Tandem Mass Spectrometry de novo Sequencing of the Skin Defense Peptides of the Central Slovenian Agile Frog Rana dalmatina
Abstract
:1. Introduction
2. Results and Discussion
| No. | Peptides | Exp. MM, Da | Theor MM, Da | Δm, ppm | Sequences and ID Numbers | Refs |
|---|---|---|---|---|---|---|
| 1 | Brevinin 1Da | 1809.096 | 1809.098 | 1.3 | IIPLLLGKVVCAITKKC-OH | [38] |
| 2 * | Brevinin 1Db | 2607.472 | 2607.468 | 1.4 | FFPAFLKVAAKVVPSILCSITKKC-OH | |
| 3 * | Brevinin 2D | 3357.852 | 3357.855 | 0.9 | GLLSGLKKVGKVVAKNVAVSLMDSLKCKISGDC-OH | |
| 4 | Brevinin 2Rd | 3011.626 | 3011.626 | 0.3 | GILDSLKNLAKNAAQILLNKASCKLSGQC-OH-P86025.1 | [48] |
| 5 | FQ-22 (MRP) | 2310.383 | 2310.383 | 0.3 | FIGSALKVLAGVLPSVISWVKQ-NH2-P56924 | [49] |
| 6 | Temporin A | 1395.896 | 1395.897 | 0.4 | FLPLIGRVLSGIL-NH2-P56917.2 | [49] |
| 7 | Temporin B | 1390.925 | 1390.928 | 1.8 | LLPIVGNLLKSLL-NH2-P79874 | [49] |
| 8 | Temporin C | 1360.877 | 1360.881 | 2.6 | LLPILGNLLNGLL-NH2-P56918 | [49] |
| 9 | Temporin D | 1376.880 | 1376.876 | 3.3 | LLPIVGNLLNSLL-NH2-P56919 | [49] |
| 10 | Temporin F | 1367.887 | 1367.890 | 2.5 | FLPLIGKVLSGIL-NH2-P56921 | [49] |
| 11 | Temporin G | 1456.890 | 1456.892 | 1.3 | FFPVIGRILNGIL-NH2-P79875 | [49] |
| 12 | Temporin H | 1095.701 | 1095.702 | 0.5 | LSPNLLKSLL-NH2-P79876 | [49] |
| 13 | Temporin K | 1121.754 | 1121.754 | 0.3 | LLPNLLKSLL-NH2-P56923 | [49] |
| 14 | Temporin M | 1452.954 | 1452.954 | 0.3 | FLPILGKVLSRVL-NH2 | [50] |
| 15 * | Temporin 1Da | 1399.896 | 1399.896 | 0.3 | FLPLIAGLLGKLF-NH2 | |
| 16 | Bradykinin | 1059.561 | 1059.561 | 0.4 | RPPGFSPFR-OH-P0DM76.1 | |
| 17 | [desArg9]Br | 903.460 | 903.460 | 0.3 | RPPGFSPF-OH-P86628.1 | |
| 18 | Br 1-7 | 756.390 | 756.392 | 2.5 | RPPGFSP-OH | |
| 19 | Br 5-9 | 652.334 | 652.333 | 1.1 | FSPFR-OH | |
| 20 | Br RI-5-10 | 765.416 | 765.417 | 1.8 | FSPFRI-OH | |
| 21 | Br RI-10 | 1172.642 | 1172.645 | 3.0 | RPPGFSPFRI-OH | |
| 22 | Br RA-1-11 | 1243.679 | 1243.683 | 2.9 | RPPGFSPFRIA-OH | |
| 23 | [desArg1]Br RA-2-11 | 1087.579 | 1087.581 | 2.3 | PPGFSPFRIA-OH | |
| 24 | Br DR-11 | 1273.653 | 1273.657 | 2.9 | DVRPPGFSPFR-OH | |
| 25 | [Thr6]Br | 1073.572 | 1073.577 | 4.7 | RPPGFTPFR-OH-C0HKA8.1 | |
| 26 | [desArg9][Thr6]Br | 917.472 | 917.476 | 4.3 | RPPGFTPF-OH | |
| 27 | [Thr6]Br 1-7 | 770.405 | 770.408 | 3.3 | RPPGFTP-OH | |
| 28 | [Thr6]Br 5-9 | 666.347 | 666.349 | 2.9 | FTPFR-OH |
3. Materials and Methods
3.1. Reagents
3.2. Skin Secretions
3.3. Mass Spectrometry
3.4. Application of BLAST (Basic Local Alignment Search Tool)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Erspamer, V. Bioactive Secretions of the Amphibian Integument. In Amphibian Biology; Heatwole, H., Barthalmus, G.T., Heatwole, A.Y., Eds.; Surrey Beatty and Sons: Chipping Norton, UK, 1994; Volume 1, pp. 178–350. [Google Scholar]
- Bevins, C.L.; Zasloff, M. Peptides from Frog Skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, L.H.; Attila, M. The Toad, Ugly and Venomous, Wears yet a Precious Jewel in His Skin. Prog. Neurobiol. 1993, 41, 473–507. [Google Scholar] [CrossRef] [PubMed]
- Kimbrell, D.A.; Beutler, B. The Evolution and Genetics of Innate Immunity. Nat. Rev. Genet. 2001, 2, 256–267. [Google Scholar] [CrossRef] [PubMed]
