Performance Research of Natural Mica Modified with Zirconium-Based Metal–Organic Frameworks for an Epoxy Resin Anti-Corrosion Coating
Abstract
:1. Introduction
| Study | Findings |
|---|---|
| Ding et al. [24] | A coating resembling skin structure was made to improve barrier properties. |
| Yu et al. [37] | Synthesis of oriented polyaniline nanofibres doped with phytic acid on a mica surface for ultra-high corrosion protection of epoxy coatings. In addition, the synergistic effect of polyaniline nanofibres and mica enhanced the adhesion of the coating. |
| Bai et al. [27] | Polyethyleneimine as binder and zinc molybdate as additive sericite were modified. This composite gives the epoxy coating self-healing properties. |
| Xiao et al. [38] | MNs surfaces can be made superhydrophobic by interacting them with natural laccase phenol (Ur) catechol groups. |
| Ye et al. [39] | Tannic acid modified mica can enhance the dispersibility of mica in epoxy resin. |
2. Results and Discussion
2.1. The Characterization of PEI-MC/UIO@MBT Nanocomposite
2.1.1. XRD Analysis
2.1.2. FTIR Analysis
2.1.3. TGA Analysis
2.1.4. BET Analysis
2.1.5. Corrosion Inhibitor Release Performance Testing
2.1.6. Dispersion Stability Testing of Composite Materials
2.1.7. Micromorphology of Composite Materials
2.2. Performance Characterisation of PMC–UIO@MBT/EP Composite Coatings
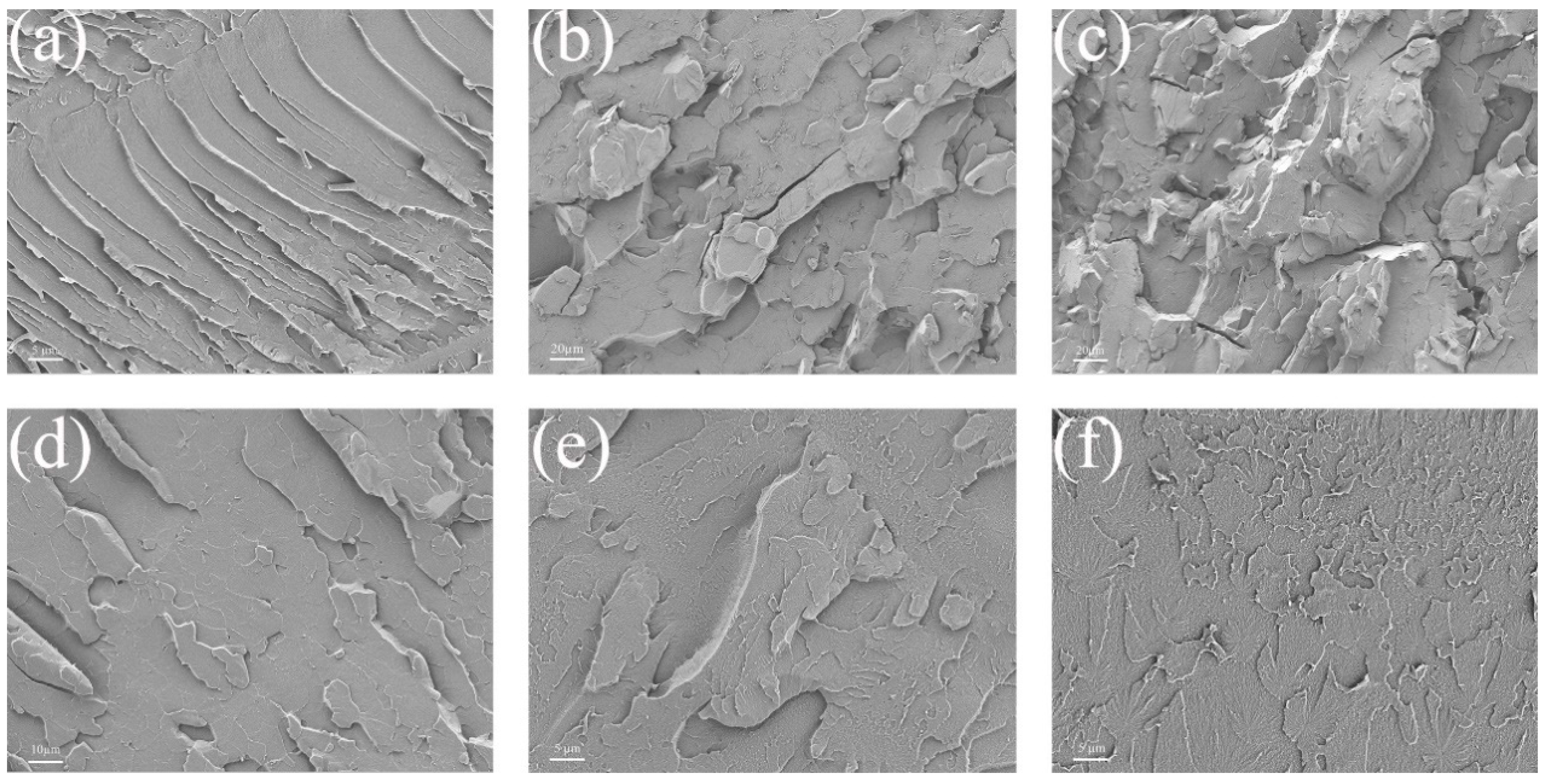
2.2.1. Micromorphology of Coated Sections
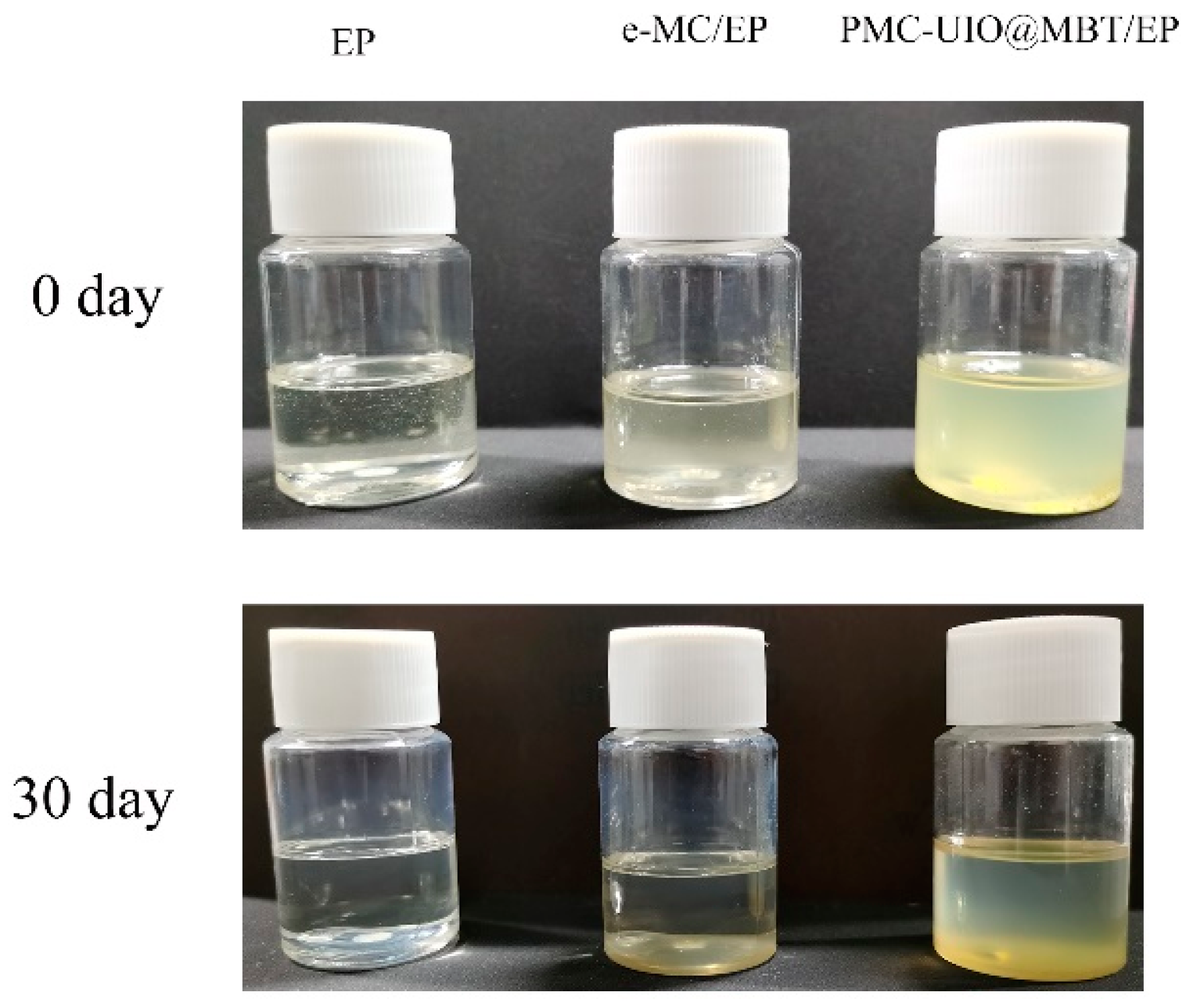
2.2.2. Compatibility of Composites in Epoxy Resins
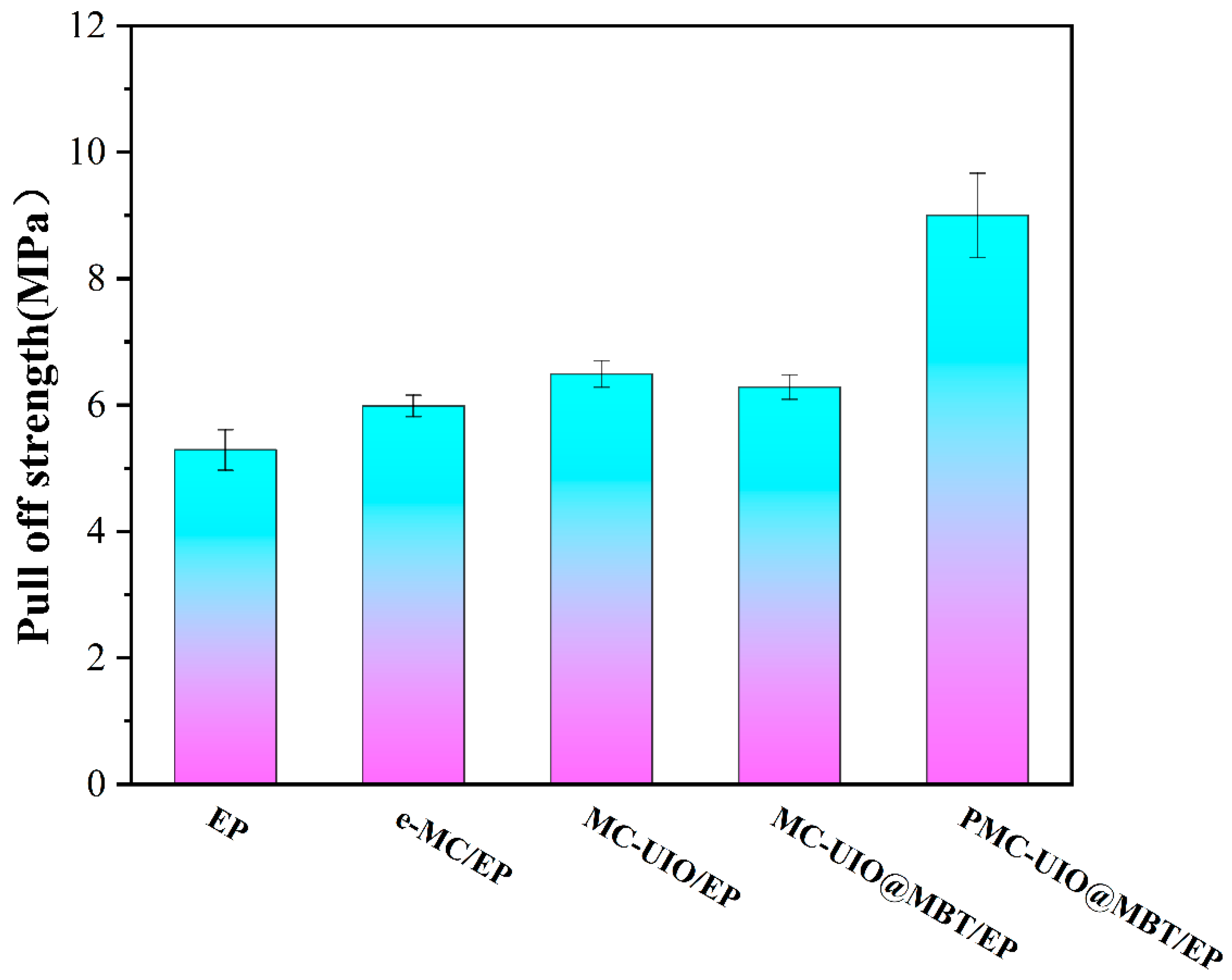
2.2.3. Adhesion Testing of Coatings
2.3. Corrosion Resistance of PMC–UIO@MBT/EP Composite Coating
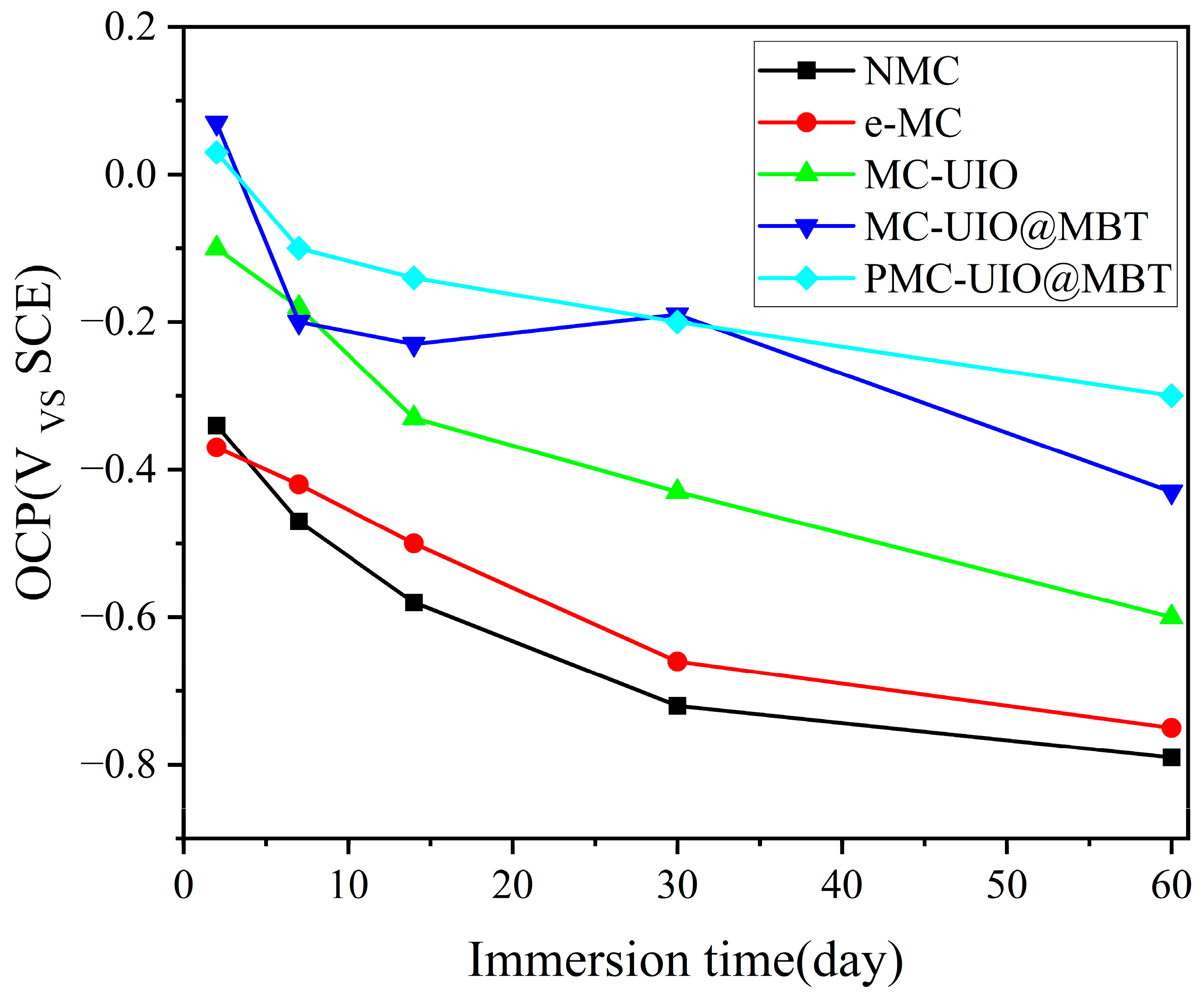
2.3.1. Corrosion Resistance of the Coating in 3.5 wt.% NaCl Solution
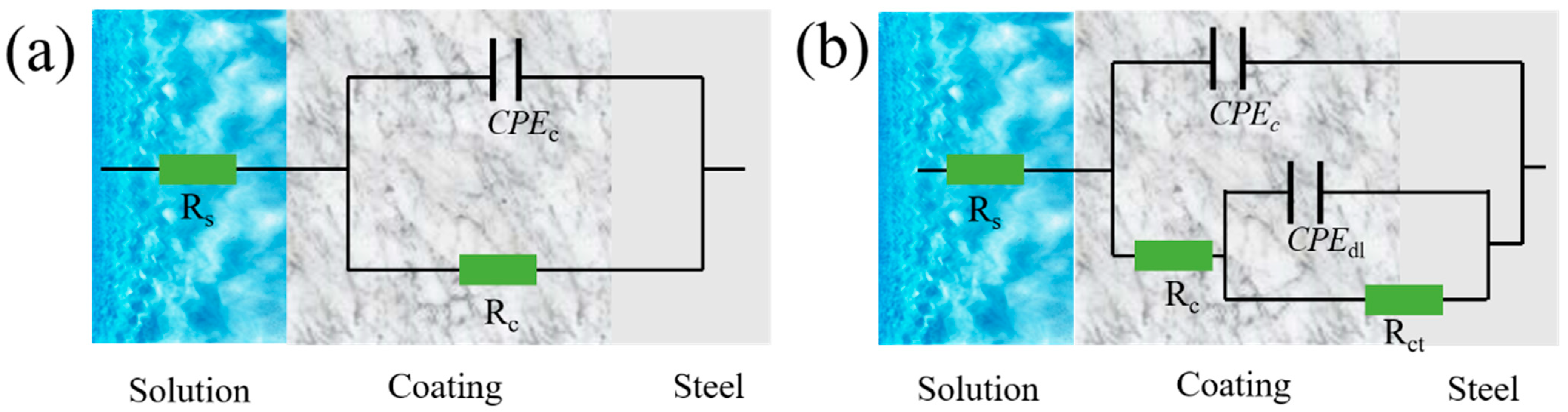
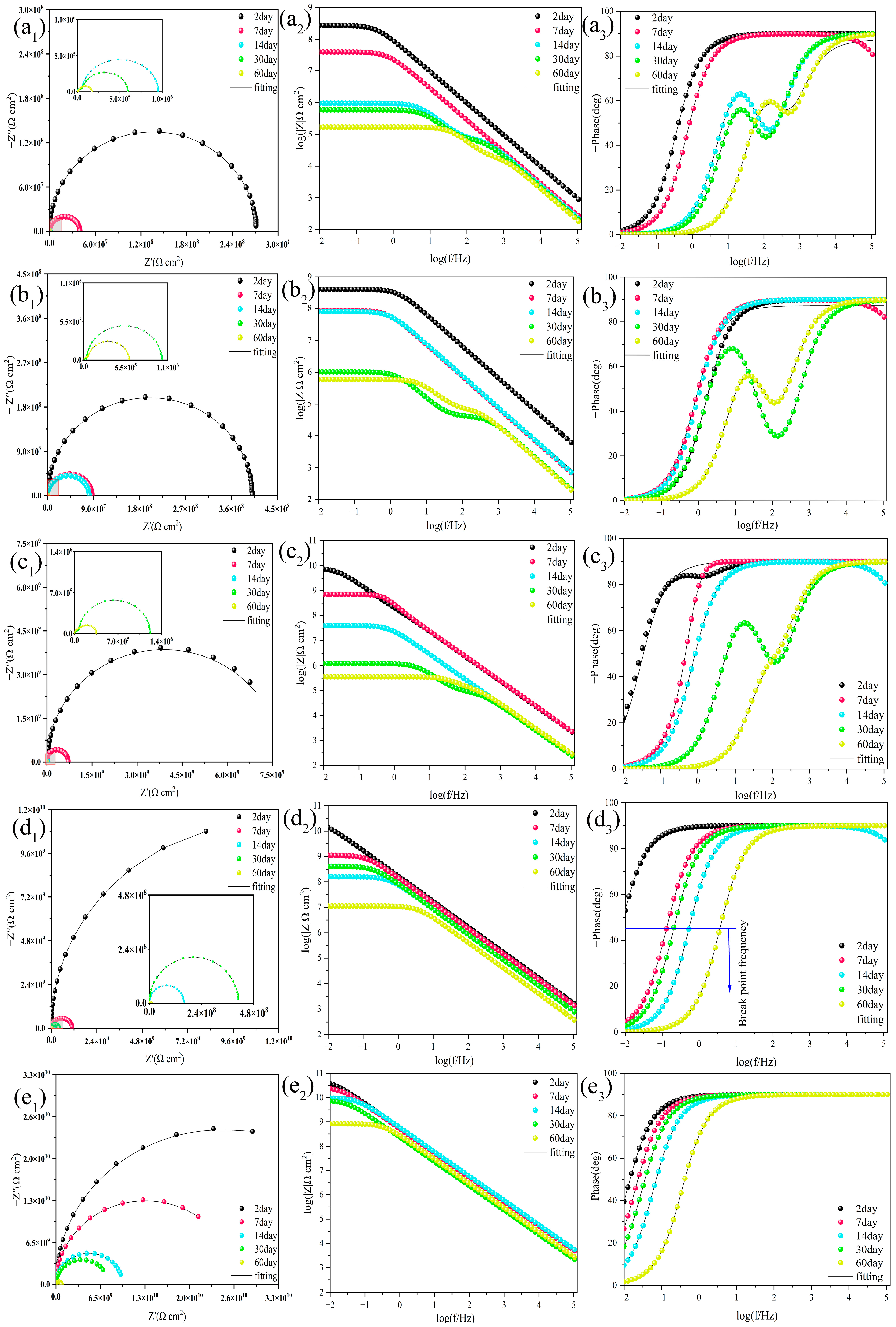
2.3.2. EIS Results for Coatings Immersed in an Alkaline 3.5% NaCl Solution
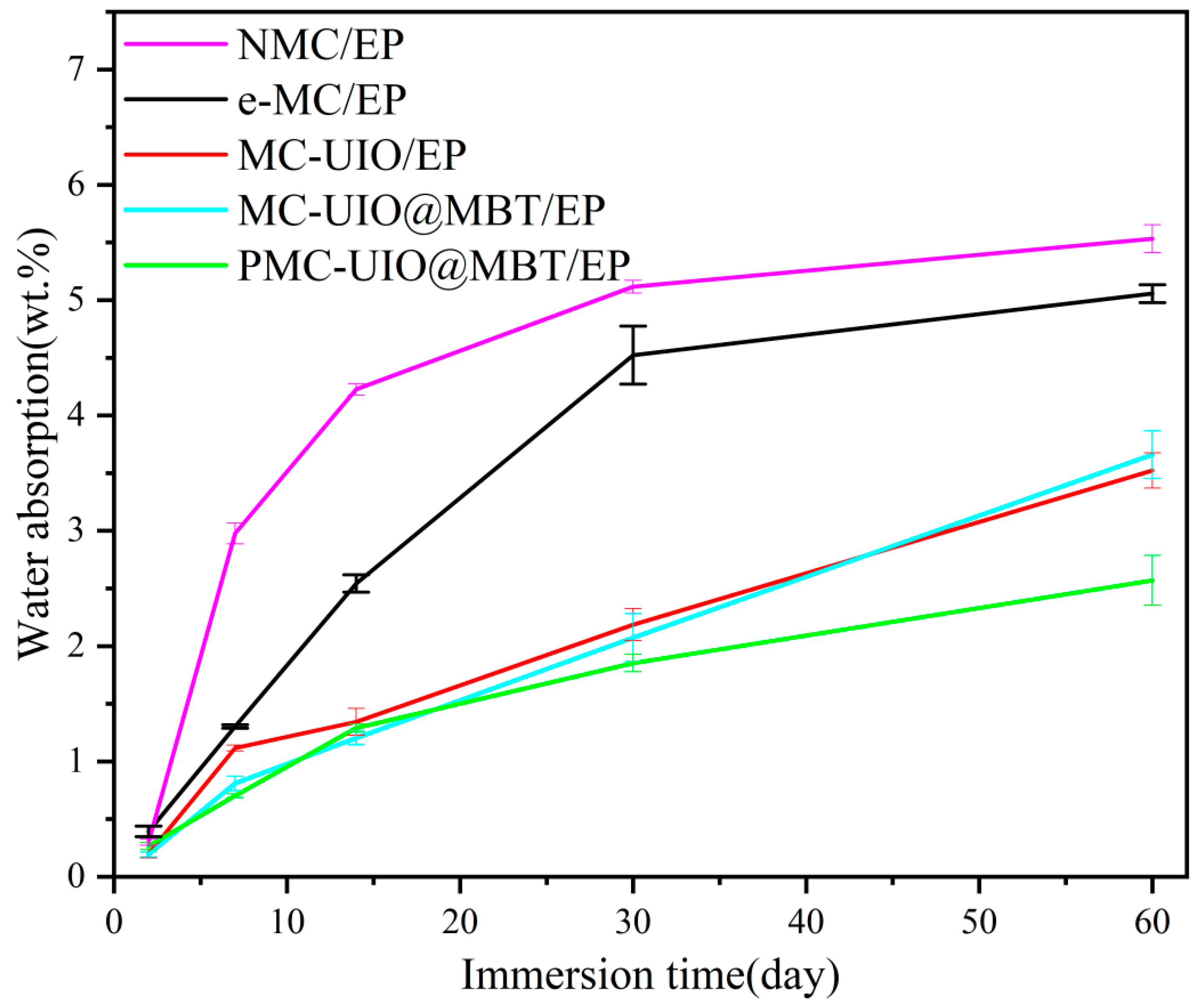
2.3.3. Water Absorption Test of Coatings
3. Mechanistic Analysis of Corrosion Resistance
4. Experiment
4.1. Material
4.2. Exfoliation of Natural Mica
4.3. Preparation of MC–UIO and Loading of Corrosion Inhibitor
4.4. Preparation of PMC@UIO@MBT Composites
4.5. Preparation of Composite Coatings
4.6. Characterization
5. Conclusions
- (1)
