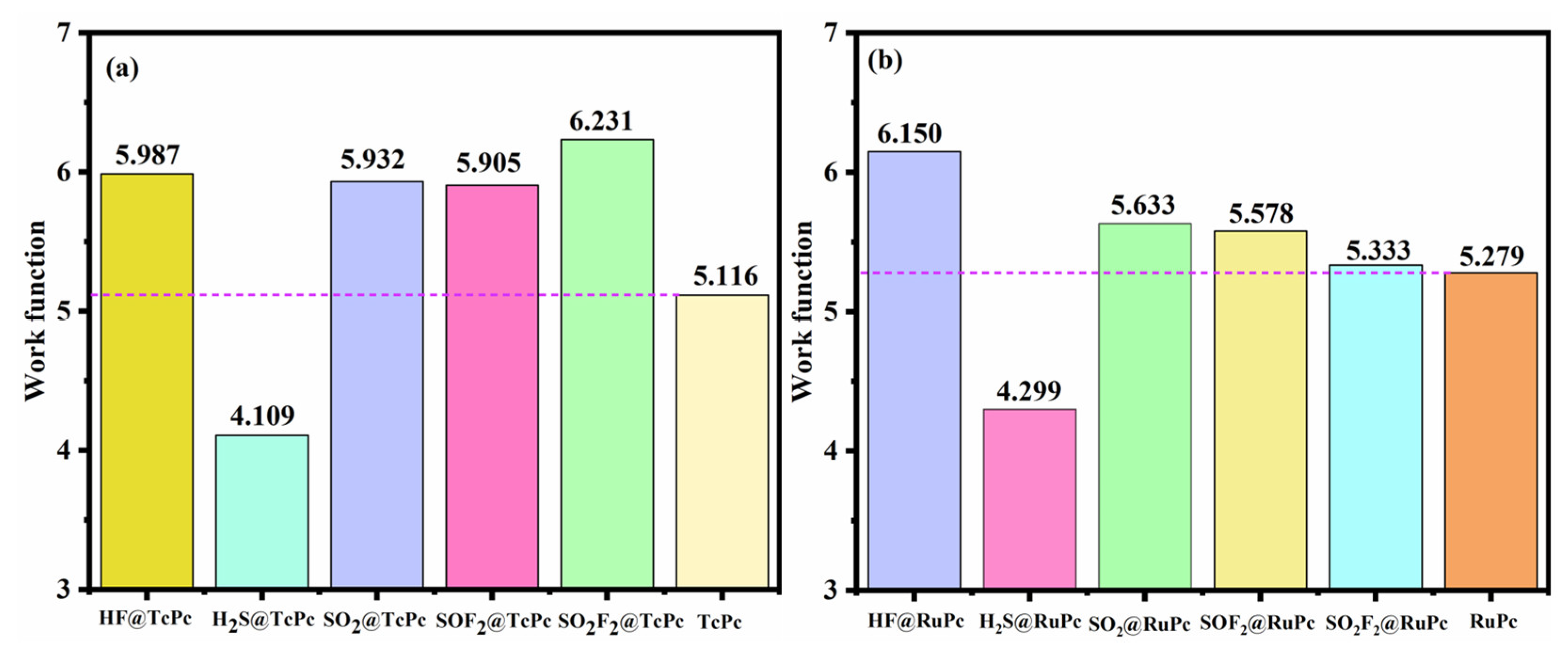
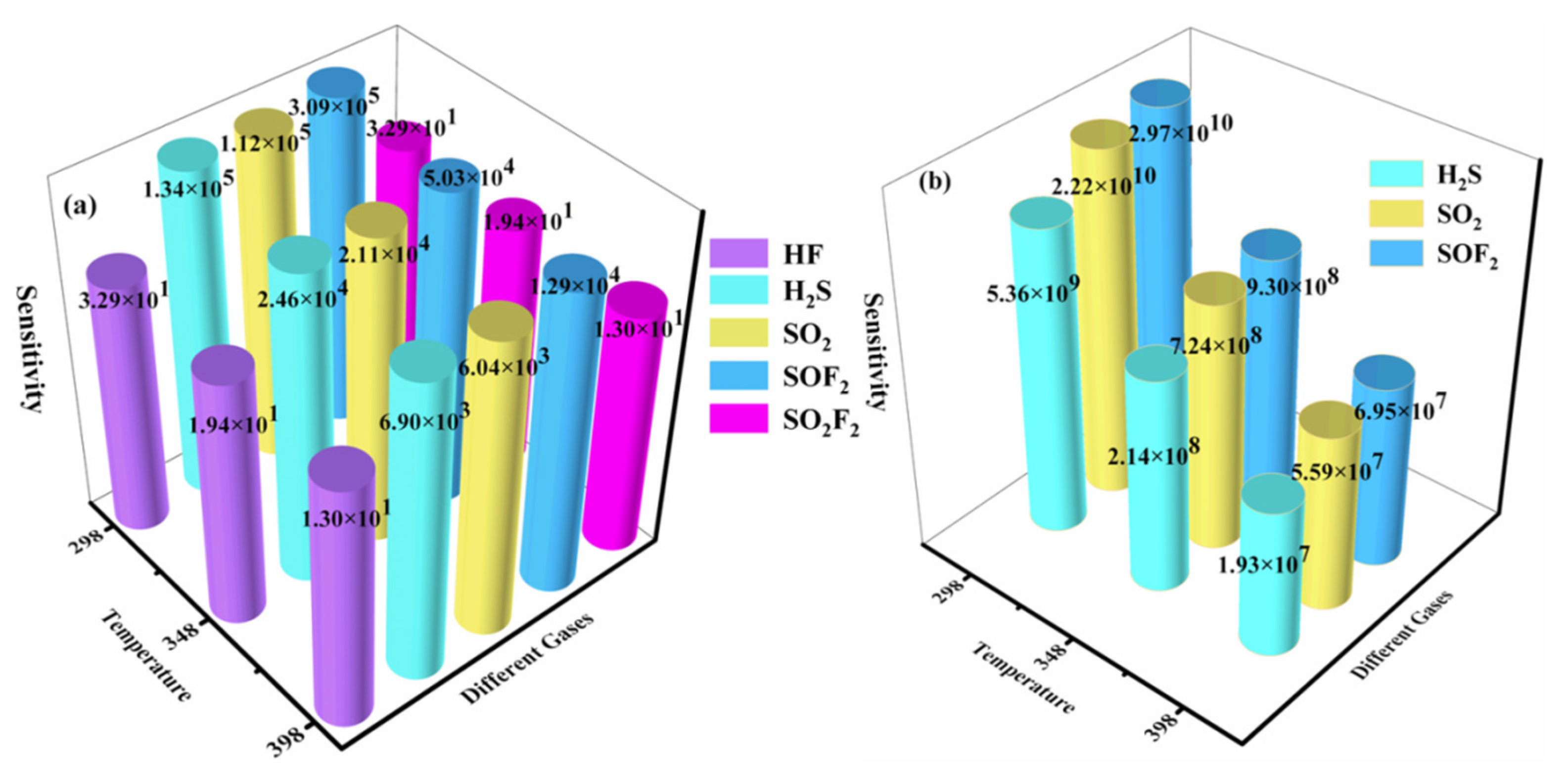
2.5. Sensing Performance Evaluation of TcPc and RuPc
3. Calculation Method and Details
4. Conclusions
- (1)
- TcPc and RuPc monolayers exhibit a semi-metallic property, and the strong hybridizations between the Tc/Ru-d orbital and N4 of Pc further demonstrate their high structural stability.
- (2)
- The TcPc monolayer exhibits a strong affinity towards H2S, SO2, SOF2, and SO2F2 due to the robust orbital hybridization between the Tc-d orbitals and S-sp orbitals of these gases.
- (3)
- The RuPc nanosheet exhibits a remarkable ability to capture H2S, SO2, and SOF2 molecules, primarily owing to the robust orbital hybridizations between the Ru-d orbitals and the S-sp orbitals of these gases. Therefore, the RuPc nanosheet holds significant promise as a scavenger for H2S, SO2, and SOF2 molecules.
