Molecularly Imprinted Polymers Using Yeast as a Supporting Substrate
Abstract
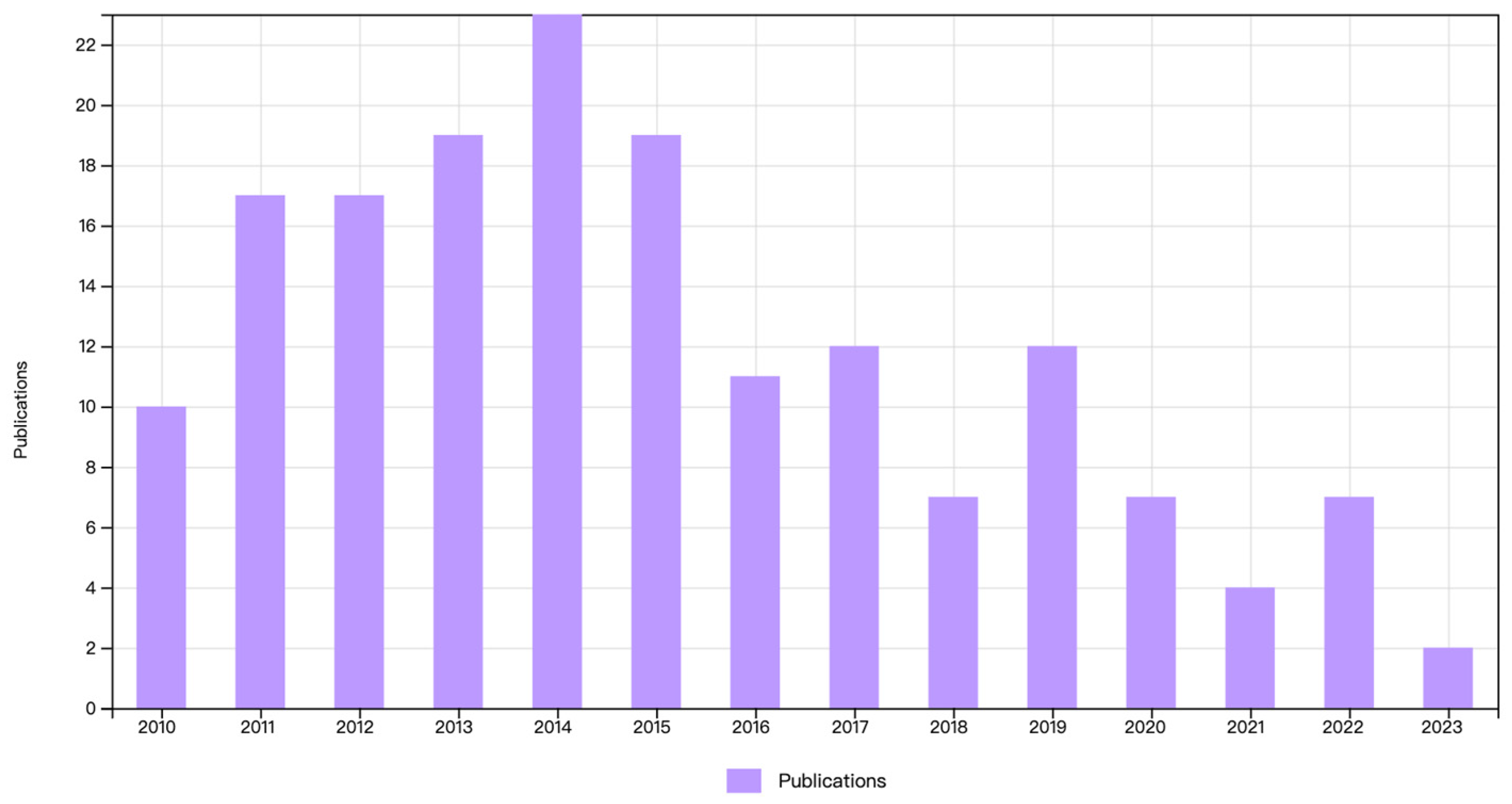
:1. Introduction
2. Research Methodology
3. Development of Yeast in Molecular Imprinting
4. Involvement of Yeast in Different Types of MIP Synthesis
4.1. Yeast’s Involvement as a Supporting Substrate in Precipitation Polymerization
4.2. Yeast’s Involvement as External and Internal Supporting Substrates in Emulsion Polymerization
