Hemolysis by Saponin Is Accelerated at Hypertonic Conditions
Abstract
1. Introduction
2. Results and Discussion
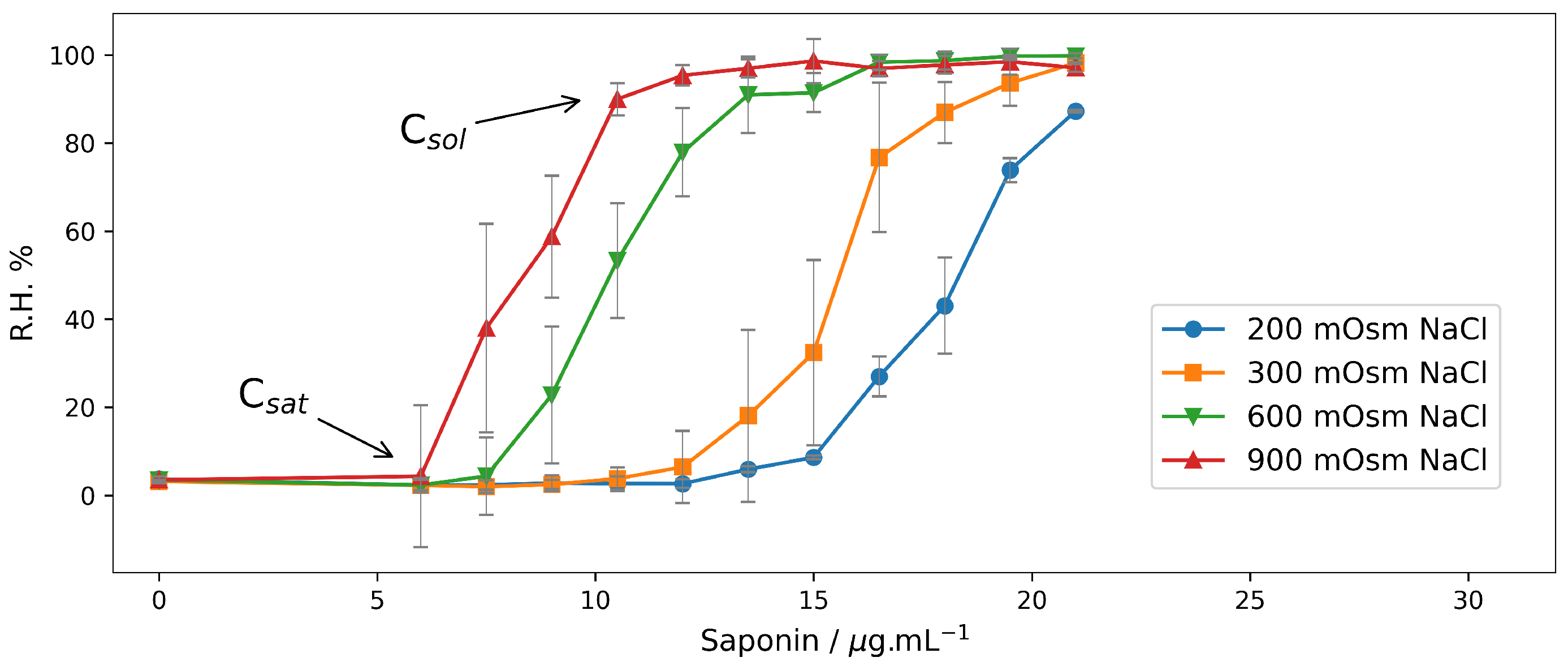
2.1. Hemolysis in NaCl Solutions at Different Osmotic Pressures
2.1.1. End-Point Assays in NaCl Solutions
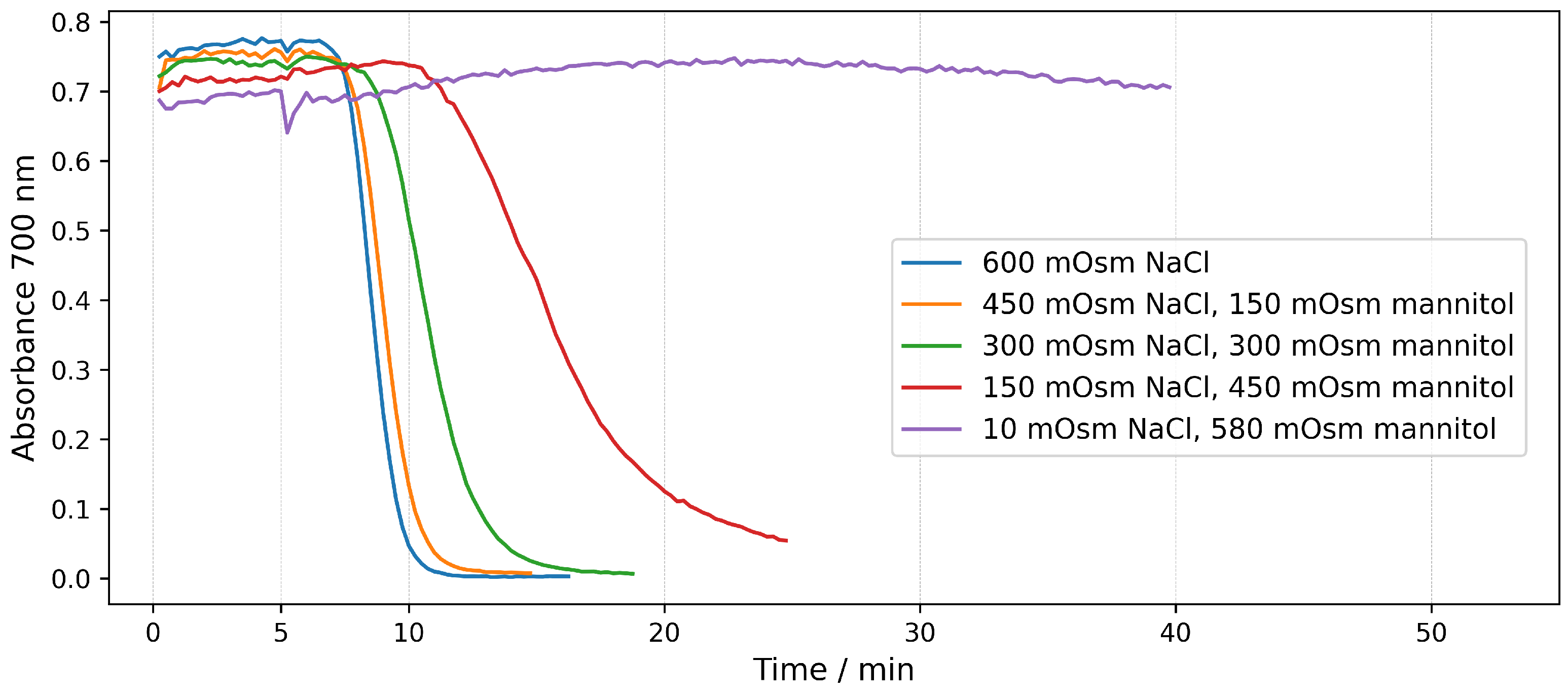
2.1.2. Real-Tme Assays in NaCl Solutions
2.2. Hemolysis in Mannitol/NaCl Solutions at Different Osmotic Pressures
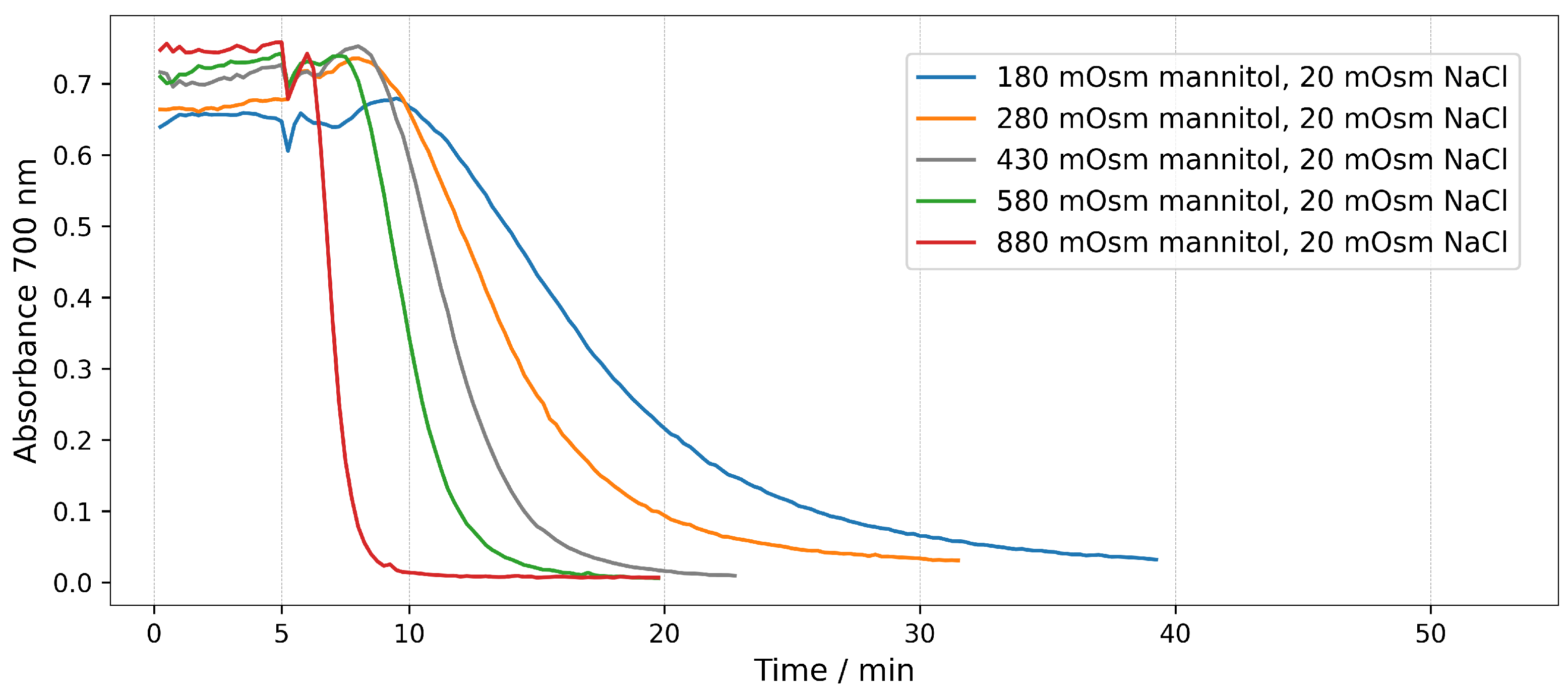
2.2.1. End-Point Assays in Mannitol/NaCl Solutions
2.2.2. Real-Time Assays in Mannitol/NaCl Solutions
3. Materials and Methods
3.1. Materials
3.2. Isolation of Erythrocytes
3.3. Assays of Hemolysis Caused by Saponin
3.3.1. End-Point Hemolysis Assays
3.3.2. Real-Time Hemolysis Assays
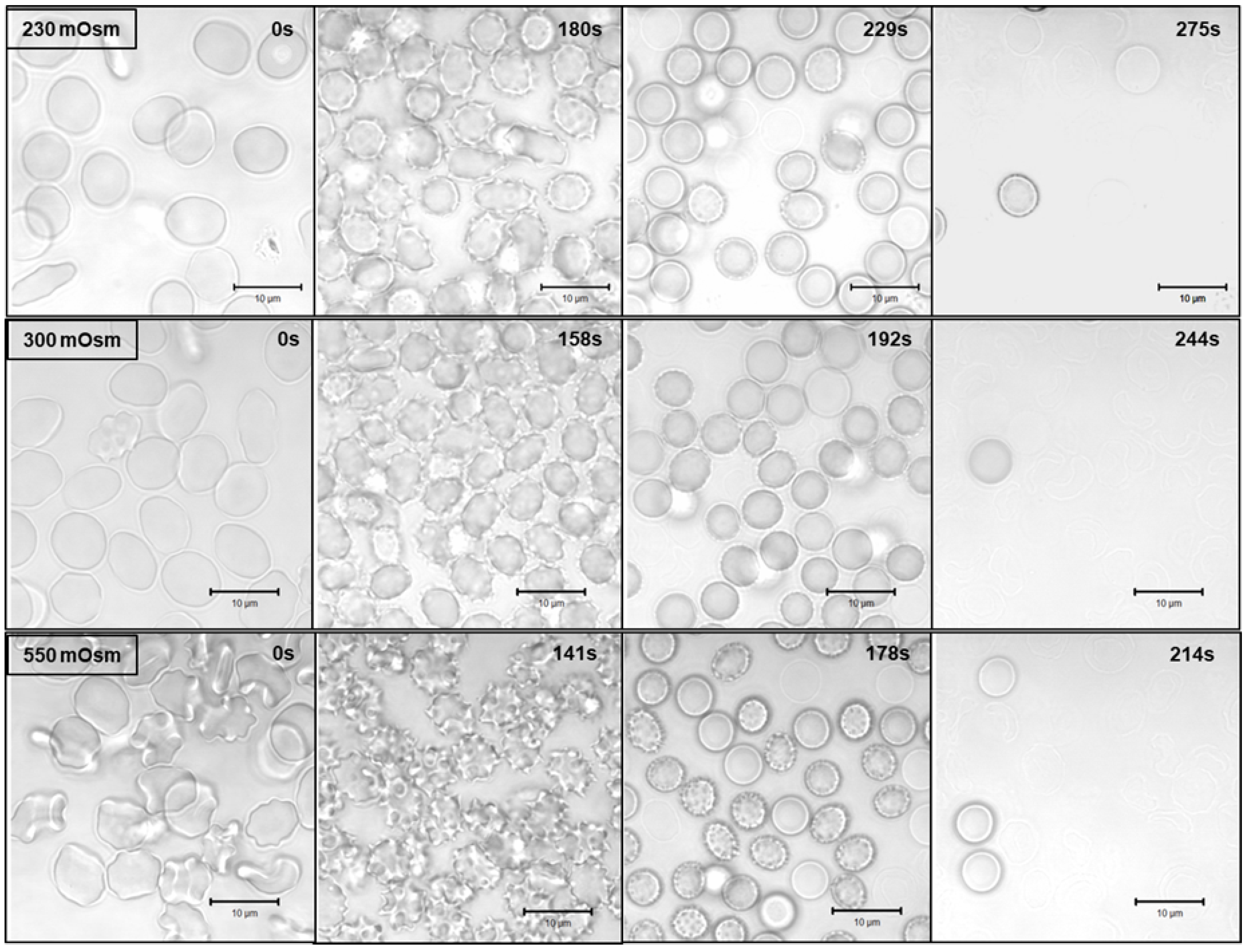
3.4. Confocal Laser Scanning Microscopy (CLSM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RBCs | Red Blood Cells |
| Hct | Hematocrit |
| R.H. | Relative hemolysis |
References
- Yang, Y.; Laval, S.; Yu, B. Chapter Two—Chemical Synthesis of Saponins. Adv. Carbohydr. Chem. Biochem. 2021, 79, 63–150. [Google Scholar] [CrossRef] [PubMed]
- NGUYEN, L.T.; Fărcaş, A.; Socaci, S.A.; Tofana, M.; Farkas, A.C.; Socaci, S.A.; Tofana, M.; Diaconeasa, Z.M.; Pop, O.L.; Salanta, L.C. An Overview of Saponins-A Bioactive Group. Bull. UASVM Food Sci. Technol. 2020, 77, 25–36. [Google Scholar] [CrossRef]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.L.; Morita, T.; Kasai, R.; Zhou, J.; Wu, C.Y.; Tanaka, O. Saponins from Chinese medicinal plants. (I). Isolation and structures of hemslosides. Planta Medica 1984, 50, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kasai, R.; Oinaka, T.; Tanaka, O.; Yang, C.R.; Zhou, J. Saponins from Chinese Folk Medicine, “Liang Wang Cha, ” Leaves and Stems of Nothopanax delavayi, Araliaceae. Chem. Pharm. Bull. 1987, 35, 1486–1490. [Google Scholar] [CrossRef]
