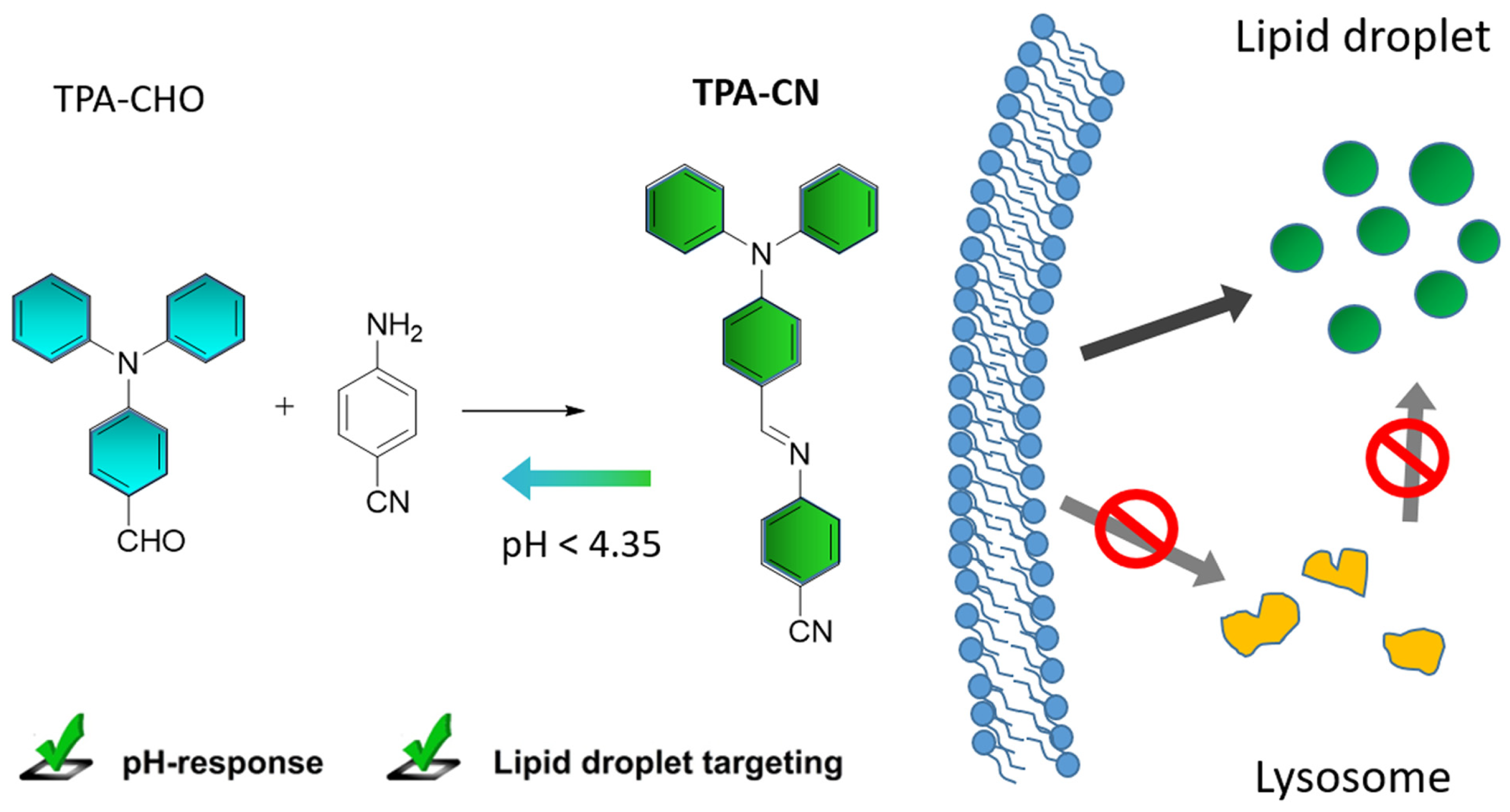
A Multifunctional Aggregation-Induced Emission Luminogen with pH-Response Detachable