4.3. Yeast’s Involvement in Surface Polymerization
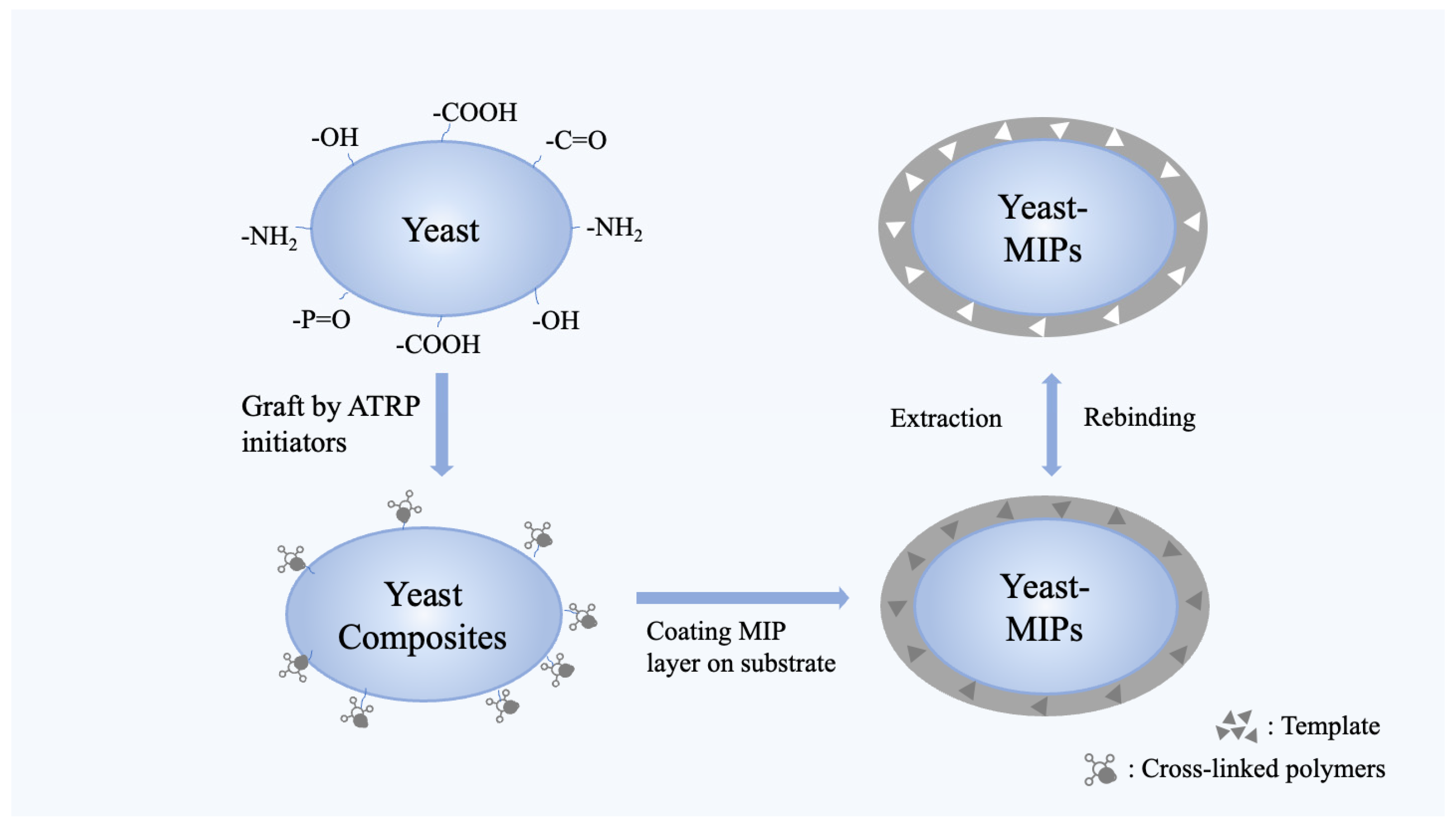
4.3.1. Yeast’s Involvement in Surface Polymerization through ATRP
4.3.2. Yeast’s Involvement in Surface Polymerization Bonded Magnetic Materials
5. Advantages and Limitations of Yeast as Supporting Substrates
- Easy availability and low cost: Yeast is easily obtainable and can be cultivated at a low cost in a short time.