- Navarro, P.; Giner, R.M.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Ríos, J.L.L.; Máez, S.; Cerdá-Nicolás, M.; Ríos, J.L.L. In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium. Life Sci. 2001, 68, 1199–1206. [Google Scholar] [CrossRef]
- Li, D.W.; Lee, E.B.; Kang, S.S.; Hyun, J.E.; Whang, W.K. Activity-Guided Isolation of Saponins from Kalopanax pictus with Anti-inflammatory Activity. Chem. Pharm. Bull. 2002, 50, 900–903. [Google Scholar] [CrossRef]
- Da Silva, B.P.; De Sousa, A.C.; Silva, G.M.; Mendes, T.P.; Parente, J.P. A new bioactive steroidal saponin from Agave attenuata. Z. Fur Naturforschung Sect. J. Biosci. 2002, 57, 423–428. [Google Scholar] [CrossRef]
- Miyakoshi, M.; Tamura, Y.; Masuda, H.; Mizutani, K.; Tanaka, O.; Ikeda, T.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antiyeast Steroidal Saponins from Yucca schidigera (Mohave Yucca), a New Anti-Food-Deteriorating Agent. J. Nat. Prod. 2000, 63, 332–338. [Google Scholar] [CrossRef]
- Iorizzi, M.; Lanzotti, V.; Ranalli, G.; De Marino, S.; Zollo, F. Antimicrobial Furostanol Saponins from the Seeds of Capsicum annuum L. Var. acuminatum§. J. Agric. Food Chem. 2002, 50, 4310–4316. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, A.R.; Vattuone, M.A. Screening antifungal activities of selected medicinal plants. J. Ethnopharmacol. 2001, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F.; Madigan, C.A.; Connolly, C.R.; Walsh, G.A.; Clark, C.; Hynes, M.J.; Timmins, B.F.; James, P.; Headon, D.R.; Power, R.F. Antimicrobial Saponins of Yucca schidigera and the Implications of Their in Vitro Properties for Their in Vivo Impact. J. Agric. Food Chem. 1998, 46, 3178–3186. [Google Scholar] [CrossRef]
- Traore, F.; Faure, R.; Ollivier, E.; Gasquet, M.; Azas, N.; Debrauwer, L.; Keita, A.; Timon-David, P.; Balansard, G. Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Medica 2000, 66, 368–371. [Google Scholar] [CrossRef]