- VanCompernolle, S.E.; Taylor, R.J.; Oswald-Richter, K.; Jiang, J.; Youree, B.E.; Bowie, J.H.; Tyler, M.J.; Conlon, J.M.; Wade, D.; Aiken, C.; et al. Antimicrobial Peptides from Amphibian Skin Potently Inhibit Human Immunodeficiency Virus Infection and Transfer of Virus from Dendritic Cells to T Cells. J. Virol. 2005, 79, 11598–11606. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Barra, D. Antimicrobial Peptides from Amphibian Skin: What Do They Tell Us? Biopolymers 1998, 47, 435–450. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Pukala, T.L.; Bowie, J.H.; Maselli, V.M.; Musgrave, I.F.; Tyler, M.J. Host-Defence Peptides from the Glandular Secretions of Amphibians: Structure and Activity. Nat. Prod. Rep. 2006, 23, 368. [Google Scholar] [CrossRef]
- Xu, X.; Lai, R. The Chemistry and Biological Activities of Peptides from Amphibian Skin Secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Vanhoye, D.; Bruston, F.; Nicolas, P.; Amiche, M. Antimicrobial Peptides from Hylid and Ranin Frogs Originated from a 150-Million-Year-Old Ancestral Precursor with a Conserved Signal Peptide but a Hypermutable Antimicrobial Domain. Eur. J. Biochem. 2003, 270, 2068–2081. [Google Scholar] [CrossRef]
- Thompson, A.H.; Bjourson, A.J.; Shaw, C.; McClean, S. Bradykinin-Related Peptides from Phyllomedusa Hypochondrialis Azurea: Mass Spectrometric Structural Characterisation and Cloning of Precursor CDNAs. Rapid Commun. Mass Spectrom. 2006, 20, 3780–3788. [Google Scholar] [CrossRef]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The Diversity and Evolution of Anuran Skin Peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.H.; Separovic, F.; Tyler, M.J. Host-Defense Peptides of Australian Anurans. Part 2. Structure, Activity, Mechanism of Action, and Evolutionary Significance. Peptides 2012, 37, 174–188. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and How Are Peptide–Lipid Interactions Utilized for Self-Defense? Magainins and Tachyplesins as Archetypes. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of Antimicrobial Pores in Membranes: Magainin and Protegrin. Biophys. J. 2000, 79, 2002–2009. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Haslam, I.S.; Roubos, E.W.; Mangoni, M.L.; Yoshizato, K.; Vaudry, H.; Kloepper, J.E.; Pattwell, D.M.; Maderson, P.F.A.; Paus, R. From Frog Integument to Human Skin: Dermatological Perspectives from Frog Skin Biology. Biol. Rev. 2014, 89, 618–655. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Vasileva, I.D.; Samgina, T.Y. FT-MS in the de Novo Top-down Sequencing of Natural Nontryptic Peptides. Mass Spectrom. Rev. 2022, 41, 284–313. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.; Denisov, E.; Lange, O.; Horning, S. Dynamic Range of Mass Accuracy in LTQ Orbitrap Hybrid Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2006, 17, 977–982. [Google Scholar] [CrossRef]
- Samgina, T.Y.; Artemenko, K.A.; Gorshkov, V.A.; Lebedev, A.T.; Nielsen, M.L.; Savitski, M.M.; Zubarev, R.A. Electrospray Ionization Tandem Mass Spectrometry Sequencing of Novel Skin Peptides from Ranid Frogs Containing Disulfide Bridges. Eur. J. Mass Spectrom. 2007, 13, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, I.D.; Samgina, T.Y.; Meng, Z.; Zubarev, R.A.; Lebedev, A.T. EThcD Benefits for the Sequencing Inside Intramolecular Disulfide Cycles of Amphibian Intact Peptides. J. Am. Soc. Mass Spectrom. 2023, 34, 1979–1988. [Google Scholar] [CrossRef]