- Rich surface chemical functional groups: The yeast surface contains abundant chemical functional groups, which greatly simplify modification steps and reduce secondary pollution. The presence of hydroxyl, amino, carboxyl, and other functional groups allows for interactions with template molecules, enhancing the recognition and selectivity of MIPs.
- Controllable morphology and structure: By adjusting cultivation conditions and synthesis parameters, MIPs with controllable morphology and structure can be synthesized on the yeast surface. This tunability enables the design and fabrication of MIPs with specific shapes, pore sizes, and surface properties to meet diverse application requirements.
- Excellent biocompatibility: Yeast is a natural biological material with good biocompatibility and biodegradability. Using yeast as a carrier for MIPs reduces adverse environmental impacts.
- Regulation of inorganic material growth: Yeast can regulate the growth of inorganic materials, providing a rich template for the synthesis of nanomaterials through template-assisted synthesis.
- Diverse microbial cell structures: Microbial cells, including yeast, possess various structures that can serve as templates for the synthesis of nanomaterials, offering a wide range of templates.
- Addition of magnetic materials: The inclusion of magnetic materials allows for easy separation of the material after use. Magnetic solid supports have the advantage of easy removal from the medium through simple magnetic separation and can be reused.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, H.; Tsai, W.-B.; Garrison, M.D.; Ferrari, S.; Ratner, B.D. Template-Imprinted Nanostructured Surfaces for Protein Recognition. Nature 1999, 398, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent Advances in Molecular Imprinting Technology: Current Status, Challenges and Highlighted Applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Li, Y.; Xu, J.; Hou, C.; Luo, Q.; Liu, J. Recent Development in the Design of Artificial Enzymes through Molecular Imprinting Technology. J. Mater. Chem. B 2022, 10, 6590–6606. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; He, X.; Liu, Z. Molecularly Imprinted Mesoporous Silica Nanoparticles for Specific Extraction and Efficient Identification of Amadori Compounds. Anal. Chim. Acta 2018, 1019, 65–73. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Molecularly Imprinted Polymer as Drug Delivery Carrier in Alginate Dressing. Mater. Lett. 2017, 201, 46–49. [Google Scholar] [CrossRef]
- Gui, R.; Guo, H.; Jin, H. Preparation and Applications of Electrochemical Chemosensors Based on Carbon-Nanomaterial-Modified Molecularly Imprinted Polymers. Nanoscale Adv. 2019, 1, 3325–3363. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical Molecularly Imprinted Polymer Based Sensors for Pharmaceutical and Biomedical Applications (Review). J. Pharmaceut. Biomed. 2022, 215, 114739. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J. Molecular Imprinting with Functional DNA. Small 2019, 15, e1805246. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef]
- Eersels, K.; Lieberzeit, P.; Wagner, P. A Review on Synthetic Receptors for Bioparticle Detection Created by Surface-Imprinting Techniques—From Principles to Applications. ACS Sens. 2016, 1, 1171–1187. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-N.; Kang, S.-H.; Li, J.; Lu, L.-N.; Luo, X.-P.; Wu, L. Comparison and Recent Progress of Molecular Imprinting Technology and Dummy Template Molecular Imprinting Technology. Anal. Methods 2021, 13, 4538–4556. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Su, L.; Teng, Y.; Hao, J.; Bi, Y. Fluorescent Nanomaterials Combined with Molecular Imprinting Polymer: Synthesis, Analytical Applications, and Challenges. Microchim. Acta 2020, 187, 399. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Liu, R.; Wu, M.; Li, Z.; Liu, B.; Wang, Z.; Gao, D.; Zhang, Z. Protein-Building Molecular Recognition Sites by Layer-by-Layer Molecular Imprinting on Colloidal Particles. Analyst 2009, 134, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Molecular Imprinting Polymers and Their Composites: A Promising Material for Diverse Applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef]