- The successful preparation of the composites and the successful encapsulation of MBT as a corrosion inhibitor in UIO-66 were demonstrated by characterization tests such as XRD, FTIR, TGA, BET, UV–VIS, and SEM. The release of MBT can be achieved due to the collapse of the crystal structure of UIO-66 under acidic and alkaline conditions. By modifying the material with polyethyleneimine, we discovered that UIO-66 is more densely loaded onto the mica flakes. This facilitates the loading of more corrosion inhibitors, as shown in the SEM results. In addition, adding polyethyleneimine and UIO-66 into the amino group improved the dispersion and compatibility of the composite in the epoxy coating;
- (2)
- In coating performance tests, the corrosion resistance of PMC–UIO@MBT/EP was significantly improved and the coating still had a low-frequency impedance modulus of 8.30 × 108 Ω after 60 days of immersion in a 3.5 wt.% NaCl solution at pH = 7. As well as the change in pH in the cathodic region due to metal corrosion, the MBT is released by the controlled UIO-66 pH response, allowing the coating to recover. Therefore, the highest low-frequency impedance modulus values are also achieved after 30 days of immersion in 3.5 wt.% NaCl solutions at pH = 11;
- (3)
- The relatively small water absorption and the adhesion of PMC–UIO@MBT/EP indicate its good barrier properties and stability. The adhesion of PMC–UIO@MBT coating was increased to 9.01 MPa; water absorption was only 2.57% after 60 days of immersion in a 3.5 wt.% NaCl solution.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Feng, C.; Cao, Y.Q.; Zhu, L.J.; Yu, Z.X.; Gao, G.H.; Song, Y.C.; Ge, H.J.; Liu, Y.X. Corrosion Behavior of Reduced-Graphene-Oxide-Modified Epoxy Coatings on N80 Steel in 10.0 wt% NaCl Solution. Int. J. Electrochem. Sci. 2020, 15, 8265–8276. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, L.; Cao, K.; Yu, Z.; Song, Y. Difunctional Silicon Dioxide Combined with Graphene Oxide Nanocomposite to Enhance the Anticorrosion Performance of Epoxy Coatings. ACS Omega 2022, 7, 24134–24144. [Google Scholar] [CrossRef]
- Peng, B.K.; Yu, Z.X.; Chen, L.G.; Liao, K.X.; Chen, H.D.; Guo, Y.C.; Liu, Y.C. Modified hydroxyapatite improves the corrosion resistance of the epoxy coating. J. Appl. Polym. Sci. 2022, 139, e52866. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Z.; Chen, L.; Liao, K.; Chen, H.; Pang, Y.; Peng, B.; Zhu, L. Preparation and performance of a composite epoxy coating based on modified hydroxyapatite. Surf. Coat. Technol. 2022, 443, 128614. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Z.; Yang, G.; Liao, K.; Peng, B.; Pang, Y.; Zhu, L.; Tang, J. A novel pH-responsive smart anticorrosion coating based on sepiolite and MOF for high-performance corrosion protection. Surf. Coat. Technol. 2022, 446, 128768. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2 MXene nanosheets toward high-performance corrosion inhibitor for epoxy coating. Prog. Org. Coat. 2019, 135, 156–167. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a Long-Term Metal Oxidation Barrier: Worse Than Nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Li, J.; Yuan, T.; Chen, B.; Ma, X.; Jiang, D.; Luo, X.; Chen, D.; Liu, Y. Epoxy composite coating with excellent anticorrosion and self-healing performances based on multifunctional zeolitic imidazolate framework derived nanocontainers. Chem. Eng. J. 2020, 385, 123835. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Yu, H. Superior to graphene: Super-anticorrosive natural mica nanosheets. Nanoscale 2020, 12, 16253–16261. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ren, S.; Qin, S.; Xue, Q.; Zhao, H.; Wang, L. Processable poly(2-butylaniline)/hexagonal boron nitride nanohybrids for synergetic anticorrosive reinforcement of epoxy coating. Corros. Sci. 2018, 131, 187–198. [Google Scholar] [CrossRef]
- Xie, L.; Wang, T.; He, C.; Sun, Z.; Peng, Q. Molecular Dynamics Simulation on Mechanical and Piezoelectric Properties of Boron Nitride Honeycomb Structures. Nanomaterials 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, X.; Wang, T.; Zhang, H.; Li, Y.; Bao, H.; Chen, M.; Wu, L. Self-Modifying Nanointerface Driving Ultrahigh Bidirectional Thermal Conductivity Boron Nitride-Based Composite Flexible Films. Nano-Micro Lett. 2023, 15, 2. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, F.; Wu, Y.; Hao, X.; Wang, Z.; Xu, X. One-Step Exfoliation and Hydroxylation of Boron Nitride Nanosheets with Enhanced Optical Limiting Performance. Adv. Opt. Mater. 2016, 4, 141–146. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Chen, Y.; Tian, X.; Liu, L.; Wang, Y.; Guo, R.; Yan, H. Dual-inhibitor composite BTA/PPy/MIL-88(Fe) for active anticorrosion of epoxy resin coatings. J. Ind. Eng. Chem. 2023, 119, 660–673. [Google Scholar] [CrossRef]
- de Poel, W.; Pintea, S.; Drnec, J.; Carla, F.; Felici, R.; Mulder, P.; Elemans, J.A.A.W.; van Enckevort, W.J.P.; Rowan, A.E.; Vlieg, E. Muscovite mica: Flatter than a pancake. Surf. Sci. 2014, 619, 19–24. [Google Scholar] [CrossRef]
