In Vitro Permeability Study of Homotaurine Using a High-Performance Liquid Chromatography with Fluorescence Detection Pre-Column Derivatization Method
Abstract
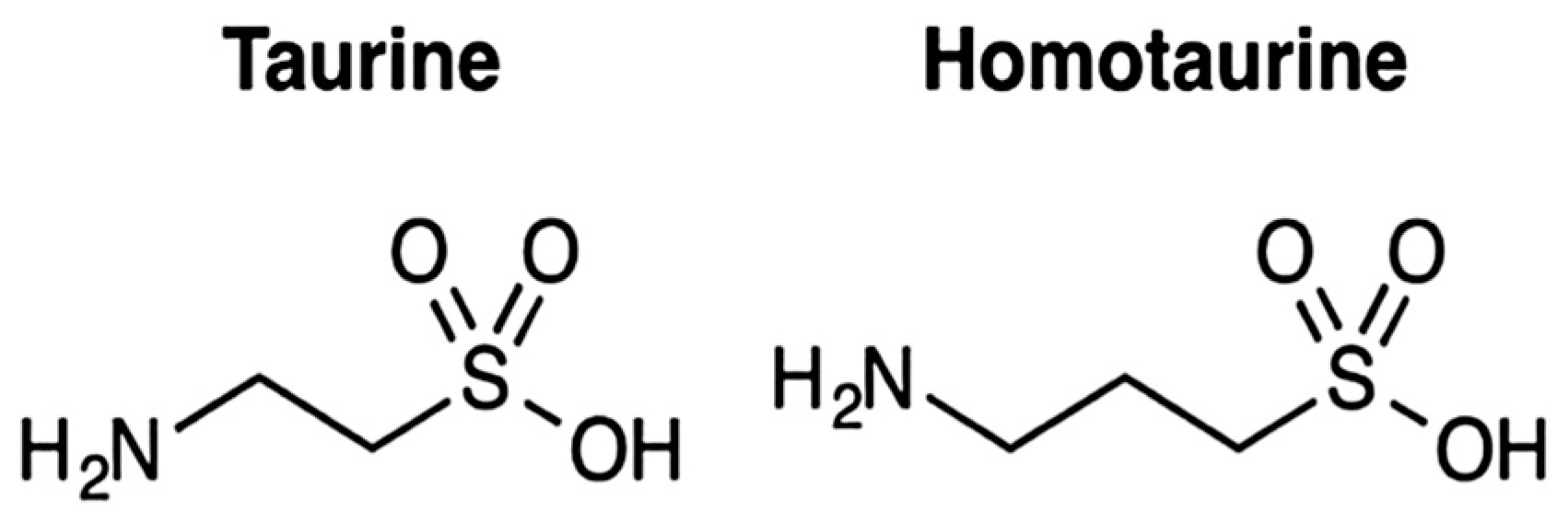
1. Introduction
2. Results and Discussion
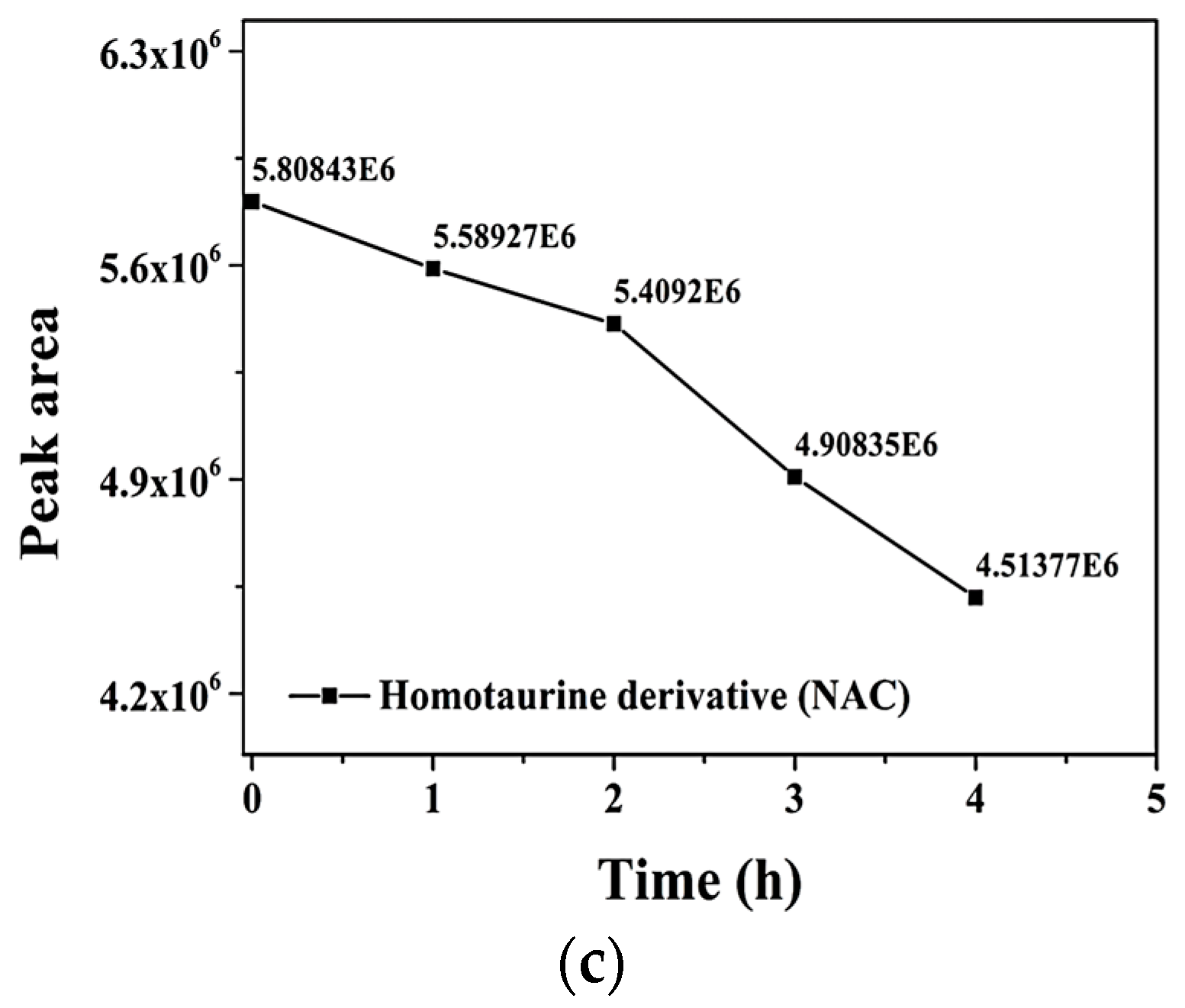
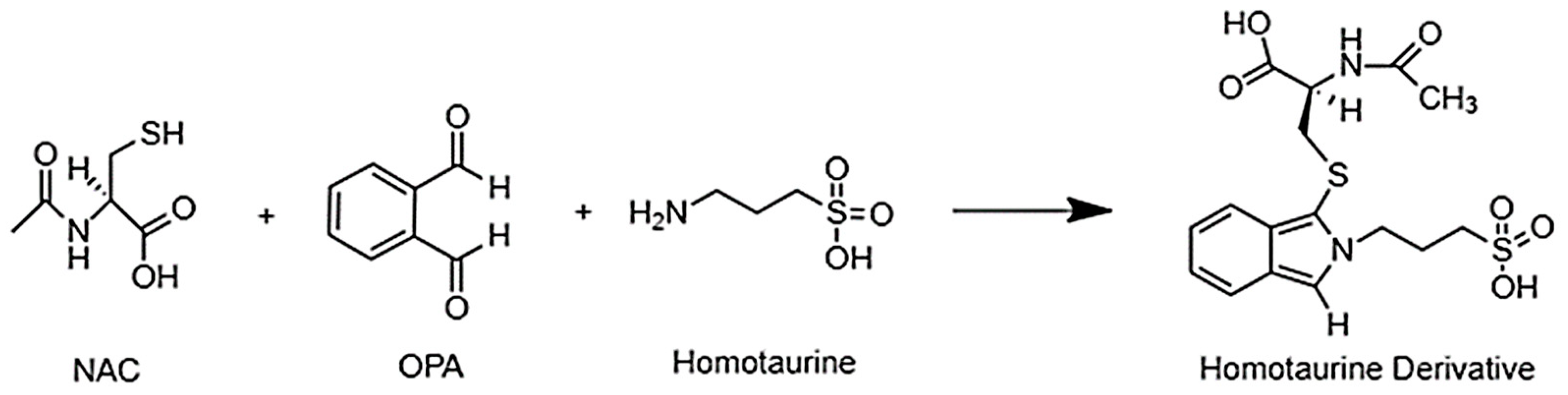
2.1. Optimization of the Derivatization Reaction
2.2. Optimization of Chromatographic Method
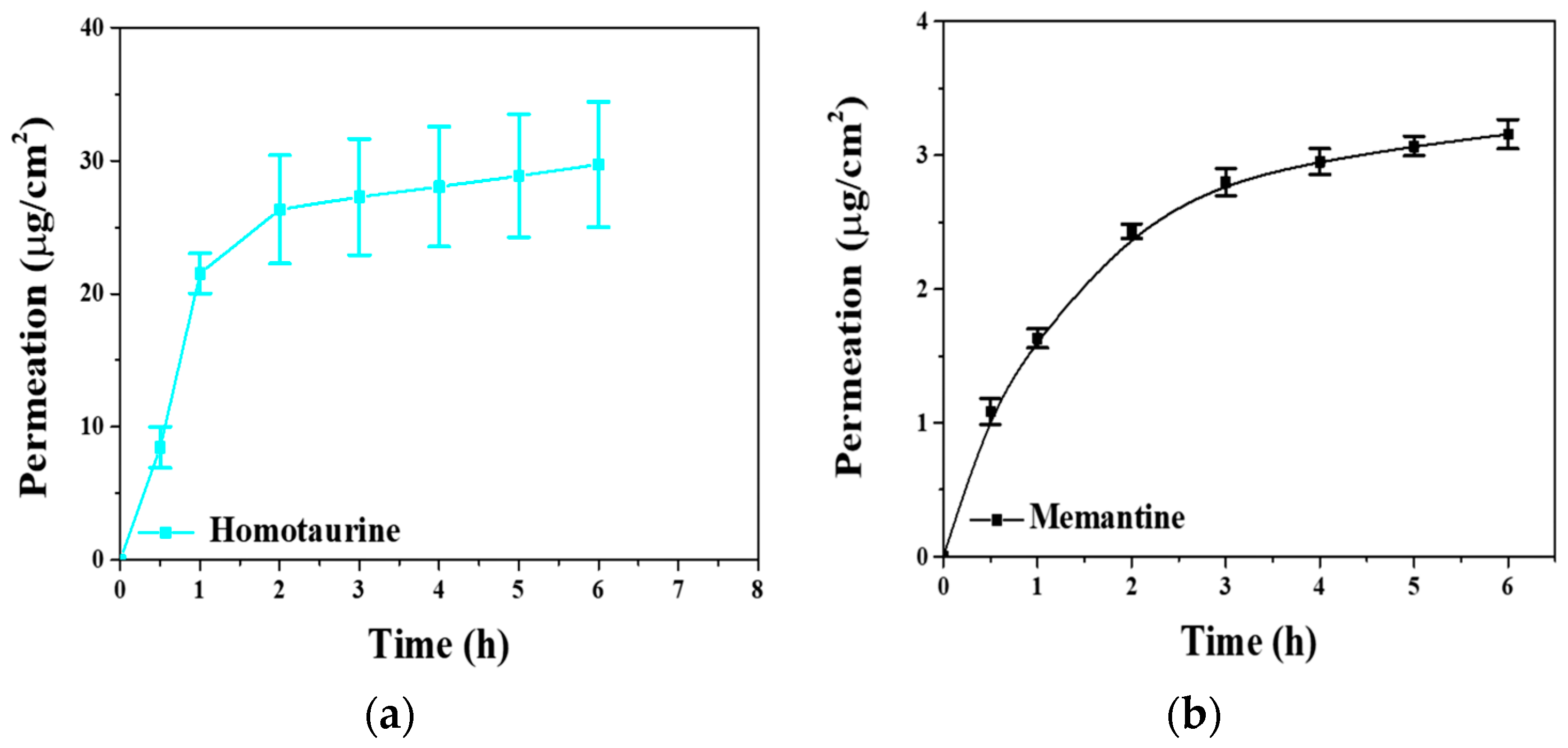
2.3. In Vitro Permeability Study
2.4. Method Validation
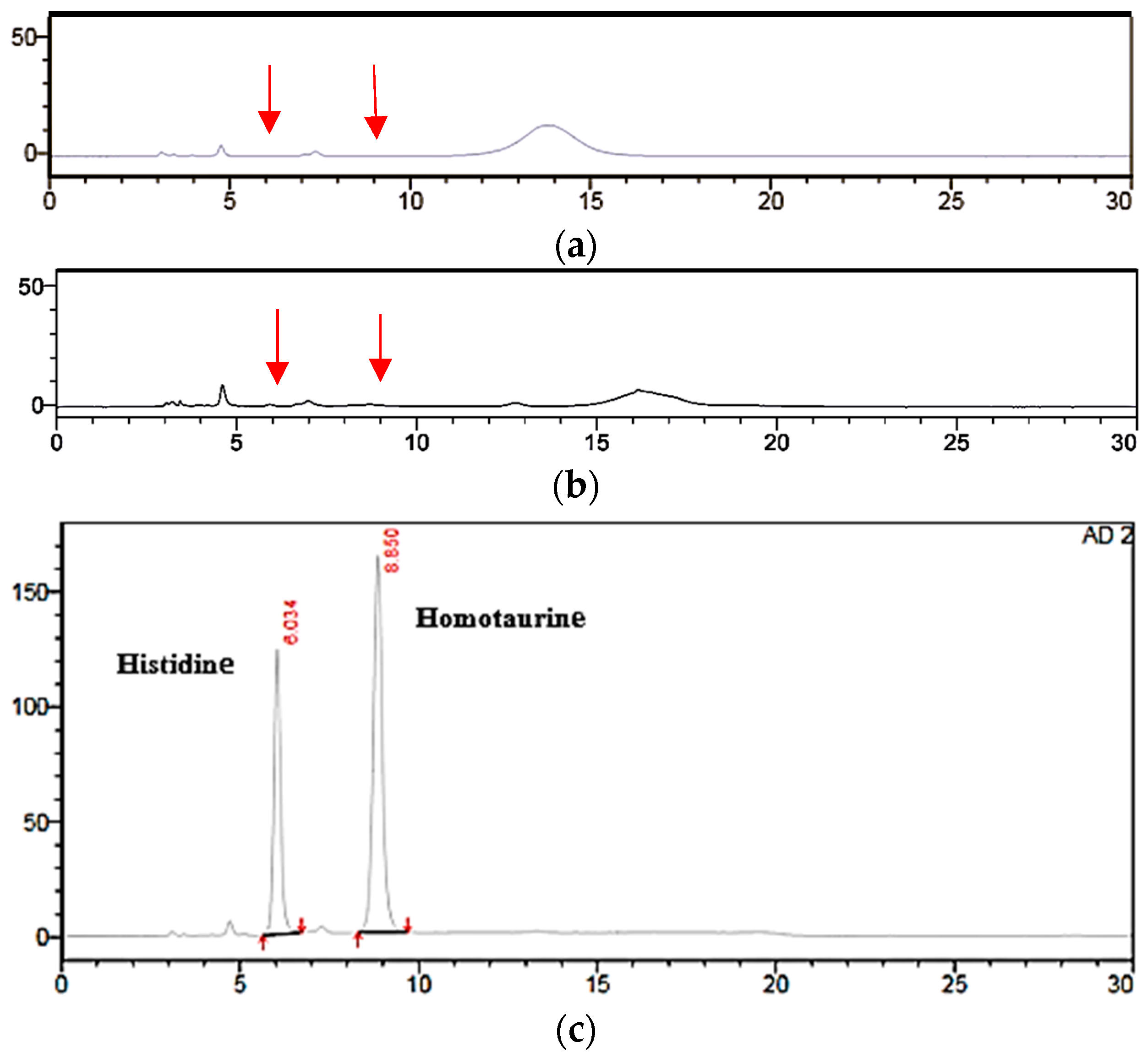
2.4.1. Selectivity
2.4.2. Linearity
2.4.3. Precision
2.4.4. Accuracy
2.4.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.5. In Vitro Permeability Study
3. Materials and Methods
3.1. Chemicals, Materials and Reagents
3.2. Derivatization Procedure
3.3. Permeability Process in the Franz Diffusion Cells
3.4. Instrumentation and Chromatographic Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kabir, M.T.; Uddin, M.S.; Jeandet, P.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Sayed, A.A.; Abdel-Daim, M.M.; Simal-Gandara, J. Anti-Alzheimer’s molecules derived from marine life: Understanding molecular mechanisms and therapeutic potential. Mar. Drugs 2021, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Aisen, P.; Ferris, S.; Saumier, D.; Duong, A.; Haine, D.; Garceau, D.; Suhy, J.; Oh, J. Effect of tramiprosate in patients eith mild-to-moderate Alzheimer’s disease: Exploratory analyses of the MRI sub-group of the alphase study. J. Nutr. Health Aging 2009, 13, 550–557. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2021, 24, 580–587. [Google Scholar] [CrossRef]
- Silva, M.; Seijas, P.; Otero, P. Exploitation of marine molecules to manage alzheimer’s disease. Mar. Drugs 2021, 19, 373. [Google Scholar] [CrossRef]