- Delmas, F.; Di Giorgio, C.; Elias, R.; Gasquet, M.; Azas, N.; Mshvildadze, V.; Dekanosidze, G.; Kemertelidze, E.; Timon-David, P. Antileishmanial activity of three saponins isolated from ivy, α- hederin, β-hederin and hederacolchiside A1, as compared to their action on mammalian cells cultured in vitro. Planta Medica 2000, 66, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Sung, M.K. Saponins as Anticarcinogens. J. Nutr. 1995, 125, 717S–724S. [Google Scholar] [CrossRef] [PubMed]
- Le Tran, Q.; Tezuka, Y.; Banskota, A.H.; Tran, Q.K.; Saiki, I.; Kadota, S. New Spirostanol Steroids and Steroidal Saponins from Roots and Rhizomes of Dracaena angustifolia and Their Antiproliferative Activity. J. Nat. Prod. 2001, 64, 1127–1132. [Google Scholar] [CrossRef]
- Lee, S.J.S.J.J.J.; Sung, J.H.H.; Lee, S.J.S.J.J.J.; Moon, C.K.K.; Lee, B.H.H. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999, 144, 39–43. [Google Scholar] [CrossRef]
- Apers, S.; Baronikova, S.; Sindambiwe, J.B.; Witvrouw, M.; De Clercq, E.; Vanden Berghe, D.; Van Marck, E.; Vlietinck, A.; Pieters, L. Antiviral, haemolytic and molluscicidal activities of triterpenoid saponins from Maesa lanceolata: Establishment of structure-activity relationships. Planta Medica 2001, 67, 528–532. [Google Scholar] [CrossRef]
- Gosse, B.; Gnabre, J.; Bates, R.B.; Dicus, C.W.; Nakkiew, P.; Huang, R.C.C. Antiviral Saponins from Tieghemella heckelii. J. Nat. Prod. 2002, 65, 1942–1944. [Google Scholar] [CrossRef]
- Kinjo, J.; Yokomizo, K.; Hirakawa, T.; Shii, Y.; Nohara, T.; Uyeda, M. Anti-herpes Virus Activity of Fabaceous Triterpenoidal Saponins. Biol. Pharm. Bull. 2000, 23, 887–889. [Google Scholar] [CrossRef][Green Version]
- Huong, N.T.T.; Matsumoto, K.; Kasai, R.; Yamasaki, K.; Watanabe, H. In Vitro Antioxidant Activity of Vietnamese Ginseng Saponin and Its Components. Biol. Pharm. Bull. 1998, 21, 978–981. [Google Scholar] [CrossRef]