- Donato, L.; Nasser, I.I.; Majdoub, M.; Drioli, E. Green Chemistry and Molecularly Imprinted Membranes. Membranes 2022, 12, 472. [Google Scholar] [CrossRef]
- Schirhagl, R.; Latif, U.; Podlipna, D.; Blumenstock, H.; Dickert, F.L. Natural and Biomimetic Materials for the Detection of Insulin. Anal. Chem. 2012, 84, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Z.; Liu, Z. Paper-Based Molecular-Imprinting Technology and Its Application. Biosensors 2022, 12, 595. [Google Scholar] [CrossRef]
- Byrne, M.E.; Salian, V. Molecular Imprinting within Hydrogels II: Progress and Analysis of the Field. Int. J. Pharm. 2008, 364, 188–212. [Google Scholar] [CrossRef]
- Takeuchi, T.; Chen, G.; Haupt, K. Introduction to New Era in Advanced Functional Materials Emerging from Molecular Imprinting and Related Techniques. J. Mater. Chem. B 2022, 10, 6570. [Google Scholar] [CrossRef]
- Urriza-Arsuaga, I.; Guadaño-Sánchez, M.; Urraca, J.L. Current Trends in Molecular Imprinting: Strategies, Applications and Determination of Target Molecules in Spain. Int. J. Mol. Sci. 2023, 24, 1915. [Google Scholar] [CrossRef]
- Roy, E.; Patra, S.; Tiwari, A.; Madhuri, R.; Sharma, P.K. Introduction of Selectivity and Specificity to Graphene Using an Inimitable Combination of Molecular Imprinting and Nanotechnology. Biosens. Bioelectron. 2017, 89, 234–248. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, Y.; Dong, W.; Zhao, Y.; Sun, L. Bio-Inspired Imprinting Materials for Biomedical Applications. Adv. Sci. 2022, 9, 2202038. [Google Scholar] [CrossRef] [PubMed]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, 1800421. [Google Scholar] [CrossRef] [PubMed]
- Martin-Yken, H. Yeast-Based Biosensors: Current Applications and New Developments. Biosensors 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Vo, D.-V.N. Advances in Biosorbents for Removal of Environmental Pollutants: A Review on Pretreatment, Removal Mechanism and Future Outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef]
- Kuroda, K.; Ueda, M. Engineering of Microorganisms towards Recovery of Rare Metal Ions. Appl. Biochem. Biotechnol. 2010, 87, 53–60. [Google Scholar] [CrossRef]
- Vartiainen, S.; Yiannikouris, A.; Apajalahti, J.; Moran, C.A. Comprehensive Evaluation of the Efficiency of Yeast Cell Wall Extract to Adsorb Ochratoxin A and Mitigate Accumulation of the Toxin in Broiler Chickens. Toxins 2020, 12, 37. [Google Scholar] [CrossRef]
- Soh, E.Y.S.; Lim, S.S.; Chew, K.W.; Phuang, X.W.; Ho, V.M.V.; Chu, K.Y.H.; Wong, R.R.; Lee, L.Y.; Tiong, T.J. Valorization of Spent Brewery Yeast Biosorbent with Sonication-Assisted Adsorption for Dye Removal in Wastewater Treatment. Environ. Res. 2022, 204, 112385. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, A.; Yongabi, D.; Deschaume, O.; Bartic, C.; Wagner, P.; Wübbenhorst, M. Cell Detection by Surface Imprinted Polymers (SIPs)—A Study of the Sensor Surface by Optical and Dielectric Relaxation Spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 816–821. [Google Scholar] [CrossRef]
- Jamieson, O.; Betlem, K.; Mansouri, N.; Crapnell, R.D.; Vieira, F.S.; Hudson, A.; Banks, C.E.; Liauw, C.M.; Gruber, J.; Zubko, M.; et al. Electropolymerised Molecularly Imprinted Polymers for the Heat-Transfer Based Detection of Microorganisms: A Proof-of-Concept Study Using Yeast. Therm. Sci. Eng. Prog. 2021, 24, 100956. [Google Scholar] [CrossRef]