- Bossù, P.; Salani, F.; Ciaramella, A.; Sacchinelli, E.; Mosca, A.; Banaj, N.; Assogna, F.; Orfei, M.D.; Caltagirone, C.; Gianni, W.; et al. Anti-inflammatory effects of homotaurine in patients with amnestic mild cognitive impairment. Front. Aging Neurosci. 2018, 10, 285. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Wallner, M.; Olsen, R.; Kaufman, D.L. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci. Rep. 2018, 8, 16555. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; De Nigris, F.; Specchia, A.; Fasano, A. Homotaurine in Parkinson’s disease. Neurol. Sci. 2015, 36, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Fekete, S.; Clarke, A.; Veuthey, J.-L.; Guillarme, D. Recent Advances in Chromatography for Pharmaceutical Analysis. Anal. Chem. 2019, 91, 210–239. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohnishi, N.; Makita, M. Electron-capture gas chromatography of taurine as its N-pentafluorobenzoyl di-n-butylamide derivative. J. Chromatogr. B Biomed. Sci. Appl. 1985, 339, 370–374. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Telesca, G.; Bianco, G. Improved determination of taurine by high-performance anion-exchange chromatography with integrated pulsed amperometric detection (HPAEC-IPAD). Anal. Bioanal. Chem. 2004, 378, 804–810. [Google Scholar] [CrossRef]
- Kelly, M.T.; Fabre, H.; Perrett, D. Determination of taurine in plasma by capillary zone electrophoresis following derivatisation with fluorescamine. Electrophoresis 2000, 21, 699–705. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Nunes, M.V.; Mendes, E.; Remião, F.; Ferreira, M.A. Development of an HPLC-UV method for determination of taurine in infant formulae and breast milk. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 1269–1278. [Google Scholar] [CrossRef]