- Artemenko, K.A.; Zubarev, A.R.; Samgina, T.Y.; Lebedev, A.T.; Savitski, M.M.; Zubarev, R.A. Two Dimensional Mass Mapping as a General Method of Data Representation in Comprehensive Analysis of Complex Molecular Mixtures. Anal. Chem. 2009, 81, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Samgina, T.Y.; Vasilieva, I.D.; Kozhevnikov, A.Y.; Meng, Z.; Zubarev, R.A.; Lebedev, A.T. Mass Spectrometry in de Novo Sequencing of the Skin Peptides from Arkhangelsk, Russia Rana Temporaria: The Variability of Secreted AMPs in Different Populations. Int. J. Mass Spectrom. 2023, 484, 116984. [Google Scholar] [CrossRef]
- Medzihradszky, K.F.; Chalkley, R.J. Lessons in de Novo Peptide Sequencing by Tandem Mass Spectrometry. Mass Spectrom. Rev. 2015, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Cortázar-Chinarro, M.; Meyer-Lucht, Y.; Van der Valk, T.; Richter-Boix, A.; Laurila, A.; Höglund, J. Antimicrobial Peptide and Sequence Variation along a Latitudinal Gradient in Two Anurans. BMC Genet. 2020, 21, 38. [Google Scholar] [CrossRef]
- Rončević, T.; Krce, L.; Gerdol, M.; Pacor, S.; Benincasa, M.; Guida, F.; Aviani, I.; Čikeš-Čulić, V.; Pallavicini, A.; Maravić, A.; et al. Membrane-Active Antimicrobial Peptide Identified in Rana Arvalis by Targeted DNA Sequencing. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 651–659. [Google Scholar] [CrossRef]
- Frese, C.K.; Altelaar, A.F.M.; Van Den Toorn, H.; Nolting, D.; Griep-Raming, J.; Heck, A.J.R.; Mohammed, S. Toward Full Peptide Sequence Coverage by Dual Fragmentation Combining Electron-Transfer and Higher-Energy Collision Dissociation Tandem Mass Spectrometry. Anal. Chem. 2012, 84, 9668–9673. [Google Scholar] [CrossRef] [PubMed]
- Samgina, T.Y.; Kovalev, S.V.; Tolpina, M.D.; Trebse, P.; Torkar, G.; Lebedev, A.T. EThcD Discrimination of Isomeric Leucine/Isoleucine Residues in Sequencing of the Intact Skin Frog Peptides with Intramolecular Disulfide Bond. J. Am. Soc. Mass Spectrom. 2018, 29, 842–852. [Google Scholar] [CrossRef]
- Zhokhov, S.S.; Kovalyov, S.V.; Samgina, T.Y.; Lebedev, A.T. An EThcD-Based Method for Discrimination of Leucine and Isoleucine Residues in Tryptic Peptides. J. Am. Soc. Mass Spectrom. 2017, 28, 1600–1611. [Google Scholar] [CrossRef]
- Frost, D.R.; Amphibian Species of the World: An Online Reference. Version 6.2. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 8 September 2023).
- Kuzmin, S.L. The Amphibians of the Former USSR, 1st ed.; Tovarischestvo Nauchnyh Izdanii KMK: Moscow, Russia, 1999. [Google Scholar]
- Lešnik, A.; Poboljšaj, K. Prispevek k poznavanju favne dvoživk (Amphibia) severovzhodne Slovenije. Nat. Slov. 1999, 1, 71–82. [Google Scholar]
- Poboljšaj, K. Dvoživke (Amphibia) slovenskega primorja. Varst. Narave 2007, 20, 110–119. [Google Scholar]
- Arnold, E.N. Reptiles and Amphibians of Britain and Europe, 2nd ed.; Collins: New York, NY, USA, 2002. [Google Scholar]
- Kaya, U.; Kuzmin, S.; Sparreboom, M.; Ugurtas, I.; Tarkhnishvili, D.; Anderson, S.; Andreone, F.; Corti, C.; Nyström, P.; Schmidt, B.; et al. Rana Dalmatina (Agile Frog). IUCN. 2022. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 5 September 2023).