- Qu, J.R.; Zhang, J.J.; Gao, Y.F.; Yang, H. Synthesis and Utilisation of Molecular Imprinting Polymer for Clean-up of Propachlor in Food and Environmental Media. Food Chem. 2012, 135, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, D.; Xiao, X.; Deng, C.; Liao, L.; Deng, J.; Lin, Y.-W. Preparation and Application of a Carbon Paste Electrode Modified with Multi-Walled Carbon Nanotubes and Boron-Embedded Molecularly Imprinted Composite Membranes. Bioelectrochemistry 2018, 121, 115–124. [Google Scholar] [CrossRef]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. Nanocomposite of Bimetallic Nanodendrite and Reduced Graphene Oxide as a Novel Platform for Molecular Imprinting Technology. Anal. Chim. Acta 2016, 918, 77–88. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Dai, J.; Dai, X.; Ou, H.; Xu, L.; Li, C.; Zhang, R. Removal of Cefalexin Using Yeast Surface-Imprinted Polymer Prepared by Atom Transfer Radical Polymerization. J. Sep. Sci. 2012, 35, 2787–2795. [Google Scholar] [CrossRef]
- Ye, L. Synthetic Strategies in Molecular Imprinting. In Molecularly Imprinted Polymers in Biotechnology; Springer: Cham, Switzerland, 2015; Volume 150, pp. 1–24. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. The Role of Binding-Site Interactions in the Molecular Imprinting of Polymers. Trends Biotechnol. 1993, 11, 85–87. [Google Scholar] [CrossRef]
- Seifi, M.; Hassanpour Moghadam, M.; Hadizadeh, F.; Ali-Asgari, S.; Aboli, J.; Mohajeri, S.A. Preparation and Study of Tramadol Imprinted Micro-and Nanoparticles by Precipitation Polymerization: Microwave Irradiation and Conventional Heating Method. Int. J. Pharm. 2014, 471, 37–44. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Lu, R.; Chen, H.; Xie, X. Fast and High-Efficiency Magnetic Surface Imprinting Based on Microwave-Accelerated Reversible Addition Fragmentation Chain Transfer Polymerization for the Selective Extraction of Estrogen Residues in Milk. J. Chromatogr. A 2018, 1562, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, C.; Chu, J.; Qi, J.; Li, X. Surface Molecular Imprinting onto Fluorescein-Coated Magnetic Nanoparticles via Reversible Addition Fragmentation Chain Transfer Polymerization: A Facile Three-in-One System for Recognition and Separation of Endocrine Disrupting Chemicals. Nanoscale 2011, 3, 280–287. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Turson, M.; Lin, S.; Jiang, P.; Dong, X. Preparation of Clenbuterol Imprinted Monolithic Polymer with Hydrophilic Outer Layers by Reversible Addition–Fragmentation Chain Transfer Radical Polymerization and Its Application in the Clenbuterol Determination from Human Serum by on-Line Solid-Phase Extraction/HPLC Analysis. Analyst 2013, 138, 3066–3074. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Husson, S.M. Surface Molecular Imprinting by Atom Transfer Radical Polymerization. Biomacromolecules 2005, 6, 1113–1121. [Google Scholar] [CrossRef]
- You, X.; Piao, C.; Chen, L. Preparation of a Magnetic Molecularly Imprinted Polymer by Atom-Transfer Radical Polymerization for the Extraction of Parabens from Fruit Juices. J. Sep. Sci. 2016, 39, 2831–2838. [Google Scholar] [CrossRef]
- Ma, W.; Li, S.; Chen, L.; Sun, J.; Yan, Y. Core–Shell Thermal-Responsive and Magnetic Molecularly Imprinted Polymers Based on Mag-Yeast for Selective Adsorption and Controlled Release of Tetracycline. J. Iran. Chem. Soc. 2017, 14, 209–219. [Google Scholar] [CrossRef]
- Liang, W.; Hu, H.; Guo, P.; Ma, Y.; Li, P.; Zheng, W.; Zhang, M. Combining Pickering Emulsion Polymerization with Molecular Imprinting to Prepare Polymer Microspheres for Selective Solid-Phase Extraction of Malachite Green. Polymers 2017, 9, 344. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cárdenas, S. Advanced Polymeric Solids Containing Nano- and Micro-Particles Prepared via Emulsion-Based Polymerization Approaches. A Review. Anal. Chim. Acta 2022, 1208, 339669. [Google Scholar] [CrossRef] [PubMed]