- Fabre, H.; Perrin, C.; Bosc, N. Determination of homotaurine as impurity in calcium acamprosate by capillary zone electrophoresis. J. Chromatogr. A 1999, 853, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Maurya, P.K.; Shinde, D.D.; Agwane, S.B. Reversed-phase liquid chromatographic determination of tramiprosate in rat plasma using evaporative light scattering detector. Biomed. Chromatogr. 2011, 25, 925–929. [Google Scholar] [CrossRef]
- Rao, R.N.; Maurya, P.K.; Shinde, D.D.; Khalid, S. Precolumn derivatization followed by liquid chromatographic separation and determination of tramiprosate in rat plasma by fluorescence detector: Application to pharmacokinetics. J. Pharm. Biomed. Anal. 2011, 55, 282–287. [Google Scholar] [CrossRef]
- Mehdinia, A.; Rostami, S.; Dadkhah, S.; Fumani, N.S. Simultaneous screening of homotaurine and taurine in marine macro-algae using liquid chromatography–fluorescence detection. J. Iran. Chem. Soc. 2017, 14, 2135–2142. [Google Scholar] [CrossRef]
- Todorova, K.A. Analytical Approaches and Methods in Quality Control Procedures of Energy Food Drinks Containing Caffeine and Taurine. Int. J. Nutr. Food Sci. 2015, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Tsanaktsidou, E.; Kanata, L.; Almpani, S.; Zacharis, C.K.; Markopoulou, C.K. Development and Validation of an HPLC-FLD Method for the Determination of NDMA and NDEA Nitrosamines in Lisinopril Using Pre-Column Denitrosation and Derivatization Procedure. Separations 2022, 9, 347. [Google Scholar] [CrossRef]