- Stanković, D.; Luznik, M.; Poboljšaj, K. Conservation and Declines of Amphibians in Slovenia. In Amphibian Biology; Heatwole, H., Wilkinson, J.W., Eds.; Pelagic Publishing: London, UK, 2015; Volume 11, pp. 32–44. [Google Scholar]
- Conlon, J.M.; Seidel, B.; Nielsen, P.F. An Atypical Member of the Brevinin-1 Family of Antimicrobial Peptides Isolated from the Skin of the European Frog Rana dalmatina. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Erspamer, V.; Bertaccini, G. Occurrence of Bradykinin in the Skin of Rana temporaria. Comp. Biochem. Physiol. 1965, 14, 43–52. [Google Scholar] [CrossRef]
- Roseghini, M.; Erspamer, G.F.; Severini, C.; Simmaco, M. Biogenic Amines and Active Peptides in Extracts of the Skin of Thirty-Two European Amphibian Species. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1989, 94, 455–460. [Google Scholar] [CrossRef]
- Xi, X.; Li, B.; Chen, T.; Kwok, H. A Review on Bradykinin-Related Peptides Isolated from Amphibian Skin Secretion. Toxins 2015, 7, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Rončević, T.; Gerdol, M.; Spazzali, F.; Florian, F.; Mekinić, S.; Tossi, A.; Pallavicini, A. Parallel Identification of Novel Antimicrobial Peptide Sequences from Multiple Anuran Species by Targeted DNA Sequencing. BMC Genomics 2018, 19, 827. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Zhang, X.; Xin, L.; Shan, B.; Li, M. De Novo Peptide Sequencing by Deep Learning. Proc. Natl. Acad. Sci. USA 2017, 114, 8247–8252. [Google Scholar] [CrossRef]
- Tran, N.H.; Qiao, R.; Xin, L.; Chen, X.; Liu, C.; Zhang, X.; Shan, B.; Ghodsi, A.; Li, M. Deep Learning Enables de Novo Peptide Sequencing from Data-Independent-Acquisition Mass Spectrometry. Nat. Methods 2019, 16, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, B. Novor: Real-Time Peptide de Novo Sequencing Software. J. Am. Soc. Mass Spectrom. 2015, 26, 1885–1894. [Google Scholar] [CrossRef]
- Novor.Cloud. Available online: https://novor.cloud/ (accessed on 6 October 2023).
- Mechkarska, M.; Kolodziejek, J.; Musale, V.; Coquet, L.; Leprince, J.; Jouenne, T.; Nowotny, N.; Conlon, J.M. Peptidomic Analysis of the Host-Defense Peptides in Skin Secretions of Rana Graeca Provides Insight into Phylogenetic Relationships among Eurasian Rana species. Comp. Biochem. Physiol. Part D Genomics Proteomics 2019, 29, 228–234. [Google Scholar] [CrossRef]
- Samgina, T.Y.; Artemenko, K.A.; Gorshkov, V.A.; Ogourtsov, S.V.; Zubarev, R.A.; Lebedev, A.T. De Novo Sequencing of Peptides Secreted by the Skin Glands of the Caucasian Green Frog Rana ridibunda. Rapid Commun. Mass Spectrom. 2008, 22, 3517–3525. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, Antimicrobial Peptides from the European Red Frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef]
- Samgina, T.Y.; Vorontsov, E.A.; Gorshkov, V.A.; Hakalehto, E.; Hanninen, O.; Zubarev, R.A.; Lebedev, A.T. Composition and Antimicrobial Activity of the Skin Peptidome of Russian Brown Frog Rana temporaria. J. Proteome Res. 2012, 11, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M. Reflections on a Systematic Nomenclature for Antimicrobial Peptides from the Skins of Frogs of the Family Ranidae. Peptides 2008, 29, 1815–1819. [Google Scholar] [CrossRef]
- Samgina, T.Y.; Vorontsov, E.A.; Gorshkov, V.A.; Artemenko, K.A.; Zubarev, R.A.; Ytterberg, J.A.; Lebedev, A.T. Collision-Induced Dissociation Fragmentation Inside Disulfide C-Terminal Loops of Natural Non-Tryptic Peptides. J. Am. Soc. Mass Spectrom. 2013, 24, 1037–1044. [Google Scholar] [CrossRef]
- Protein BLAST. Available online: https://blast.ncbi.nlm.nih.gov/ (accessed on 8 September 2023).