- Rigg, A.; Champagne, P.; Cunningham, M.F. Polysaccharide-Based Nanoparticles as Pickering Emulsifiers in Emulsion Formulations and Heterogenous Polymerization Systems. Macromol. Rapid Commun. 2022, 43, 2100493. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ye, L. Interfacial Molecular Imprinting in Nanoparticle-Stabilized Emulsions. Macromolecules 2011, 44, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Xu, J.; Huang, L.; Qiu, T.; Zhong, S. Interconnectivity of Macroporous Molecularly Imprinted Polymers Fabricated by Hydroxyapatite-Stabilized Pickering High Internal Phase Emulsions-Hydrogels for the Selective Recognition of Protein. Colloids Surf. B 2017, 155, 142–149. [Google Scholar] [CrossRef]
- He, J.; Ma, Z.; Yang, Y.; Hemar, Y.; Zhao, T. Extraction of Tetracycline in Food Samples Using Biochar Microspheres Prepared by a Pickering Emulsion Method. Food Chem. 2020, 329, 127162. [Google Scholar] [CrossRef]
- Hang, H.; Li, C.; Pan, J.; Li, L.; Dai, J.; Dai, X.; Yu, P.; Feng, Y. Selective Separation of Lambdacyhalothrin by Porous/Magnetic Molecularly Imprinted Polymers Prepared by Pickering Emulsion Polymerization. J. Sep. Sci. 2013, 36, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Wongkongkatep, P.; Manopwisedjaroen, K.; Tiposoth, P.; Archakunakorn, S.; Pongtharangkul, T.; Suphantharika, M.; Honda, K.; Hamachi, I.; Wongkongkatep, J. Bacteria Interface Pickering Emulsions Stabilized by Self-Assembled Bacteria–Chitosan Network. Langmuir 2012, 28, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, W.; Li, C.; Pan, J.; Dai, X.; Gan, M.; Qu, Q.; Zhang, Y. Magnetic Molecularly Imprinted Microspheres via Yeast Stabilized Pickering Emulsion Polymerization for Selective Recognition of λ-Cyhalothrin. Colloids Surf. A 2014, 453, 27–36. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Meng, M.; Song, Z.; Pan, J.; Yan, Y.; Li, C. Surface Molecularly Imprinted Polymers Based on Yeast Prepared by Atom Transfer Radical Emulsion Polymerization for Selective Recognition of Ciprofloxacin from Aqueous Medium. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Wang, R.; Zhang, P.; Zhang, Y.; Randell, E.; Zhang, M.; Jia, Q. A Review: Development and Application of Surface Molecularly Imprinted Polymers toward Amino Acids, Peptides, and Proteins. Anal. Chim. Acta 2022, 1234, 340319. [Google Scholar] [CrossRef]
- Li, X. Selective Removal and Persulfate Catalytic Decomposition of Diethyl Phthalate from Contaminated Water on Modified MIL100 through Surface Molecular Imprinting. Chemosphere 2020, 240, 124875. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, H.; Suo, Z.; Li, F.; Jin, Q.; Zhu, X.; Chen, Q. A Molecularly Imprinted Electrochemical Sensor Based on Surface Imprinted Polymerization and Boric Acid Affinity for Selective and Sensitive Detection of P-Glycoproteins. Anal. Chim. Acta 2022, 1207, 339797. [Google Scholar] [CrossRef]
- Pan, J.; Hang, H.; Li, X.; Zhu, W.; Meng, M.; Dai, X.; Dai, J.; Yan, Y. Fabrication and Evaluation of Temperature Responsive Molecularly Imprinted Sorbents Based on Surface of Yeast via Surface-Initiated AGET ATRP. Appl. Surf. Sci. 2013, 287, 211–217. [Google Scholar] [CrossRef]
- Guan, W.; Lei, J.; Wang, X.; Zhou, Y.; Lu, C.; Sun, S. Selective Recognition of Beta-Cypermethrin by Molecularly Imprinted Polymers Based on Magnetite Yeast Composites. J. Appl. Polym. Sci. 2013, 129, 1952–1958. [Google Scholar] [CrossRef]
- Qiu, L.; Jaria, G.; Gil, M.V.; Feng, J.; Dai, Y.; Esteves, V.I.; Otero, M.; Calisto, V. Core-Shell Molecularly Imprinted Polymers on Magnetic Yeast for the Removal of Sulfamethoxazole from Water. Polymers 2020, 12, 1385. [Google Scholar] [CrossRef] [PubMed]