- McMahon, G.P.; O’Kennedy, R.; Kelly, M.T. High-performance liquid chromatographic determination of taurine in human plasma using pre-column extraction and derivatization. J. Pharm. Biomed. Anal. 1996, 14, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Ding, X.; Liu, Y. Separation methods for taurine analysis in biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 781, 251–267. [Google Scholar] [CrossRef]
- Synaridou, M.S.; Tsamis, V.; Sidiropoulou, G.; Zacharis, C.K.; Panderi, I.; Markopoulou, C.K. Fluorimetric analysis of five amino acids in chocolate: Development and validation. Molecules 2021, 26, 4325. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. The Molecular Constituents of the Blood-Brain Barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef]
- Teixeira, L.d.S.; Chagas, T.V.; Alonso, A.; Gonzalez-Alvarez, I.; Bermejo, M.; Polli, J.; Rezende, K.R. Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs. Pharmaceutics 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, A.; Tai, H.-C. Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols. Molecules 2021, 26, 7416. [Google Scholar] [CrossRef] [PubMed]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. A sensitive HPLC-FLD method combined with multivariate analysis for the determination of amino acids in l-citrulline rich vegetables. J. Food Drug Anal. 2019, 27, 717–728. [Google Scholar] [CrossRef]
- Zeng, F.; Ou, J.; Huang, Y.; Li, Q.; Xu, G.; Liu, Z.; Yang, S. Determination of 21 Free Amino Acids in Fruit Juices by HPLC Using a Modification of the 6-Aminoquinolyl-N-hydroxysuccinimidyl Carbamate (AQC) Method. Food Anal. Methods 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Rolle, T.; Dallorto, L.; Rossatto, S.; Curto, D.; Nuzzi, R. Assessing the Performance of Daily Intake of a Homotaurine, Carnosine, Forskolin, Vitamin B2, Vitamin B6, and Magnesium Based Food Supplement for the Maintenance of Visual Function in Patients with Primary Open Angle Glaucoma. J. Ophthalmol. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hu, D.; Jin, Y.; Hou, X.; Zhu, Y.; Chen, D.; Tai, J.; Chen, Q.; Shi, C.; Ye, J.; Wu, M.; et al. Application of Marine Natural Products against Alzheimer’s Disease: Past, Present and Future. Mar. Drugs 2023, 21, 43. [Google Scholar] [CrossRef]
- Takagi, T.; Masada, T.; Minami, K.; Kataoka, M.; Yamashita, S. Development of an In Vitro Methodology to Assess the Bioequivalence of Orally Disintegrating Tablets Taken without Water. Pharmaceutics 2023, 15, 2192. [Google Scholar] [CrossRef]
- European Medicines Agency. Validation of Analytical Procedures Q2(R2); European Medicines Agency: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Jurca, T.; Józsa, L.; Suciu, R.; Pallag, A.; Marian, E.; Bácskay, I.; Mureșan, M.; Stan, R.L.; Cevei, M.; Cioară, F.; et al. Formulation of topical dosage forms containing synthetic and natural anti-inflammatory agents for the treatment of rheumatoid arthritis. Molecules 2020, 26, 24. [Google Scholar] [CrossRef]
- Gieszinger, P.; Kiss, T.; Szabó-Révész, P.; Ambrus, R. The development of an in vitro horizontal diffusion cell to monitor nasal powder penetration inline. Pharmaceutics 2021, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Shafaati, A.; Foroutan, S.M.; Khoddam, A.; Madadian, B. Sensitive and rapid HPLC method for determination of memantine in human plasma using OPA derivatization and fluorescence detection: Application to pharmacokinetic studies. Sci Pharm 2010, 78, 847–856. [Google Scholar] [CrossRef] [PubMed]


| 50 μL | 100 μL | 200 μL | ||||
|---|---|---|---|---|---|---|
| Homotaurine | Histidine | Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 6.778 | 5.159 | 6.658 | 5.041 | 6.772 | 5.149 |