- Samgina, T.Y.; Vasileva, I.D.; Trebse, P.; Torkar, G.; Surin, A.K.; Meng, Z.; Zubarev, R.A.; Lebedev, A.T. Mass Spectrometry Differentiation between Rana Arvalis Populations Based on Their Skin Peptidome Composition. J. Am. Soc. Mass Spectrom. 2022, 33, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Barra, D.; Bossa, F. Antimicrobial Peptides from Skin Secretions of Rana Esculenta. Molecular Cloning of CDNAs Encoding Esculentin and Brevinins and Isolation of New Active Peptides. J. Biol. Chem. 1994, 269, 11956–11961. [Google Scholar] [CrossRef]
- Conlon, J.M.; Musale, V.; Attoub, S.; Mangoni, M.L.; Leprince, J.; Coquet, L.; Jouenne, T.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Rinaldi, A.C. Cytotoxic Peptides with Insulin-Releasing Activities from Skin Secretions of the Italian Stream Frog Rana Italica (Ranidae). J. Pept. Sci. 2017, 23, 769–776. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Mignogna, G.; Andreu, D.; Shai, Y.; Barra, D.; Simmaco, M. Ranacyclins, a New Family of Short Cyclic Antimicrobial Peptides: Biological Function, Mode of Action, and Parameters Involved in Target Specificity. Biochemistry 2003, 42, 14023–14035. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L. Temporins, Anti-Infective Peptides with Expanding Properties. Cell. Mol. Life Sci. 2006, 63, 1060–1069. [Google Scholar] [CrossRef]
- Musale, V.; Casciaro, B.; Mangoni, M.L.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Conlon, J.M. Assessment of the Potential of Temporin Peptides from the Frog Rana Temporaria (Ranidae) as Anti-Diabetic Agents. J. Pept. Sci. 2018, 24, e3065. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.M.; Cardillo, A.B.; Martínez Ceron, M.C.; Camperi, S.A.; Giudicessi, S.L. Temporins: An Approach of Potential Pharmaceutic Candidates. Surg. Infect. 2020, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Shen, Y.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Kunitzins: Prototypes of a New Class of Protease Inhibitor from the Skin Secretions of European and Asian Frogs. Biochem. Biophys. Res. Commun. 2016, 477, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yin, W.; Chen, Y.; Chen, X.; Jiang, Y.; Wang, T.; Ma, C.; Zhou, M.; Chen, T.; Shaw, C.; et al. Conjugation of a Cationic Cell-Penetrating Peptide with a Novel Kunitzin-like Trypsin Inhibitor: New Insights for Enhancement of Peptide Bioactivities. Pharmaceutics 2022, 14, 1805. [Google Scholar] [CrossRef]