Connector for Lipid Droplet-Specific Imaging and Tracing
Abstract
:1. Introduction
2. Results
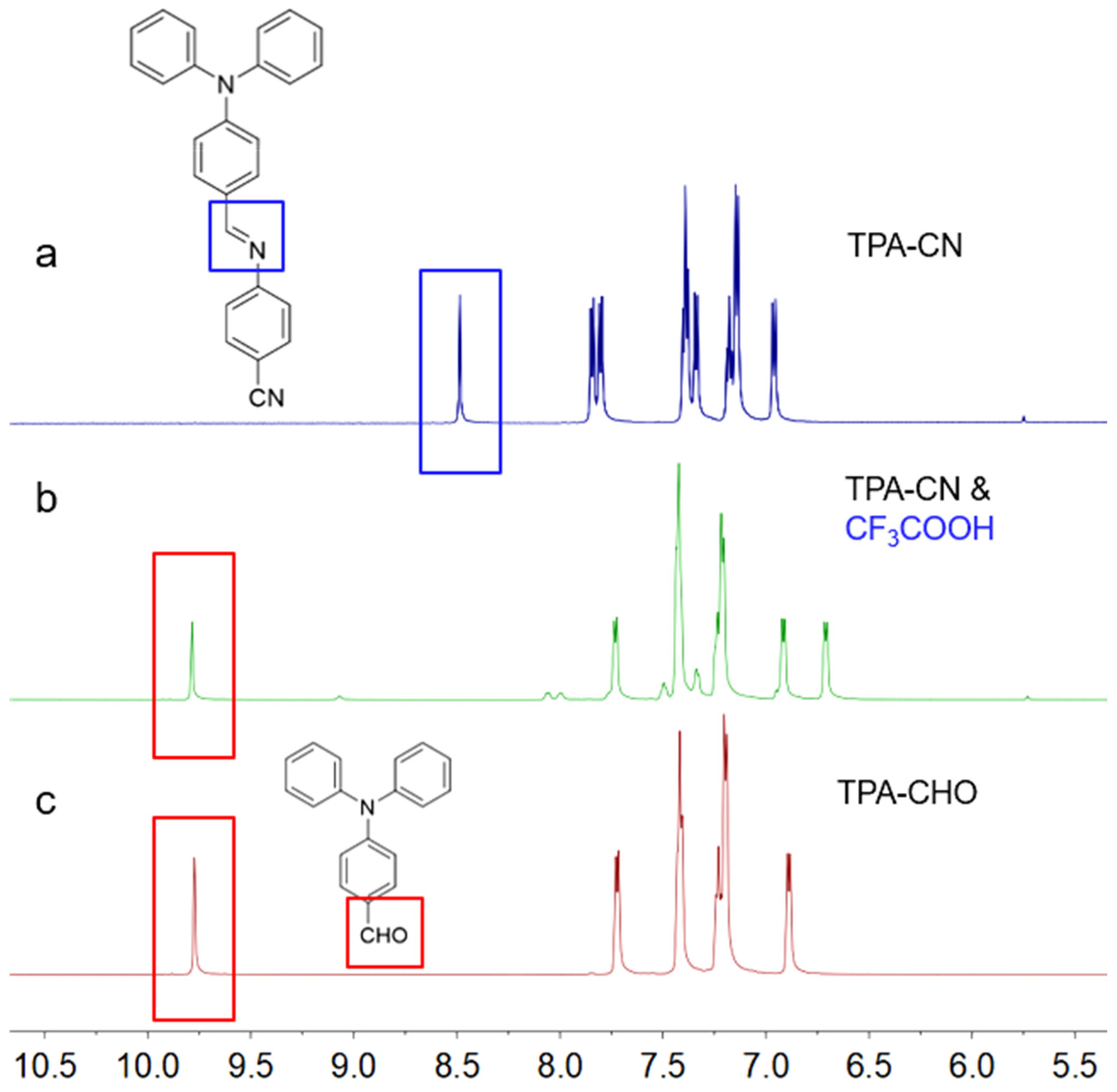