| Peak area | 9,291,676 | 3,302,120 | 9,170,223 | 3,497,763 | 8,922,560 | 3,237,679 |
| Tailing factor | 2.795 | 1.990 | 2.395 | 1.976 | 2.325 | 1.937 |
| pH = 8.5 | pH = 9 | |||
| Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 7.144 | 5.208 | 7.766 | 5.534 |
| Peak area | 9,745,111 | 3,490,905 | 8,962,345 | 3,216,106 |
| Tailing factor | 2.048 | 2.100 | 1.852 | 1.724 |
| pH = 10.5 | pH = 11 | |||
| Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 6.658 | 5.041 | 6.721 | 5.111 |
| Peak area | 9,170,223 | 3,497,763 | 9,071,322 | 2,224,509 |
| Tailing factor | 2.415 | 1.976 | 2.721 | 1.931 |
| t = 0 min | t = 5 min | |||
| Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 6.635 | 5.045 | 6.539 | 5.006 |
| Peak area | 9,329,892 | 2,760,075 | 8,994,530 | 3,337,056 |
| Tailing factor | 2.660 | 2.034 | 2.599 | 1.985 |
| t = 10 min | t = 30 min | |||
| Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 6.567 | 4.999 | 6.526 | 5.012 |
| Peak area | 9,069,873 | 3,189,284 | 8,851,468 | 3,268,445 |
| Tailing factor | 2.689 | 1.952 | 2.863 | 1.983 |
| pH = 3 | pH = 5 | pH = 7 | ||||
|---|---|---|---|---|---|---|
| Homotaurine | Histidine | Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 6.506 | 4.888 | 8.166 | 5.497 | 8.552 | 5.911 |
| Peak area | 804,412 | 210,781 | 9,368,871 | 2,069,597 | 9,442,442 | 3,918,684 |
| Tailing factor | 1.477 | 1.158 | 2.029 | 2.491 | 1.920 | 2.264 |
| Resolution | 1.795 | 5.203 | 4.365 | |||
| C = 20 mM | C = 40 mM | C = 60 mM | ||||
|---|---|---|---|---|---|---|
| Homotaurine | Histidine | Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 7.766 | 5.534 | 8.079 | 5.745 | 8.552 | 5.911 |
| Peak area | 8,962,345 | 3,216,106 | 8,952,314 | 3,096,572 | 9,463,550 | 3,124,075 |
| Tailing factor | 1.852 | 1.724 | 1.887 | 1.873 | 1.845 | 1.753 |