- Pan, X.-F.; Gao, H.-L.; Lu, Y.; Wu, C.-Y.; Wu, Y.-D.; Wang, X.-Y.; Pan, Z.-Q.; Dong, L.; Song, Y.-H.; Cong, H.-P.; et al. Transforming ground mica into high-performance biomimetic polymeric mica film. Nat. Commun. 2018, 9, 2974. [Google Scholar] [CrossRef]
- Sun, W.-B.; Han, Z.-M.; Yue, X.; Zhang, H.-Y.; Yang, K.-P.; Liu, Z.-X.; Li, D.-H.; Zhao, Y.-X.; Ling, Z.-C.; Yang, H.-B.; et al. Nacre-Inspired Bacterial Cellulose/Mica Nanopaper with Excellent Mechanical and Electrical Insulating Properties by Biosynthesis. Adv. Mater. 2023, 35, 2300241. [Google Scholar] [CrossRef]
- Shang, K.; Lin, G.-D.; Jiang, H.-J.; Jin, X.; Zhao, J.; Liu, D.; Zhao, B.; Yang, J.-J.; Fu, T.; Wang, J.-S. Flame retardancy, combustion, and ceramization behavior of ceramifiable flame-retardant room temperature vulcanized silicone rubber foam. Fire Mater. 2023. [Google Scholar] [CrossRef]
- Bitla, Y.; Chu, Y.-H. Development of magnetoelectric nanocomposite for soft technology. J. Phys. D-Appl. Phys. 2018, 51, 234006. [Google Scholar] [CrossRef]
- He, Y.; Dong, H.; Meng, Q.; Jiang, L.; Shao, W.; He, L.; Hu, W. Mica, a Potential Two-Dimensional-Crystal Gate Insulator for Organic Field-Effect Transistors. Adv. Mater. 2011, 23, 5502–5507. [Google Scholar] [CrossRef] [PubMed]
- Van Khai, T.; Gil Na, H.; Sub Kwak, D.; Jung Kwon, Y.; Ham, H.; Bo Shim, K.; Woo Kim, H. Synthesis and characterization of single- and few-layer mica nanosheets by the microwave-assisted solvothermal approach. Nanotechnology 2013, 24, 145602. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Yu, H. Epidermis microstructure inspired mica-based coatings for smart corrosion protection. Prog. Org. Coat. 2021, 152, 106126. [Google Scholar] [CrossRef]
- Meng, F.; Liu, L.; Tian, W.; Wu, H.; Li, Y.; Zhang, T.; Wang, F. The influence of the chemically bonded interface between fillers and binder on the failure behaviour of an epoxy coating under marine alternating hydrostatic pressure. Corros. Sci. 2015, 101, 139–154. [Google Scholar] [CrossRef]
- Li, D.; Ma, L.; Zhang, B.; Chen, S. Large-scale fabrication of a durable and self-healing super-hydrophobic coating with high thermal stability and long-term corrosion resistance. Nanoscale 2021, 13, 7810–7821. [Google Scholar] [CrossRef]
- Bai, Z.; Sun, Y.; Li, C.; Zhang, M.; Cui, Y.; Zhu, Y.; Wang, H. Novel intelligent self-responsive function fillers to enhance the durable anticorrosion performance of epoxy coating. Prog. Org. Coat. 2022, 170, 106963. [Google Scholar] [CrossRef]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Zou, X.D.; Forsberg, K. Hierarchical porous zeolitic imidazolate frameworks nanoparticles for efficient adsorption of rare-earth elements. Microporous Mesoporous Mater. 