- Oakenfull, D. Saponins in food—A review. Food Chem. 1981, 7, 19–40. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef]
- Sato, Y.; Yamakose, H.; Suzuki, Y. Participation of Band 3 Protein in Hypotonic Hemolysis of Human Erythrocytes. Biol. Pharm. Bull. 1993, 16, 188–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shalel, S.; Streichman, S.; Marmur, A. The Mechanism of Hemolysis by Surfactants: Effect of Solution Composition. J. Colloid Interface Sci. 2002, 252, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Söderlind, E.; Karlsson, L. Haemolytic activity of maltopyranoside surfactants. Eur. J. Pharm. Biopharm. 2006, 62, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by surfactants—A review. Adv. Colloid Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Action of Saponin on Biological Cell Membranes. Nature 1962, 196, 952–953. [Google Scholar] [CrossRef]
- Glauert, A.M.; Dingle, J.T.; Lucy, J.A. Action of Saponin on Biological Cell Membranes. Nature 1962, 196, 953–955. [Google Scholar] [CrossRef]
- Shany, S.; Bernheimer, A.W.; Grushoff, P.S.; Kim, K.S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol. Cell. Biochem. 1974, 3, 179–186. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, N.; Makky, A.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic investigation of interactions between steroidal saponin digitonin and cell membrane models. J. Phys. Chem. 2014, 118, 14632–14639. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Cheng, D.; Iles, G.H. Structure of membrane holes in osmotic and saponin hemolysis. J. Cell Biol. 1973, 56, 519–527. [Google Scholar] [CrossRef]
- Baumann, E.; Stoya, G.; Völkner, A.; Richter, W.; Lemke, C.; Linss, W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000, 102, 21–35. [Google Scholar] [CrossRef]
- Abe, H.; Sakaguchi, M.; Anno, M.; Arichi, S. Erythrocyte membrane stabilization by plant saponins and sapogenins. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1981, 316, 262–265. [Google Scholar] [CrossRef]