- (4)
- The adsorption of H2S, SO2, and SOF2 induces significant changes in the bandgap and work function of the TcPc and RuPc monolayers, highlighting the strong sensitivity of these monolayers to H2S, SO2, and SOF2 molecules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, S.; Zhang, X.; Tang, J.; Liu, S. A review on SF6 substitute gases and research status of CF3I gases. Energy. Rep. 2018, 4, 486–496. [Google Scholar] [CrossRef]
- Chuah, C.; Lee, Y.; Bae, T. Potential of adsorbents and membranes for SF6 capture and recovery: A review. Chem. Eng. J. 2021, 404, 126577. [Google Scholar] [CrossRef]
- Kim, S.; Nagorny, P. Electrochemical synthesis of glycosyl fluorides using sulfur(VI) hexafluoride as the fluorinating agent. Org. Lett. 2022, 24, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Maiss, M.; Steele, L.; Francey, R.; Fraser, P.; Langenfelds, R.; Trivett, N.; Levin, I. Sulfur hexafluoride—A powerful new atmospheric tracer. Atmos. Environ. 1996, 30, 1621–1629. [Google Scholar] [CrossRef]
- Beyer, C.; Jenett, H.; Klockow, D. Influence of reactive SFx gases on electrode surfaces after electrical discharges under SF6 atmosphere. IEEE. T. Dielect. El. In. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE. T. Dielect. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Y.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.D.; Hayashi, R.; Ochi, K.; Suehiro, J.; Imasaka, K.; Hara, M.; Sano, N.; Nagao, E.; Minagawa, T. Analysis of PD-generated SF6 decomposition gases adsorbed on carbon nanotubes. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1200–1207. [Google Scholar] [CrossRef]
- Chen, D.C.; Zhang, X.X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A: Mater. Sci. Process. 2018, 124, 194. [Google Scholar] [CrossRef]
- Yong, Y.; Gao, R.; Wang, X.; Yuan, X.; Hu, S.; Zhao, Z.; Li, X.; Kuang, Y. Highly sensitive and selective room-temperature gas sensors based on B6N6H6 monolayer for sensing SO2 and NH3: A first-principles study. Results Phys. 2022, 33, 105208. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.; Ma, R.; Sang, T.; Chen, Q.; Ma, L.; Chen, Y.; Liao, Y.; Yang, G.; Huang, Y.; et al. The electronic properties and adsorption mechanism of Agn, Aun (n = 1–4) modified GeSe monolayer towards hazardous gases (H2S, NH3, NO2 and SOF2): A first-principles study. Surf. Interfaces 2022, 32, 102150. [Google Scholar] [CrossRef]
- Lin, L.; Hu, C.; Deng, C.; Xu, Y.; Tao, H.; Zhang, Z. Adsorption behavior of transition metal (Pd, Pt, Ag and Au) doped SnS monolayers on SF6 decomposed species and the effects of applied electric field and biaxial strain. FlatChem 2022, 36, 100438. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Xu, L.; Long, J.; Cheng, Q.; Zeng, W. Sc doped WSe2 monolayer: A candidate for enhanced adsorption and detection of SF6 decomposition gases. J. Mater. Res. Technol. 2022, 17, 1786–1798. [Google Scholar] [CrossRef]
- Cui, H.; Feng, Z.; Wang, W.; Peng, X.; Hu, J. Adsorption Behavior of Pd-doped PtS2 monolayer upon SF6 decomposed species and the effect of applied electric field. IEEE Sens. J. 2022, 22, 6764–6771. [Google Scholar] [CrossRef]
- Zhu, W.G.; Liu, Y.; Huang, W.; Zhang, C.; Wei, L.; Peng, J. Conductive 2D phthalocyanine-based metal-organic framework as a photoelectrochemical sensor for N-acetyl-L-cysteine detection. Sens. Actuators. B 2022, 367, 132028. [Google Scholar]
- Tanaka, Y.; Mishra, P.; Tateishi, R.; Cuong, N.T.; Orita, H.; Otani, M.; Nakayama, T.; Uchihashi, T.; Sakamoto, K. Highly ordered cobalt-phthalocyanine chains on fractional atomic steps: One-dimensionality and electron hybridization. ACS Nano 2013, 7, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Bazarnik, M.; Brede, J.; Decker, R.; Wiesendanger, R. Tailoring molecular selfassembly of magnetic phthalocyanine molecules on Fe- and Co-intercalated graphene. ACS Nano 2013, 7, 11341–11349. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.M. Surface chemistry of porphyrins and phthalocyanines. Surf. Sci. Rep. 2015, 70, 259–379. [Google Scholar] [CrossRef]