2019, 278, 175–184. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Liu, Y.; Chen, R.; Li, W. Zeolitic imidazolate framework-8 was coated with silica and investigated as a flame retardant to improve the flame retardancy and smoke suppression of epoxy resin. RSC Adv. 2018, 8, 2575–2585. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; He, L.; Liu, Y.; Liu, Y.; Chen, C.; Tang, Z.J.A.M. Core–shell upconversion nanoparticle@ metal–organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv. Mater. 2015, 27, 4075–4080. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tuninetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of ammonia-borane over Ni/ZIF-8 nanocatalyst: High efficiency, mechanism, and controlled hydrogen release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [PubMed]
- Kiey, S.A.A.; El-Shahat, M.; Abdelhameed, R.M. Role of different metal precursors based MOFs for boosting an corrosion performance of mild steel in acid media. Mater. Today Sustain. 2023, 23, 100460. [Google Scholar] [CrossRef]
- Ren, B.; Chen, Y.; Li, Y.; Li, W.; Gao, S.; Li, H.; Cao, R. Rational design of metallic anti-corrosion coatings based on zinc gluconate@ZIF-8. Chem. Eng. J. 2020, 384, 123389. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Synthesis of graphene oxide nanosheets decorated by nanoporous zeolite-imidazole (ZIF-67) based metal-organic framework with controlled-release corrosion inhibitor performance: Experimental and detailed DFT-D theoretical explorations. J. Hazard. Mater. 2021, 404, 124068. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, Z.; Yang, G.; Liao, K.; Peng, B.; Guo, Y.; Zhu, L. A hydrophobic smart coating based on hexagonal boron nitride/metal-organic frameworks for high-performance corrosion protection. Prog. Org. Coat. 2022, 172, 107154. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, M.; Xu, F.; Meng, L.; Wei, Y.; Chang, Y.; Fu, D.; Wang, H. A multiple synergistic composite coating with ultrahigh anti-corrosion performance under harsh oxygen environment. Corros. Sci. 2022, 208, 110696. [Google Scholar] [CrossRef]
- Xiao, X.; Ye, Z.; Meng, G.; Gu, L. Mussel-inspired preparation of superhydrophobic mica nanosheets for long-term anticorrosion and self-healing performance of epoxy coatings. Prog. Org. Coat. 2023, 178, 107456. [Google Scholar] [CrossRef]