- Duda, T.F.; Vanhoye, D.; Nicolas, P. Roles of Diversifying Selection and Coordinated Evolution in the Evolution of Amphibian Antimicrobial Peptides. Mol. Biol. Evol. 2002, 19, 858–864. [Google Scholar] [CrossRef]
- Tennessen, J.A. Adaptive Diversity and Divergence At Frog Antimicrobial Peptide Loci. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2009. [Google Scholar]
- Tennessen, J.A. Molecular Evolution of Animal Antimicrobial Peptides: Widespread Moderate Positive Selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef]
- Tennessen, J.A.; Blouin, M.S. Selection for Antimicrobial Peptide Diversity in Frogs Leads to Gene Duplication and Low Allelic Variation. J. Mol. Evol. 2007, 65, 605–615. [Google Scholar] [CrossRef]
- Tennessen, J.A.; Woodhams, D.C.; Chaurand, P.; Reinert, L.K.; Billheimer, D.; Shyr, Y.; Caprioli, R.M.; Blouin, M.S.; Rollins-Smith, L.A. Variations in the Expressed Antimicrobial Peptide Repertoire of Northern Leopard Frog (Rana Pipiens) Populations Suggest Intraspecies Differences in Resistance to Pathogens. Dev. Comp. Immunol. 2009, 33, 1247–1257. [Google Scholar] [CrossRef]
- Tyler, M.J. Frogs and Cane Toad Skin Secretions. In Toxic Plants and Animals: A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: South Brisbane, Australia, 1987; pp. 329–330. [Google Scholar]
- Tyler, M.J.; Stone, D.J.M.; Bowie, J.H. A Novel Method for the Release and Collection of Dermal, Glandular Secretions from the Skin of Frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Steinborner, S.T.; Waugh, R.J.; Bowie, J.H.; Wallace, J.C.; Tyler, M.J.; Ramsay, S.L. New Caerin Antibacterial Peptides from the Skin Glands of the Australian Tree FrogLitoria Xanthomera. J. Pept. Sci. 1997, 3, 181–185. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Mechkarska, M.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Nielsen, P.F.; Nowotny, N.; King, J.D. Host Defense Peptides from Lithobates Forreri, Hylarana Luctuosa, and Hylarana Signata (Ranidae): Phylogenetic Relationships Inferred from Primary Structures of Ranatuerin-2 and Brevinin-2 Peptides. Comp. Biochem. Physiol. Part D Genomics Proteomics 2014, 9, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.C. Collecting Arthropod and Amphibian Secretions for Chemical Analyses. In Behavioral and Chemical Ecology; Nova Science Pub. Inc.: New York, NY, USA, 2009; pp. 1–46. [Google Scholar]