- Ribeiro, B.; Lacchini, E.; Bicalho, K.U.; Mertens, J.; Arendt, P.; Bossche, R.V.; Calegario, G.; Gryffroy, L.; Ceulemans, E.; Buitink, J.; et al. A Seed-Specific Regulator of Triterpene Saponin Biosynthesis in Medicago truncatula. Plant Cell 2020, 32, 2020–2042. [Google Scholar] [CrossRef]
- Ondevilla, J.C.; Hanashima, S.; Mukogawa, A.; Miyazato, D.G.; Umegawa, Y.; Murata, M. Effect of the number of sugar units on the interaction between diosgenyl saponin and membrane lipids. Biochim. Biophys. Acta (BBA)—Biomembr. 2023, 1865, 184145. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, H.; Wang, M.; Han, L.; Liu, Y.; Zhu, Y.; Yang, S. Synthesis, cytotoxicity and haemolytic activity of Pulsatilla saponin A, D derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 2550–2554. [Google Scholar] [CrossRef]
- Lin, F.; Wang, R. Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. J. Mol. Model. 2010, 16, 107–118. [Google Scholar] [CrossRef]
- Garza, D.L.; Hanashima, S.; Umegawa, Y.; Murata, M.; Kinoshita, M.; Matsumori, N.; Greimel, P. Behavior of Triterpenoid Saponin Ginsenoside Rh2 in Ordered and Disordered Phases in Model Membranes Consisting of Sphingomyelin, Phosphatidylcholine, and Cholesterol. Langmuir 2022, 38, 10478–10491. [Google Scholar] [CrossRef] [PubMed]
- Orczyk, M.; Wojciechowski, K.; Brezesinski, G. The influence of steroidal and triterpenoid saponins on monolayer models of the outer leaflets of human erythrocytes, E. coli and S. cerevisiae cell membranes. J. Colloid Interface Sci. 2020, 563, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Yokosuka, A.; Kuroda, M.; Sashida, Y. Cytotoxic Activities and Structure-Cytotoxic Relationships of Steroidal Saponins. Biol. Pharm. Bull. 2001, 24, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Rondon-Berrios, H.; Argyropoulos, C.; Ing, T.S.; Raj, D.S.; Malhotra, D.; Agaba, E.I.; Rohrscheib, M.; Khitan, Z.J.; Murata, G.H.; Shapiro, J.I.; et al. Hypertonicity: Clinical entities, manifestations and treatment. World J. Nephrol. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim. Biophys. Acta (BBA)—Biomembr. 1985, 821, 470–478. [Google Scholar] [CrossRef]