- Ye, Z.; Tang, Z.; Meng, G.; Yuan, X.; Gu, L. Mussel-inspired preparation of dispersible mica nanosheets for waterborne epoxy coatings with reinforced anticorrosive performance. Prog. Org. Coat. 2023, 175, 107379. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Liu, H.Y.; Huan, S.-Y.; Li, J. Ultrathin reduced graphene oxide/MOF nanofiltration membrane with improved purification performance at low pressure. Chemosphere 2018, 204, 378–389. [Google Scholar] [CrossRef]
- Younes, H.A.; Taha, M.; Mahmoud, R.; Mahmoud, H.M.; Abdelhameed, R.M. High adsorption of sodium diclofenac on post-synthetic modified zirconium-based metal-organic frameworks: Experimental and theoretical studies. J. Colloid Interface Sci. 2022, 607, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiong, W.; Feng, X.; Cheng, G.; Shi, L.; Chen, D.; Zhang, Y. Highly recyclable cysteamine-modified acid-resistant MOFs for enhancing Hg (II) removal from water. Environ. Technol. 2020, 41, 3094–3104. [Google Scholar] [CrossRef] [PubMed]
- Adewunmi, A.A.; Ismail, S.; Sultan, A.S. Laboratory scale study on rheological behavior, morphological and structural properties of crosslinked polyacrylamide composite hydrogels embedded with date seed powder. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Hu, B.; Hu, Q.; Xu, D.; Chen, C. Macroscopic and microscopic investigation on adsorption of Sr(II) on sericite. J. Mol. Liq. 2017, 225, 563–568. [Google Scholar] [CrossRef]
- Asadi, N.; Naderi, R.; Mahdavian, M. Synergistic effect of imidazole dicarboxylic acid and Zn2+ simultaneously doped in halloysite nanotubes to improve protection of epoxy ester coating. Prog. Org. Coat. 2019, 132, 29–40. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Low cost and efficient synthesis of magnetic iron oxide/activated sericite nanocomposites for rapid removal of methylene blue and crystal violet dyes. Mater. Charact. 2020, 163, 110275. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, X.; He, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Antifouling, High-Flux Nanofiltration Membranes Enabled by Dual Functional Polydopamine. ACS Appl. Mater. Interfaces 2014, 6, 5548–5557. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Yan, S.; Li, H.; Chen, J.; Zhang, C.; Li, C.; Zhao, Y.; Fan, Y.; Guo, C. A novel silk fibroin-graphene oxide hybrid for reinforcing corrosion protection performance of waterborne epoxy coating. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 634, 127959. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Wu, Y.; Liu, G.; Dong, J.; Zhou, K. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: Synthesis and anti-corrosion applications. Appl. Surf. Sci. 2019, 479, 963–973. [Google Scholar] [CrossRef]