| T = 25 °C | T = 30 °C | T = 35 °C | ||||
|---|---|---|---|---|---|---|
| Homotaurine | Histidine | Homotaurine | Histidine | Homotaurine | Histidine | |
| Retention time | 7.270 | 5.200 | 6.757 | 4.962 | 6.068 | 4.683 |
| Peak area | 9,442,442 | 3,918,684 | 9,653,686 | 3,616,461 | 9,176,446 | 2,671,120 |
| Tailing factor | 1.920 | 2.491 | 1.722 | 2.161 | 2.438 | 1.965 |
| Resolution | 4.365 | 3.824 | 2.914 | |||
| Linearity | |||||||
|---|---|---|---|---|---|---|---|
| Cinitial (μg/mL) | 0.05 | 0.075 | 0.1 | 0.5 | 1 | 2 | 5 |
| Cfinal (ng/mL) | 4.55 | 6.82 | 9.09 | 45.45 | 90.91 | 181.82 | 454.55 |
| Regression Equation | Y = 0.033x + 0.1108 | ||||||
| R2 | 0.9999 | ||||||
| Slope | 0.033 | ||||||
| Standard Error | 0.05750 | ||||||
| LOD (ng/mL) | 0.455 | ||||||
| LOQ (ng/mL) | 1.365 | ||||||
| Ctheoritical (ng/mL) | Cexperimental (ng/mL) | % Recovery | Mean % Recovery | Standard Deviation | Limits of Confidence |
| 4.55 | 4.61 | 101.58 | 101.33 | 1.03 | 101.33 ± 1.28 |
| 45.45 | 46.57 | 102.47 | |||
| 90.91 | 92.59 | 101.85 | |||
| 181.82 | 183.60 | 100.98 | |||
| 454.55 | 453.44 | 99.75 | |||
| Ctheoritical (ng/mL) | Cexperimental (ng/mL) | % Recovery | Mean % Recovery | Standard Deviation | |
| 4.55 | 4.76 | 104.8 | 98.7 | 6.5 | |
| 4.55 | 4.63 | 101.8 | |||
| 4.55 | 5.10 | 101.5 | |||
| 4.55 | 4.00 | 88.0 | |||
| 4.55 | 4.43 | 97.4 | |||
| Intra-day and Inter-day precision | |||||
| Day 1 | |||||
| Concentration level (ng/mL) | 4.55 | 45.45 | 454.55 | ||
| % RSD | 2.0% | 1.1% | 1.4% | ||
| Mean % RSD | 1.5% | ||||
| Day 2 | |||||
| Concentration level (ng/mL) | 4.55 | 45.45 | 454.55 | ||
| % RSD | 3.5% | 1.6% | 2.9% | ||
| Mean % RSD | 2.7% | ||||
| Day 3 | |||||
| Concentration level (ng/mL) | 4.55 | 45.45 | 454.55 | ||