- Malheiros, S.V.P.; Meirelles, N.C.; De Paula, E. Pathways involved in trifluoperazine-, dibucaine- and praziquantel-induced hemolysis. Biophys. Chem. 2000, 83, 89–100. [Google Scholar] [CrossRef]
- Winter, W. Mechanism of Saponin-Induced Red-Cell Hemolysis-Evidence for The Involvement of Aquaporin CHIP28. In Blood; W.B. SAUNDERS COMPANY: Philadelphia, PA, USA, 1994; Volume 84, p. A115. [Google Scholar]
- Huang, Y.X.; Wu, Z.J.; Huang, B.T.; Luo, M. Pathway and Mechanism of pH Dependent Human Hemoglobin Tetramer-Dimer-Monomer Dissociations. PLoS ONE 2013, 8, e81708. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochemistry Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Snyder, K.A. Calculation of ionic diffusion coefficients on the basis of migration test results. Mater. Struct. 2003, 36, 156–165. [Google Scholar] [CrossRef]
- Grigera, J.R. Conformation of polyols in water. Molecular-dynamics simulation of mannitol and sorbitol. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1988, 84, 2603–2608. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; de Castro, W.V.; Castro, A.H.F.; Duarte-Almeida, J.M. Validated spectrophotometric method for quantification of total triterpenes in plant matrices. DARU J. Pharm. Sci. 2020, 28, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Iyekowa, O.; Momo, D.O.; Agbonifo, E.; Ngbodin, J.; Akinyemi, K. Bioactive Chemical Constituents Screening and Inhibitory Activities of Methanol Extract of Oecophylla Longinoda (Tailor Ant) Against Some Pathogens. Nipes J. Sci. Technol. Res. 2022, 4, 80–86. [Google Scholar]


| NaCl, mOsm | , g/mL | , g/mL |
|---|---|---|