- Li, X.; Bandyopadhyay, P.; Thanh Tuan, N.; Park, O.-K.; Lee, J.H. Fabrication of functionalized graphene oxide/maleic anhydride grafted polypropylene composite film with excellent gas barrier and anticorrosion properties. J. Membr. Sci. 2018, 547, 80–92. [Google Scholar] [CrossRef]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective dye adsorption by highly water stable metal-organic framework: Long term stability analysis in aqueous media. Appl. Surf. Sci. 2018, 445, 424–436. [Google Scholar] [CrossRef]
- Dong, J.; Pan, W.; Luo, J.; Liu, R. Synthesis of inhibitor-loaded polyaniline microcapsules with dual anti-corrosion functions for protection of carbon steel. Electrochim. Acta 2020, 364, 137299. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pK(a) Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, F.; Yang, H.; Xu, S.; Xie, Q.; Zhang, M.; Chen, T.; Hu, G.; Wang, J. Effect of specific functional groups on oil adhesion from mica substrate: Implications for low salinity effect. J. Ind. Eng. Chem. 2017, 56, 342–349. [Google Scholar] [CrossRef]
- Yang, H.-R.; Li, S.-S.; An, Q.-D.; Zhai, S.-R.; Xiao, Z.-Y.; Zhang, L.-P. Facile transformation of carboxymethyl cellulose beads into hollow composites for dye adsorption. Int. J. Biol. Macromol. 2021, 190, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Sohrabi, M.R.; Motiee, F. Preparation and characterization of a new sawdust/MNP/PEI nanocomposite and its applications for removing Pb (II) ions from aqueous solution. Water Sci. Technol. 2018, 78, 2469–2480. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mandavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Makarewicz, E.; Michalik, A.; Shyichuk, O. Hybrid coatings of epoxy resin and polyvinylbutyral with vinyl polymers. Przem. Chem. 2012, 91, 550–554. [Google Scholar]
- Okoro, C.; Mohammed, Z.; Jeelani, S.; Rangari, V. Plasticizing effect of biodegradable dipropylene glycol bibenzoate and epoxidized linseed oil on diglycidyl ether of bisphenol A based epoxy resin. J. Appl. Polym. Sci. 2021, 138, 50661. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Zhu, R.; Sheng, X.; Xie, D.; Mei, Y. Facile modification of graphene oxide with Lysine for improving anti-corrosion performances of water-borne epoxy coatings. Prog. Org. Coat. 2019, 136, 105200. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Aragon, E.; Merlatti, C.; Pébère, N. Delaminated areas beneath organic coating: A local electrochemical impedance approach. Corros. Sci. 2006, 48, 1779–1790. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.H.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Jia, F.F.; Yang, L.; Wang, Q.M.; Song, S.X. Correlation of natural muscovite exfoliation with interlayer and solvation forces. RSC Adv. 2017, 7, 1082–1088. [Google Scholar] [CrossRef]
- Wang, L.-L.; Tong, Y.-P.; Zhang, L.-Q.; Tian, M. Comparison of Ethylene-Propylene Diene Terpolymer Composites Filled with Natural and Synthesized Micas. J. Appl. Polym. Sci. 2010, 116, 3184–3192. [Google Scholar] [CrossRef]
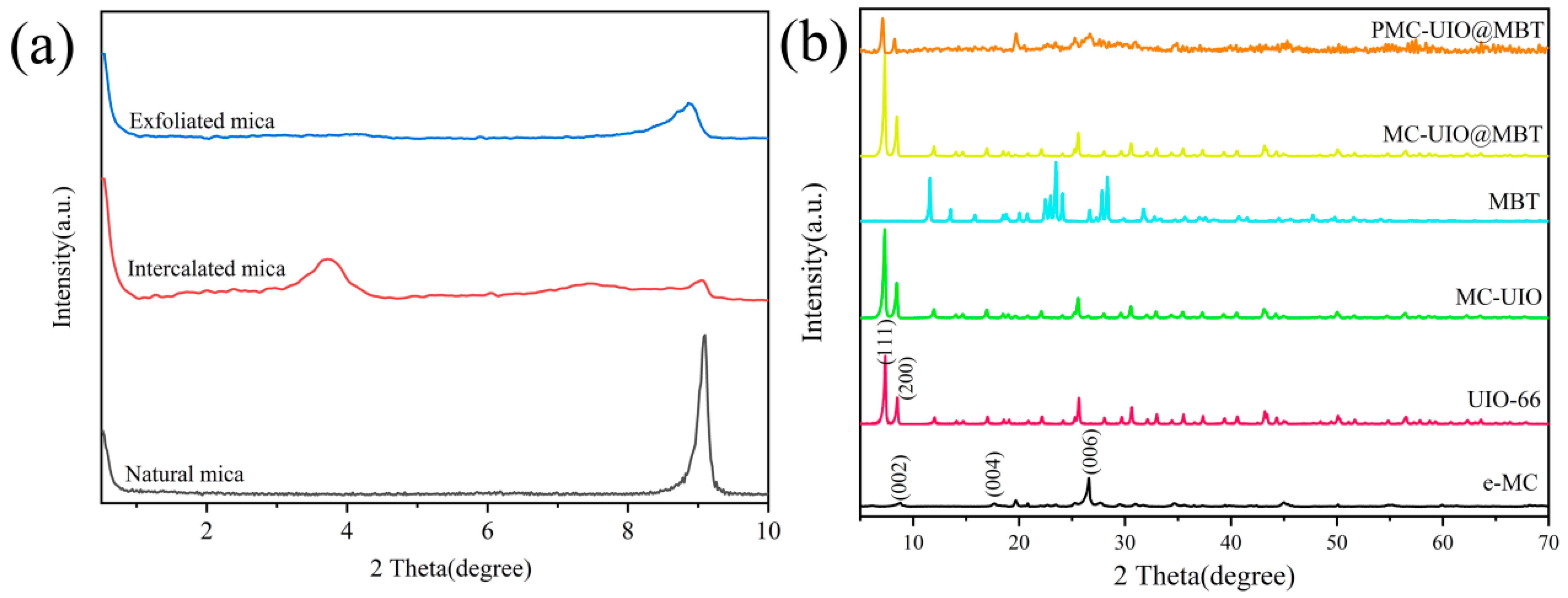



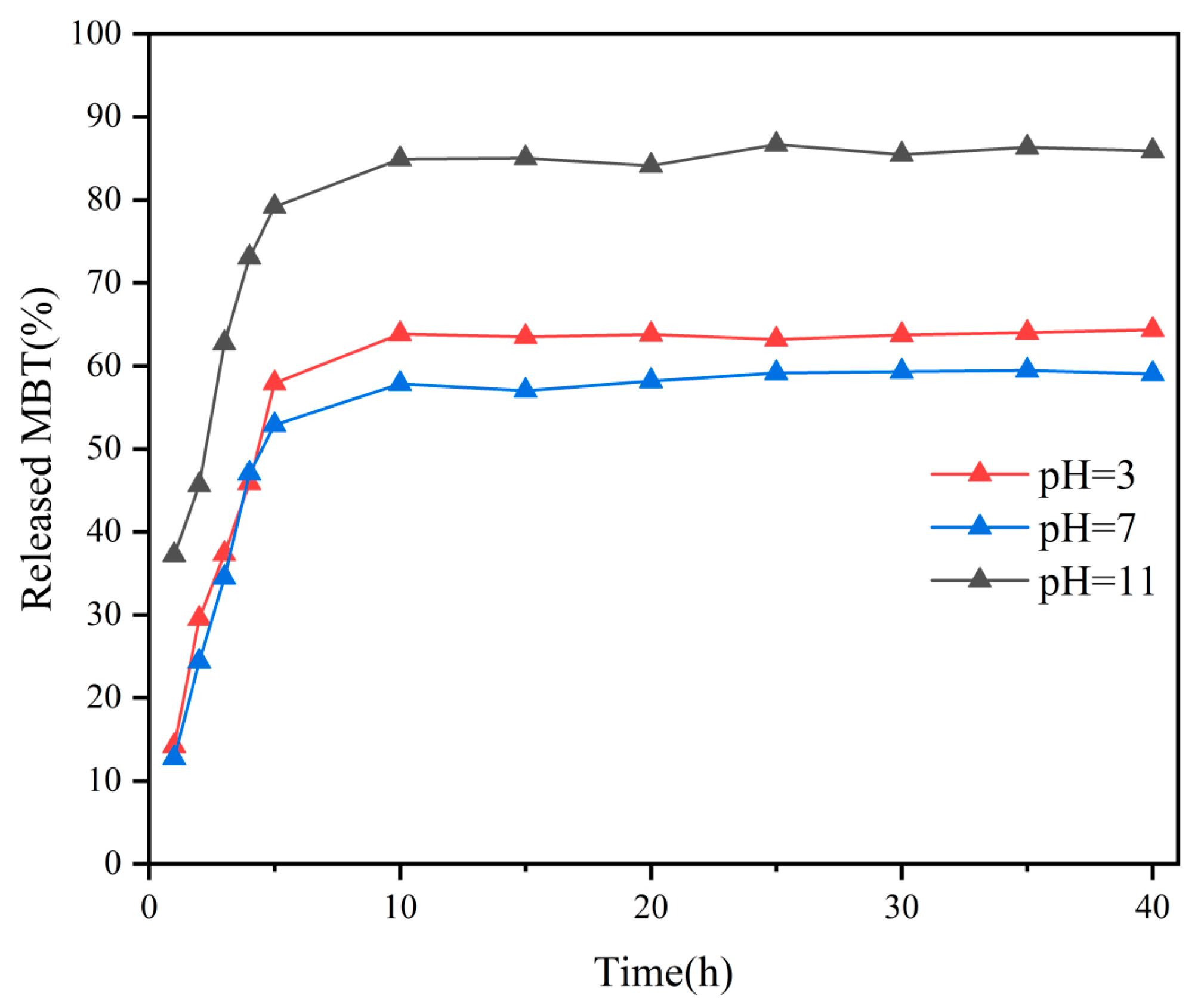


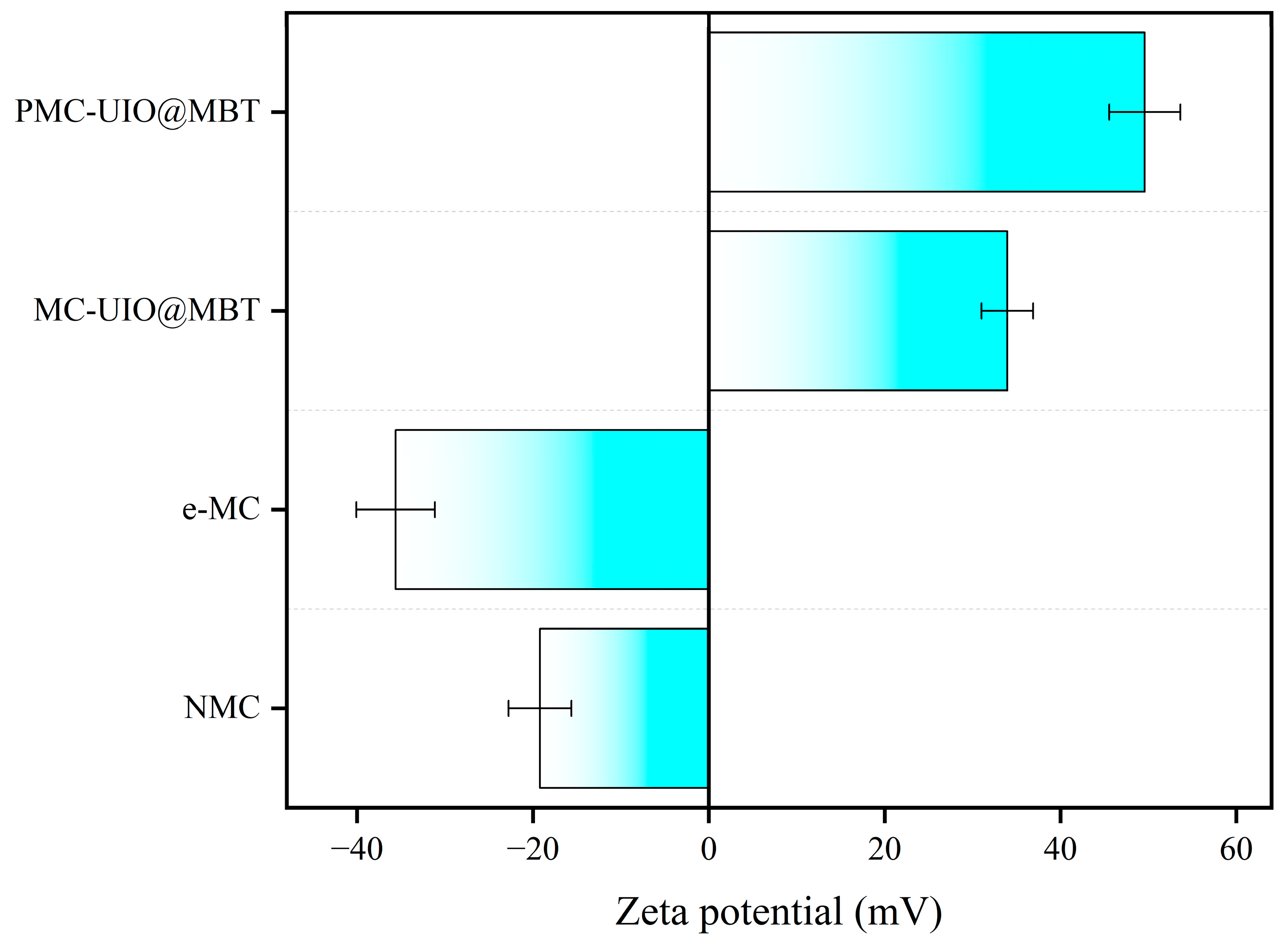
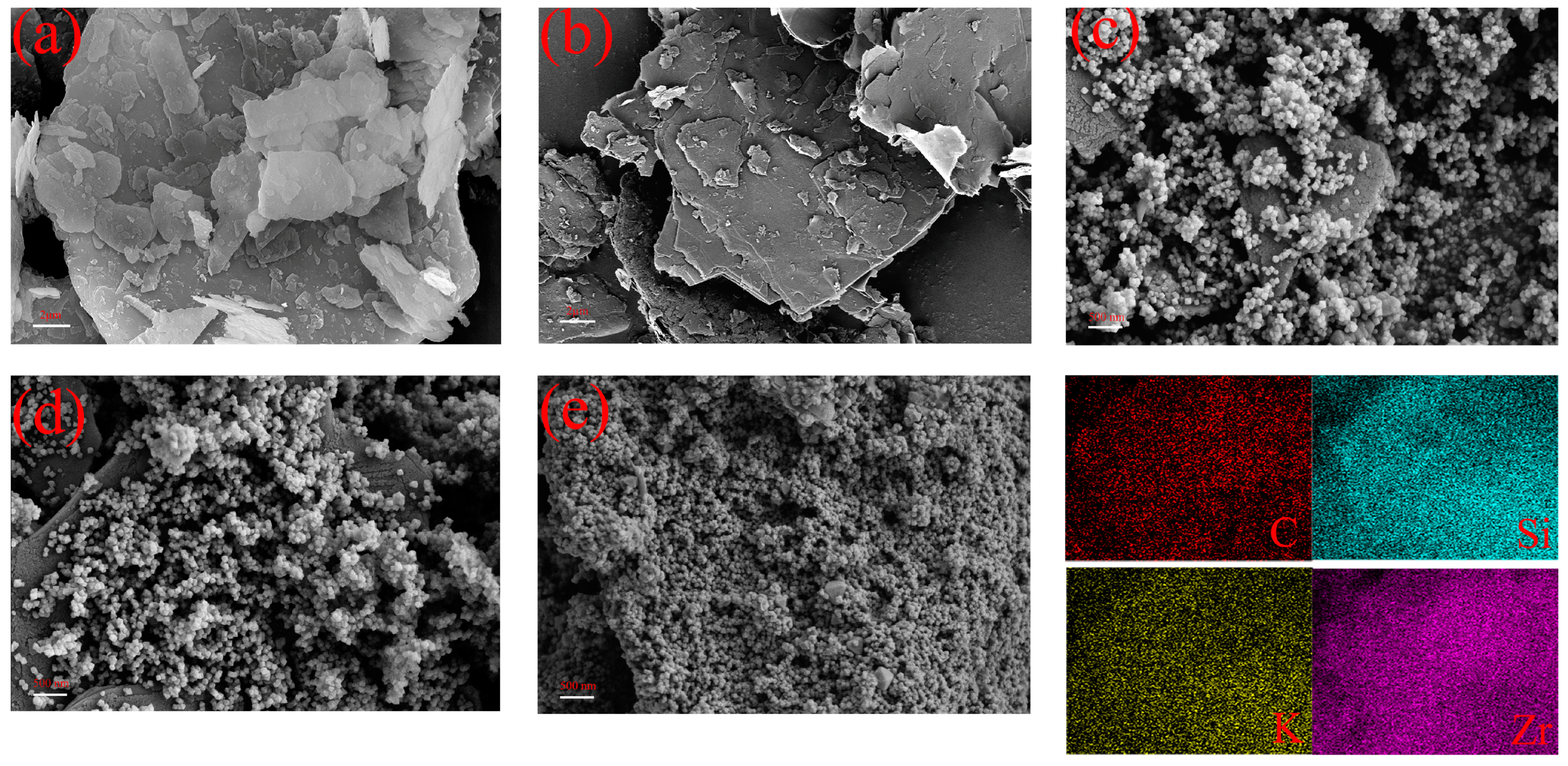



| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| UIO-66 | 727.7943 | 0.6474 | 3.56 |
| MC–UIO@MBT | 452.3264 | 0.4952 | 4.38 |
| Abbreviated Name | Implication |
|---|---|
| MC | Mica |
| NMC | Natural mica |
| e-MC | Exfoliated mica |
| MC–UIO | UIO-66 loaded on e-MC |
| MC–UIO@MBT | Loading of corrosion inhibitor MBT on MC–UIO |
| PMC–UIO@MBT | MC–UIO@MBT modified by polyethyleneimine |
| Abbreviated name/EP | The composites that corresponded were dissolved in epoxy resin to create the coatings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Feng, C.; Peng, B.; Hui, X.; Bai, X.; Yu, Z. Performance Research of Natural Mica Modified with Zirconium-Based Metal–Organic Frameworks for an Epoxy Resin Anti-Corrosion Coating. Molecules 2023, 28, 7106. https://doi.org/10.3390/molecules28207106
Zhu L, Feng C, Peng B, Hui X, Bai X, Yu Z. Performance Research of Natural Mica Modified with Zirconium-Based Metal–Organic Frameworks for an Epoxy Resin Anti-Corrosion Coating. Molecules. 2023; 28(20):7106. https://doi.org/10.3390/molecules28207106
Chicago/Turabian StyleZhu, Lijuan, Chun Feng, Bokai Peng, Xuezhi Hui, Xiaofeng Bai, and Zongxue Yu. 2023. "Performance Research of Natural Mica Modified with Zirconium-Based Metal–Organic Frameworks for an Epoxy Resin Anti-Corrosion Coating" Molecules 28, no. 20: 7106. https://doi.org/10.3390/molecules28207106
APA StyleZhu, L., Feng, C., Peng, B., Hui, X., Bai, X., & Yu, Z. (2023). Performance Research of Natural Mica Modified with Zirconium-Based Metal–Organic Frameworks for an Epoxy Resin Anti-Corrosion Coating. Molecules, 28(20), 7106. https://doi.org/10.3390/molecules28207106