- Urraca, J.L.; Chamorro-Mendiluce, R.; Orellana, G.; Moreno-Bondi, M.C. Molecularly Imprinted Polymer Beads for Clean-up and Preconcentration of β-Lactamase-Resistant Penicillins in Milk. Anal. Bioanal. Chem. 2016, 408, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Hammad, S.F.; Abdella, A.A.; Mansour, F.R. Chitosan-Based Molecular Imprinted Polymer for Extraction and Spectrophotometric Determination of Ketorolac in Human Plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118668. [Google Scholar] [CrossRef]
- Bagheri, N.; Habibi, B.; Khataee, A.; Hassanzadeh, J. Application of Surface Molecular Imprinted Magnetic Graphene Oxide and High Performance Mimetic Behavior of Bi-Metal ZnCo MOF for Determination of Atropine in Human Serum. Talanta 2019, 201, 286–294. [Google Scholar] [CrossRef] [PubMed]


| Target Molecule | Yeast Type | Type of Polymerization | Polymerization Technique | Imprinting System | Thickness * | Applications | Ref. * |
|---|---|---|---|---|---|---|---|
| Cefalexin | Yeast powder | Surface imprinting technique | ATRP * | CuCl; MAA *; EGDMA * | 0.63 μm | Selective recognition and adsorption of cefalexin | [37] |
| Beta-cypermethrin | Yeast powder | Surface imprinting technique | None | MAA; EGDMA; M@Y * | 1.0–1.1 μm | Selective recognition and separation of beta-cypermethrin from wastewater samples | [38] |
| Cefalexin | Yeast powder | Surface imprinting technique | AGET ATRP * | Aam *; EGDMA; CuCl2; AsAc * | None | Selective recognition and adsorption of cefalexin | [39] |
| Ciprofloxacin | Yeast powder | Surface imprinting technique | ATRP | MAA; HEMA *; EGDMA; CuBr | 0.365 μm | Selective recognition and removal of Ciprofloxacin from aqueous media | [40] |
| λ-cyhalothrin | Yeast powder | Pickering emulsions | Thermally initiated radical polymerization | EGDMA; MAA; AIBME * | None | Selective recognition and separation of λ-cyhalothrin | [41] |
| Tetracycline antibiotics | Yeast powder | Precipitation polymerization | None | MAA; EGDMA; AIBN * | None | Selective adsorption and release of tetracycline from aqueous solution | [42] |
| Sulfamethoxazole | Yeast cells | Surface imprinting technique | One-step in situ polymerization | AIBN; EGDMA | None | Selective removal of sulfamethoxazole from water | [43] |
| Supporting Substrate | Target Molecule | Application | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Silica beads | β-lactamase-resistant penicillins | Determination of beta-lactamase-resistant penicillin residues in complex matrices including milk | The detection limit is much lower than the standard, and the specific recognition effect is good | The preparation process is complex, and the silica is treated in multiple steps | [65] |
| Chitosan | ketorolac | Determination of ketorolac in human plasma | Non-toxicity, bioavailability, and biocompatibility | — | [66] |
| Graphene | atropine | Determination of atropine in human serum | Highly selective and sensitive analytical assay for atropine | Interaction between the adjacent graphene sheets or the interaction between the graphene sheets need to be strengthened | [67] |
| Yeast | cefalexin | Selective recognition and adsorption of cefalexin | Low cost, easily available source, and abundant active biomolecule on the cell wall without further modification process | — | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Dong, Z.; Shen, X.; Wu, B. Molecularly Imprinted Polymers Using Yeast as a Supporting Substrate. Molecules 2023, 28, 7103. https://doi.org/10.3390/molecules28207103
Wang Z, Dong Z, Shen X, Wu B. Molecularly Imprinted Polymers Using Yeast as a Supporting Substrate. Molecules. 2023; 28(20):7103. https://doi.org/10.3390/molecules28207103
Chicago/Turabian StyleWang, Zhigang, Zhuangzhuang Dong, Xiantao Shen, and Bin Wu. 2023. "Molecularly Imprinted Polymers Using Yeast as a Supporting Substrate" Molecules 28, no. 20: 7103. https://doi.org/10.3390/molecules28207103
APA StyleWang, Z., Dong, Z., Shen, X., & Wu, B. (2023). Molecularly Imprinted Polymers Using Yeast as a Supporting Substrate. Molecules, 28(20), 7103. https://doi.org/10.3390/molecules28207103