- Conlon, J.M.; Mechkarska, M.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Measey, G.J. Evidence from Peptidomic Analysis of Skin Secretions That Allopatric Populations of Xenopus Gilli (Anura:Pipidae) Constitute Distinct Lineages. Peptides 2015, 63, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xi, X.; Ge, L.; Yang, N.; Hou, X.; Ma, J.; Ma, C.; Wu, Y.; Guo, X.; Li, R.; et al. Bradykinin-Related Peptides (BRPs) from Skin Secretions of Three Genera of Phyllomedusine Leaf Frogs and Their Comparative Pharmacological Effects on Mammalian Smooth Muscles. Peptides 2014, 52, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T. Active Peptides in Amphibian Skin. Trends Pharmacol. Sci. 1981, 2, 202–205. [Google Scholar] [CrossRef]
- Gibson, B.W.; Poulter, L.; Williams, D.H.; Maggio, J.E. Novel Peptide Fragments Originating from PGLa and the Caerulein and Xenopsin Precursors from Xenopus Laevis. J. Biol. Chem. 1986, 261, 5341–5349. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Woodhams, D.C.; Reinert, L.K.; Vredenburg, V.T.; Briggs, C.J.; Nielsen, P.F.; Michael Conlon, J. Antimicrobial Peptide Defenses of the Mountain Yellow-Legged Frog (Rana Muscosa). Dev. Comp. Immunol. 2006, 30, 831–842. [Google Scholar] [CrossRef]
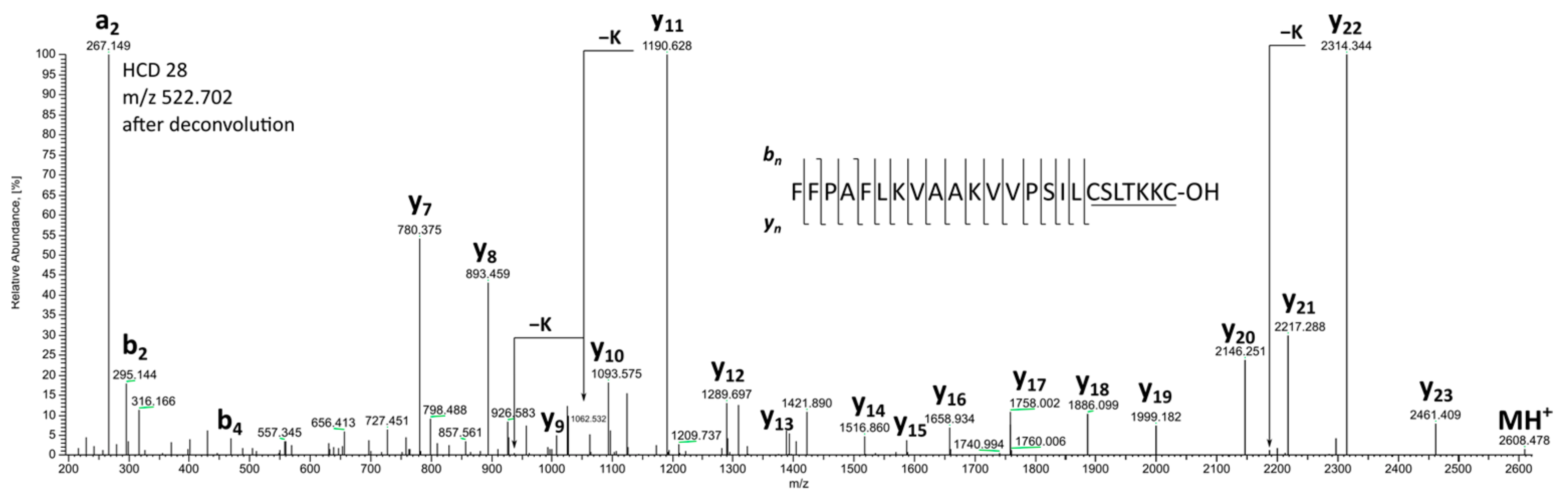
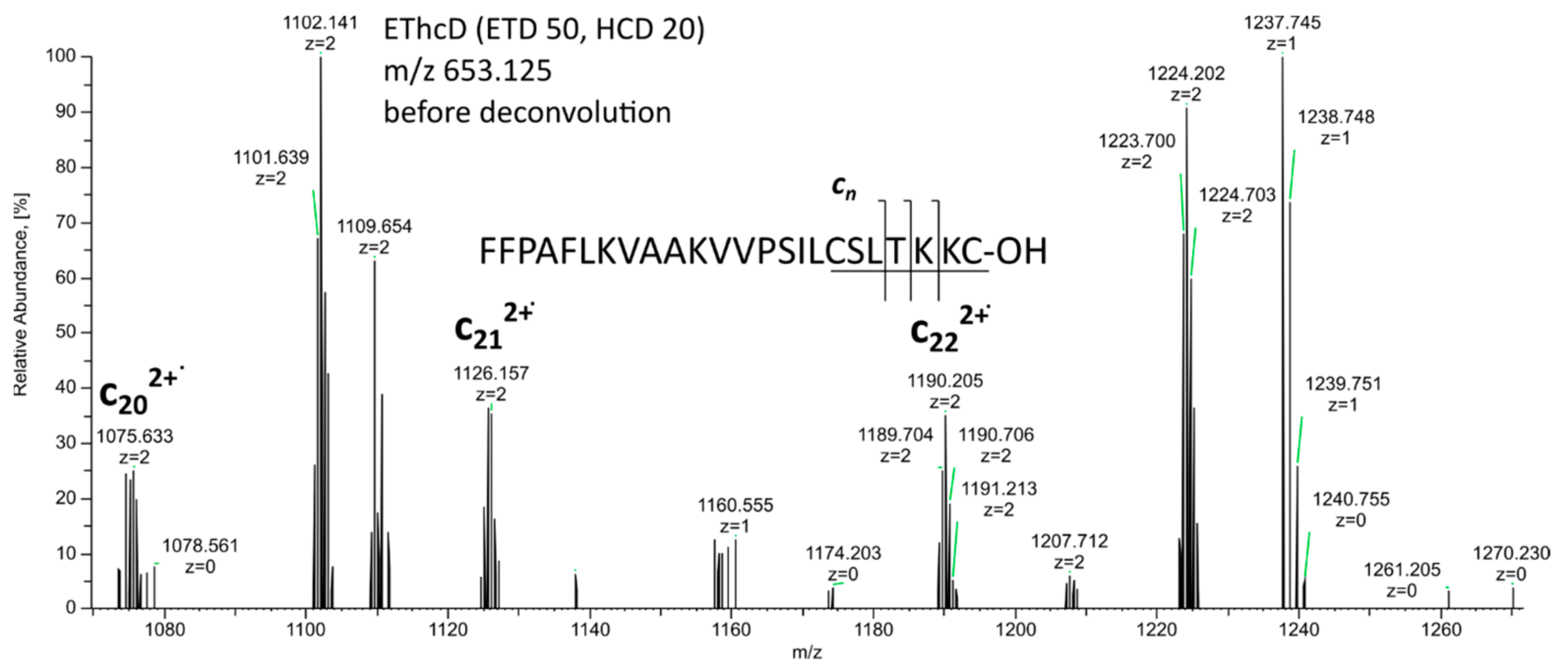

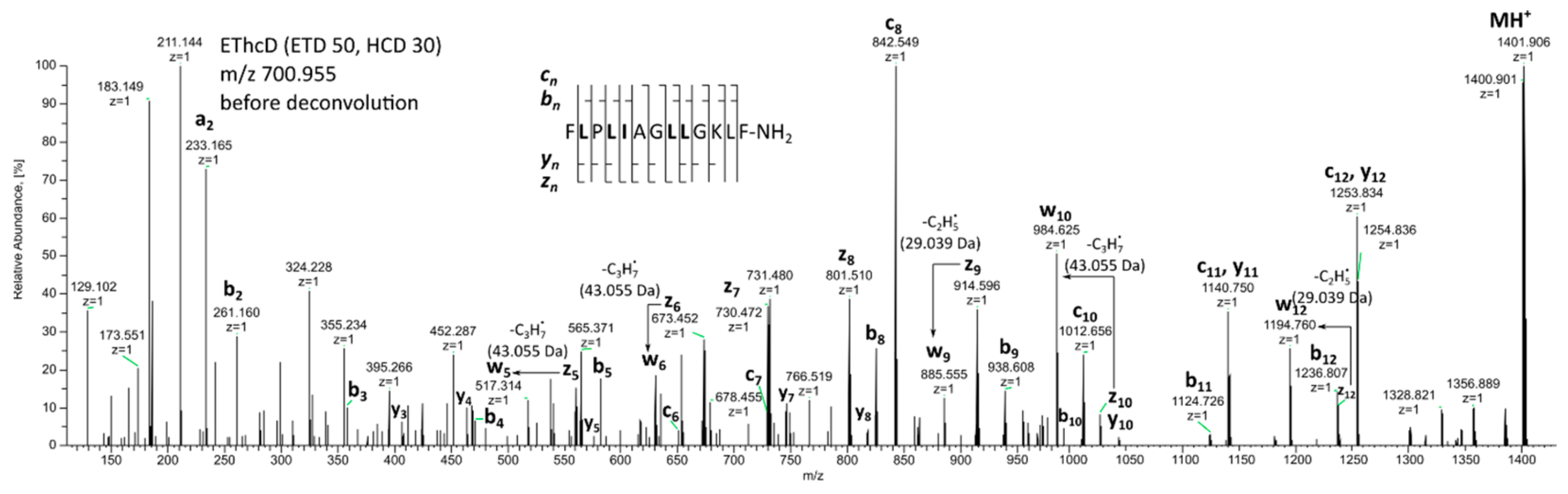
| No. | Peptide Code | cDNA Cloning Sequence | Reference * |
|---|---|---|---|
| Group 1—Shot linear ** | |||
| 1 | rdal_51_25/1-15 | FLPVIAGVLSKLFGK | Temp 1Da; R. dalmat. V4→L4; V8→L8 S10→G10 |
| 2 | rdal_21_605/1-6 | ILPLIGKVLSGILAK | Temp F; R. temporar. I1→F1 [49] |
| 3 | rdal_44_43/1-15 | LLPIVGNLLNDLLGK | Temp D, R. temp Asp11→Ser11 [49] Temp AV R. arv [54] |
| 4 | rdal_226_12/1-14 | FLPIVTNLLLRFVG | |
| 5 | rdal-8-600/1-18 | FLGFVGQALNALLGKLGK | |
| Group 2—Shot linear ranabox | |||
| 6 | rdal-38-11/1-23 | FFPAIFRLVAKVVPSIICSVTKN | Brev 1R, P. ridibundus CSVTKKC [49] |
| 7 | rdal_103_32/1-24 | FLPLLAGLAANFLPKIFCKITRKC | Brev 1E, P. escul [55] |
| 8 | rdal-3-1108/1-20 | IVPILLGVVPQLVCAITKKC | Brev1Ita, R. italica I4→F4; V8→M8 Q11→K11; A15→L15 [56] |
| 9 | rdal-53-31/1-17 | IIPLLLGKVVCAITKKC | Brev 1Da, R. dalmat [38] |
| Group 3—Brevinin helical ranabox | |||