- Ji, W.; Wang, T.X.; Ding, X.; Lei, S.; Han, B.H. Porphyrin- and phthalocyanine-based porous organic polymers: From synthesis to application. Coord. Chem. Rev. 2021, 439, 213875. [Google Scholar] [CrossRef]
- Lazarev, N.M.; Petrov, B.I.; Bochkarev, M.N.; Arapova, A.V.; Kukinov, A.A.; Cherkasov, A.V. Silicon phthalocyanine: Vapor pressure and photovoltaic properties. Synth. Met. 2020, 266, 116398. [Google Scholar] [CrossRef]
- Jin, L.; Chen, D. Enhancement in photovoltaic performance of phthalocyanine sensitized solar cells by attapulgite nanoparticles. Electrochim. Acta 2012, 72, 40–45. [Google Scholar] [CrossRef]
- Alosabi, A.Q.; Al-Muntaser, A.A.; El-Nahass, M.M.; Oraby, A.H. Structural, optical and DFT studies of disodium phthalocyanine thin films for optoelectronic devices applications. Opt. Laser Technol. 2022, 155, 108372. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Ali, H.A.M.; El-Zaidia, E.F.M.; Alfadhli, S.; El-Bashir, B.O.; Alatawi, R.A.S.; Eisa, A.A.A.; Yahia, I.S. Linear and nonlinear optical characteristics of manganese phthalocyanine chloride/polyacetate sheet: Towards flexible optoelectronic devices. Opt. Mater. 2021, 114, 110988. [Google Scholar] [CrossRef]
- Huang, S.; Chen, K.; Li, T.T. Porphyrin and phthalocyanine based covalent organic frameworks for electrocatalysis. Coord. Chem. Rev. 2022, 464, 214563. [Google Scholar] [CrossRef]
- Shumba, M.; Nyokong, T. Development of nanocomposites of phosphorus-nitrogen co-doped graphene oxide nanosheets and nanosized cobalt phthalocyanines for electrocatalysis. Electrochim. Acta 2016, 213, 529–539. [Google Scholar] [CrossRef]
- Zemła, M.R.; Czelej, K.; Majewski, J.A. Graphene–iron(II) phthalocyanine hybrid systems for scalable molecular spintronics. J. Phys. Chem. C 2020, 124, 27645–27655. [Google Scholar] [CrossRef]
- Annese, E.; Mori, T.J.A.; Schio, P.; Salles, B.R.; Cezar, J.C. Fe-phthalocyanine nanoclusters on La0.67Sr0.33MnO3 ferromagnetic substrate for spintronics application. ACS Appl. Nano Mater. 2020, 3, 1516–1525. [Google Scholar] [CrossRef]
- Lessard, B.H. The rise of silicon phthalocyanine: From organic photovoltaics to organic thin film transistors. ACS Appl. Mater. Interfaces 2021, 13, 31321–31330. [Google Scholar] [CrossRef]
- Aziz, T.; Sun, Y.; Wu, Z.H.; Haider, M.; Qu, T.Y.; Khan, A.; Zhen, C.; Liu, Q.; Cheng, H.M.; Sun, D.M. A flexible nickel phthalocyanine resistive random access memory with multi-level data storage capability. J. Mater. Sci. Technol. 2021, 86, 151–157. [Google Scholar] [CrossRef]
- Wang, H.; Mauthoor, S.; Din, S.; Gardener, J.A.; Chang, R.; Warner, M.; Aeppli, G.; McComb, D.W.; Ryan, M.P.; Wu, W.; et al. Ultralong copper phthalocyanine nanowires with new crystal structure and broad optical absorption. ACS Nano 2010, 4, 3921–3926. [Google Scholar] [CrossRef]
- Chow, S.Y.S.; Ng, D.K.P. Synthesis of an ABCD-type phthalocyanine by intramolecular cyclization reaction. Org. Lett. 2016, 18, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, S.; Wakamatsu, H.; Nishikata, O.; Mori, A.; Inoue, T. Growth of copperphthalocyanine nano-crystallite epitaxially grown on KCl (001) substrate. J. Cryst. Growth 2005, 275, 1993–1996. [Google Scholar] [CrossRef]
- Kumar, A.; Alami Mejjati, N.; Meunier-Prest, R.; Krystianiak, A.; Heintz, O.; Lesniewska, E.; Devillers, C.H.; Bouvet, M. Tuning of interfacial charge transport in polyporphine/phthalocyanine heterojunctions by molecular geometry control for an efficient gas sensor. Chem. Eng. J. 2022, 429, 132453. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B 2022, 357, 131352. [Google Scholar] [CrossRef]