| 200 | 12 | >21 |
| 300 | 10.5 | 21 |
| 600 | 7.5 | 15 |
| 900 | 6 | 12 |
| Osmotic Pressure | NaCl | Manitol | Saponin |
|---|---|---|---|
| mOsm | mM | mM | g/mL |
| Hemolysis in NaCl solutions | |||
| 200 | 100 | - | |
| 300 | 150 | - | 0 ÷ 21 |
| 600 | 300 | - | Step 1.5 |
| 900 | 450 | - | |
| Hemolysis in manitol at low ionic strength | |||
| 200 | 10 | 180 | |
| 300 | 10 | 280 | 0 ÷ 45 |
| 600 | 10 | 580 | Step 3.0 |
| 900 | 10 | 880 | |
| Osmotic Pressure | NaCl | Manitol | Saponin |
|---|---|---|---|
| mOsm | mM | mM | g/mL |
| Hemolysis in NaCl solutions | |||
| 200 | 100 | - | 15 |
| 300 | 150 | - | |
| 450 | 225 | - | |
| 600 | 300 | - | |
| 900 | 450 | - | |
| Hemolysis in manitol at low ionic strength | |||
| 200 | 10 | 180 | 30 |
| 300 | 10 | 280 | |
| 450 | 10 | 430 | |
| 600 | 10 | 580 | |
| 900 | 10 | 880 | |
| Hemolysis in NaCl/manitol solutions | |||
| 600 | 300 | 0 | 15 |
| 600 | 225 | 150 | |
| 600 | 150 | 300 | |
| 600 | 75 | 450 | |
| 600 | 10 | 580 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paarvanova, B.; Tacheva, B.; Savova, G.; Karabaliev, M.; Georgieva, R. Hemolysis by Saponin Is Accelerated at Hypertonic Conditions. Molecules 2023, 28, 7096. https://doi.org/10.3390/molecules28207096
Paarvanova B, Tacheva B, Savova G, Karabaliev M, Georgieva R. Hemolysis by Saponin Is Accelerated at Hypertonic Conditions. Molecules. 2023; 28(20):7096. https://doi.org/10.3390/molecules28207096
Chicago/Turabian StylePaarvanova, Boyana, Bilyana Tacheva, Gergana Savova, Miroslav Karabaliev, and Radostina Georgieva. 2023. "Hemolysis by Saponin Is Accelerated at Hypertonic Conditions" Molecules 28, no. 20: 7096. https://doi.org/10.3390/molecules28207096
APA StylePaarvanova, B., Tacheva, B., Savova, G., Karabaliev, M., & Georgieva, R. (2023). Hemolysis by Saponin Is Accelerated at Hypertonic Conditions. Molecules, 28(20), 7096. https://doi.org/10.3390/molecules28207096