| 10 | rdal_15_33/1-34 | GILLDKLKNFAKTAGKGVLQSLLNTASCKISGQC | Brev 2Ec, R. escul [6] |
| Group 4—Esculentin helical ranabox | |||
| 11 | rdal-6-971/1-31 | FLWETVKNFGKTFTLNILDKLKCKIGGECPP | Brev 2Tc, R. tempor V6→I6; T12→K12; D19→H19; E28→G28 [6] |
| 12 | rdal_120_5/1-37 | GILSLVKGIAKLAGKGLAKEGGKFGLELMACKIAKQC | Escul 2a; P. escul. I9→V9; I29→M29 [55] |
| Group 5—likely beta hairpin | |||
| 13 | rdal_108_19/1-19 | GALRGCWTKSIPPKPCKGK | Ranacyc T; R tempor I11→ Y11 [57] |
| 14 | rdal-32-284/1-24 | VPQLCFKFQKVIYCEINKTLPNFA | |
| Group 6—Kunetzin | |||
| 15 | rdal-58-29/1-17 | AAKIILNPKFRCKAAFC | Ranat 2Ra, P. ridib [48] |
| Group 7 Pro-rich predicted by cloning No peptides in that group | |||
| Group 8 Undefined | |||
| 16 | rdal-77-6/1-35 | FLPLVLGKTHSFSEQAELLSWKSSNVEYHLPKCTDV | |
| 17 | rdal_7_282/1-9 | GIVEAWPLR | |
| 18 | rdal_40_14/1-15 | GLEVLGKILSGLLGK | Temp 1AVa; R. arv [54] |
| 19 | rdal-39-24/1-32 | NLLGFLQGAKDILKECEADNYQGWLCESSYKPQ | |
| 20 | rdal-110-77/1-21 | LANRAARNTQNVLNAITCTL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samgina, T.Y.; Vasileva, I.D.; Trebše, P.; Torkar, G.; Surin, A.K.; Meng, Z.; Zubarev, R.A.; Lebedev, A.T. Tandem Mass Spectrometry de novo Sequencing of the Skin Defense Peptides of the Central Slovenian Agile Frog Rana dalmatina. Molecules 2023, 28, 7118. https://doi.org/10.3390/molecules28207118
Samgina TY, Vasileva ID, Trebše P, Torkar G, Surin AK, Meng Z, Zubarev RA, Lebedev AT. Tandem Mass Spectrometry de novo Sequencing of the Skin Defense Peptides of the Central Slovenian Agile Frog Rana dalmatina. Molecules. 2023; 28(20):7118. https://doi.org/10.3390/molecules28207118
Chicago/Turabian StyleSamgina, Tatiana Yu., Irina D. Vasileva, Polonca Trebše, Gregor Torkar, Alexey K. Surin, Zhaowei Meng, Roman A. Zubarev, and Albert T. Lebedev. 2023. "Tandem Mass Spectrometry de novo Sequencing of the Skin Defense Peptides of the Central Slovenian Agile Frog Rana dalmatina" Molecules 28, no. 20: 7118. https://doi.org/10.3390/molecules28207118
APA StyleSamgina, T. Y., Vasileva, I. D., Trebše, P., Torkar, G., Surin, A. K., Meng, Z., Zubarev, R. A., & Lebedev, A. T. (2023). Tandem Mass Spectrometry de novo Sequencing of the Skin Defense Peptides of the Central Slovenian Agile Frog Rana dalmatina. Molecules, 28(20), 7118. https://doi.org/10.3390/molecules28207118