- Baygu, Y.; Capan, R.; Erdogan, M.; Ozkaya, C.; Acikbas, Y.; Kabay, N.; Gok, Y. Synthesis, characterization and chemical sensor properties of a novel Zn(II) phthalocyanine containing 15-membered dioxa-dithia macrocycle moiety. Synth. Met. 2021, 280, 116870. [Google Scholar] [CrossRef]
- Yahya, M.; Nural, Y.; Seferoglu, Z. Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: A review. Dyes Pigm. 2022, 198, 109960. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Chu, W.; Wang, L.W. Computational screening of transition metal-doped phthalocyanine monolayers for oxygen evolution and reduction. Nanoscale Adv. 2020, 2, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Shati, K.; Javaid, S.; Khan, R.T.A.; Akhtar, M.J. Electronic structure and spin state of fluorinated metal phthalocyanine molecules. J. Magn. Magn. Mater. 2020, 494, 165775. [Google Scholar] [CrossRef]
- Prasongkit, J.; Tangsukworakhun, S.; Jaisutti, R.; Osotchan, T. Highly sensitive and selective sensing of acetone and hydrogen sulfide using metal phthalocyanine carbon nanotube hybrids. Appl. Surf. Sci. 2020, 532, 147314. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, L.M.; Ganz, E. Efficient and selective electroreduction of CO2 by single-atom catalyst two-dimensional TM–Pc monolayers. ACS Sustain. Chem. Eng. 2018, 6, 15494–15502. [Google Scholar] [CrossRef]
- Zou, D.; Zhao, W.; Cui, B.; Li, D.; Liu, D. Adsorption of gas molecules on a manganese phthalocyanine molecular device and its possibility as a gas sensor. Phys. Chem. Chem. Phys. 2018, 20, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Aldahhak, H.; Powroźnik, P.; Pander, P.; Jakubik, W.; Dias, F.B.; Schmidt, W.G.; Gerstmann, U.; Krzywiecki, M. Toward efficient toxic-gas detectors: Exploring molecular interactions of sarin and dimethyl methylphosphonate with metal-centered phthalocyanine structures. J. Phys. Chem. C 2020, 124, 6090–6102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, L.; Su, X.; Zhou, F.; Duan, G. Interfacial self-assembly of CoPc thin films with their high sensing use as NO2 sensors. Mater. Chem. Phys. 2019, 234, 94–101. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, T.; Chen, X.; Li, B.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; Yang, Z. Enhancing room-temperature NO2 detection of cobalt phthalocyanine based gas sensor at an ultralow laser exposure. Phys. Chem. Chem. Phys. 2020, 22, 18499–18506. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, H.; Yan, C.; Li, H.; Si, R.; Li, M.; Xiao, J.; Wang, G.; Bao, X. Synergistic catalysis over iron-nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv. Mater. 2019, 31, 1903470. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Welding metallophthalocyanines into bimetallic molecular meshes for ultrasensitive, low-power chemo resistive detection of gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Liu, B.; Zhang, H.; Qin, J. Theoretical insight into two-dimensional M-Pc monolayer as an excellent material for formaldehyde and phosgene sensing. Appl. Surf. Sci. 2021, 543, 148805. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.J.; Guo, Q.J.; Dai, E.R.; Nie, Z.F. Metal-Decorated Phthalocyanine Monolayer as a Potential Gas Sensing Material for Phosgene: A First-Principles Study. ACS Omega 2022, 7, 21994–22002. [Google Scholar] [CrossRef]
- Ma, D.W.; He, C.Z.; Ma, B.Y.; Lu, Z.W.; Tang, Y.A.; Lu, Z.S.; Yang, Z.X. Interaction between H2O, N2, CO, NO, NO2 and N2O molecules and a defective WSe2 monolayer. Phys. Chem. Chem. Phys. 2017, 19, 26022–26033. [Google Scholar] [CrossRef]