| % RSD | 6.9% | 1.7% | 0.98% | ||
| Mean % RSD | 3.2% | ||||
| Overall of 3 days | 2.5% | ||||
| Cell | J (μg/cm2/h) Homotaurine | J (μg/cm2/h) Memantine | Papp (h/cm2) Homotaurine | Papp (h/cm2) × 10−3 Memantine |
|---|---|---|---|---|
| a | 1.197 | 0.114 | 5.983 | 0.571 |
| b | 0.936 | 0.129 | 4.679 | 0.648 |
| c | 0.858 | 0.113 | 4.290 | 0.566 |
| Mean | 0.997 | 0.189 | 4.984 | 0.595 |
| Standard Deviation | 0.180 | 0.009 | 0.887 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntorkou, M.; Tsanaktsidou, E.; Chachlioutaki, K.; Fatouros, D.G.; Markopoulou, C.K. In Vitro Permeability Study of Homotaurine Using a High-Performance Liquid Chromatography with Fluorescence Detection Pre-Column Derivatization Method. Molecules 2023, 28, 7086. https://doi.org/10.3390/molecules28207086
Ntorkou M, Tsanaktsidou E, Chachlioutaki K, Fatouros DG, Markopoulou CK. In Vitro Permeability Study of Homotaurine Using a High-Performance Liquid Chromatography with Fluorescence Detection Pre-Column Derivatization Method. Molecules. 2023; 28(20):7086. https://doi.org/10.3390/molecules28207086
Chicago/Turabian StyleNtorkou, Marianna, Eleni Tsanaktsidou, Konstantina Chachlioutaki, Dimitrios G. Fatouros, and Catherine K. Markopoulou. 2023. "In Vitro Permeability Study of Homotaurine Using a High-Performance Liquid Chromatography with Fluorescence Detection Pre-Column Derivatization Method" Molecules 28, no. 20: 7086. https://doi.org/10.3390/molecules28207086
APA StyleNtorkou, M., Tsanaktsidou, E., Chachlioutaki, K., Fatouros, D. G., & Markopoulou, C. K. (2023). In Vitro Permeability Study of Homotaurine Using a High-Performance Liquid Chromatography with Fluorescence Detection Pre-Column Derivatization Method. Molecules, 28(20), 7086. https://doi.org/10.3390/molecules28207086