- Deng, P.; Cheng, L.; Jiang, P.; Zeng, Z.; Li, A.; Liao, C. Sensing performance of CdPc monolayer toward the SF6 decomposition gases: A DFT study. Chem. Phys. Lett. 2022, 806, 140030. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yue, L.J.; Gong, F.L.; Li, F.; Zhang, H.L.; Chen, J.L. Highly enhanced H2S gas sensing and magnetic performances of metal doped hexagonal ZnO monolayer. Vacuum 2017, 141, 109–115. [Google Scholar] [CrossRef]
- Flietner, B.; Doll, T.; Lechner, J.; Leu, M.; Eisele, I. Reliable hybrid gasfets for work function measurements with arbitrary materials. Sens. Actuators B Chem. 1994, 22, 109–113. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Roondhe, B.; Jha, P.K.; Ahuja, R. Haeckelite boron nitride as sensor for the detection of hazardous methyl mercury. Appl. Surf. Sci. 2020, 506, 144860–144872. [Google Scholar] [CrossRef]
- Xia, S.Y.; Tao, L.Q.; Jiang, T.; Sun, H.; Li, J. Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study. Appl. Surf. Sci. 2021, 536, 147965.47. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the Dmol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B. 2002, 66, 155125–155134. [Google Scholar] [CrossRef]
- Vozzi, C.; Negro, M.; Calegari, F.; Sansone, G.; Nisoli, M.; De Silvestri, S.; Stagira, S. Generalized molecular orbital tomography. Nat. Phys. 2011, 7, 822–826. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Xiong, H.H.; Zhang, H.N.; Dong, J.H. Adhesion strength and stability of TiB2/TiC interface in composite coatings by first principles calculation. Comp. Mater. Sci. 2017, 127, 244–250. [Google Scholar] [CrossRef]






| Adsorption System | Orientation | Eads/eV | Qt/e | Bg/eV |
|---|---|---|---|---|
| HF@H2Pc | H-end | −0.15 | −0.08 | 1.106 |
| H2S@H2Pc | S-end | −0.14 | 0.027 | 1.125 |
| SO2@H2Pc | S-end | −0.33 | −0.054 | 1.092 |
| SOF2@H2Pc | S-end | −0.27 | −0.309 | 1.106 |
| SO2F2@H2Pc | S-end | −0.24 | 0.043 | 1.121 |
| Adsorption System | Orientation | Eads/eV | D/Å | Qt/e | Bg/eV |
|---|---|---|---|---|---|
| HF@TcPc | H-end | −0.23 | 2.368 | −0.140 | 0.000 |
| H2S@TcPc | S-end | −1.43 | 2.308 | 0.298 | 0.787 |
| SO2@TcPc | S-end | −1.97 | 2.160 | −0.099 | 0.778 |
| SOF2@TcPc | S-end | −1.78 | 2.132 | −0.073 | 0.830 |
| SO2F2@TcPc | S-end | −0.96 | 2.353 | −0.256 | 0.000 |
| Adsorption System | Orientation | Eads/eV | D/Å | Qt/e | Bg/eV |
|---|---|---|---|---|---|
| HF@RuPc | H-end | −0.28 | 2.268 | −0.165 | 0.000 |
| H2S@RuPc | S-end | −1.26 | 2.243 | 0.275 | 1.150 |
| SO2@RuPc | S-end | −1.64 | 2.110 | −0.071 | 1.223 |
| SOF2@RuPc | S-end | −1.53 | 2.088 | −0.039 | 1.238 |
| SO2F2@RuPc | S-end | −0.33 | 3.411 | 0.003 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, R.; Jiang, W.; He, X.; Xiong, H.; Xie, G.; Nie, Z. The Adsorption Mechanisms of SF6-Decomposed Species on Tc- and Ru-Embedded Phthalocyanine Surfaces: A Density Functional Theory Study. Molecules 2023, 28, 7137. https://doi.org/10.3390/molecules28207137
Xue R, Jiang W, He X, Xiong H, Xie G, Nie Z. The Adsorption Mechanisms of SF6-Decomposed Species on Tc- and Ru-Embedded Phthalocyanine Surfaces: A Density Functional Theory Study. Molecules. 2023; 28(20):7137. https://doi.org/10.3390/molecules28207137
Chicago/Turabian StyleXue, Rou, Wen Jiang, Xing He, Huihui Xiong, Gang Xie, and Zhifeng Nie. 2023. "The Adsorption Mechanisms of SF6-Decomposed Species on Tc- and Ru-Embedded Phthalocyanine Surfaces: A Density Functional Theory Study" Molecules 28, no. 20: 7137. https://doi.org/10.3390/molecules28207137
APA StyleXue, R., Jiang, W., He, X., Xiong, H., Xie, G., & Nie, Z. (2023). The Adsorption Mechanisms of SF6-Decomposed Species on Tc- and Ru-Embedded Phthalocyanine Surfaces: A Density Functional Theory Study. Molecules, 28(20), 7137. https://doi.org/10.3390/molecules28207137