2.1. Synthesis and Characterization of TPA-CN
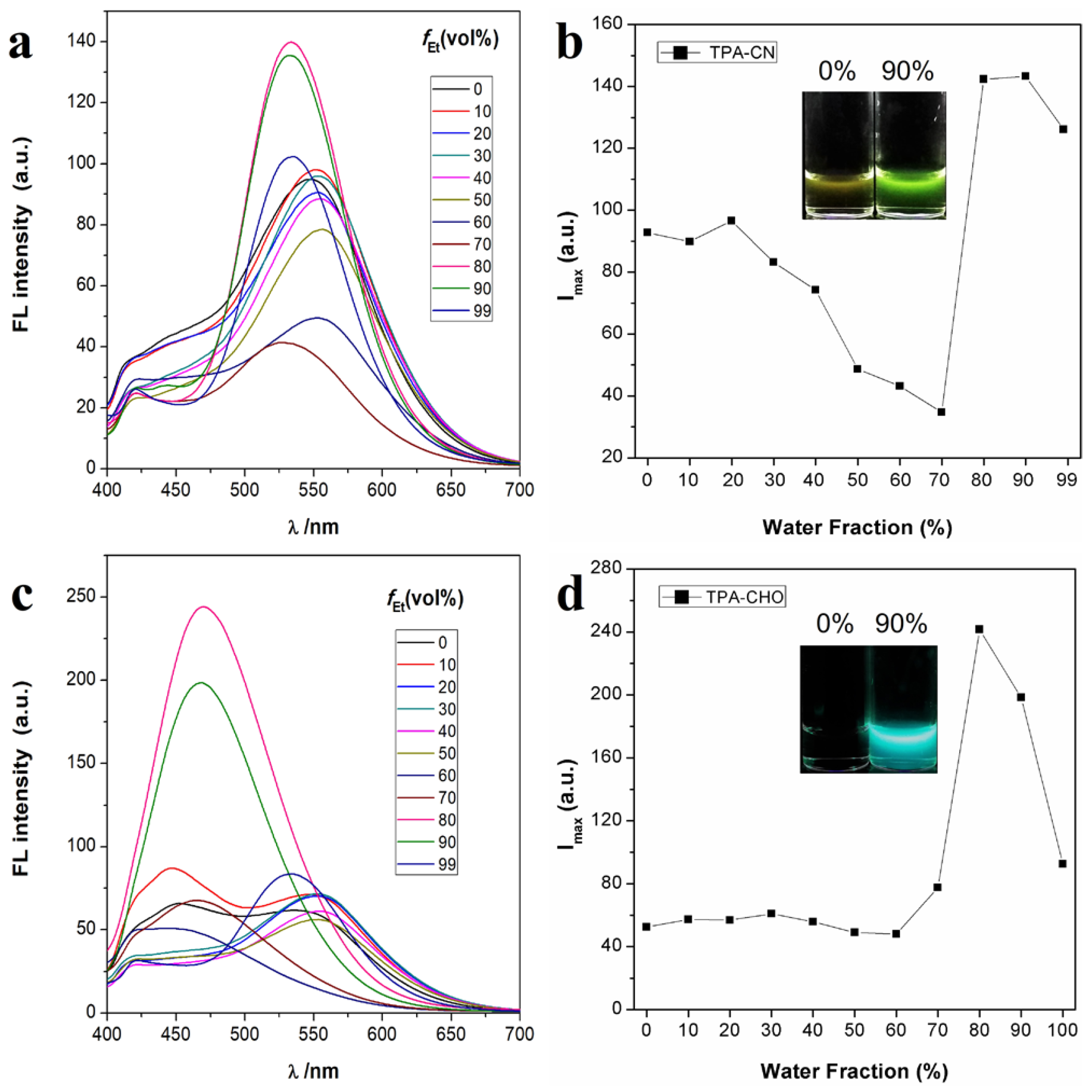
2.2. Acid Response Property of TPA-CN
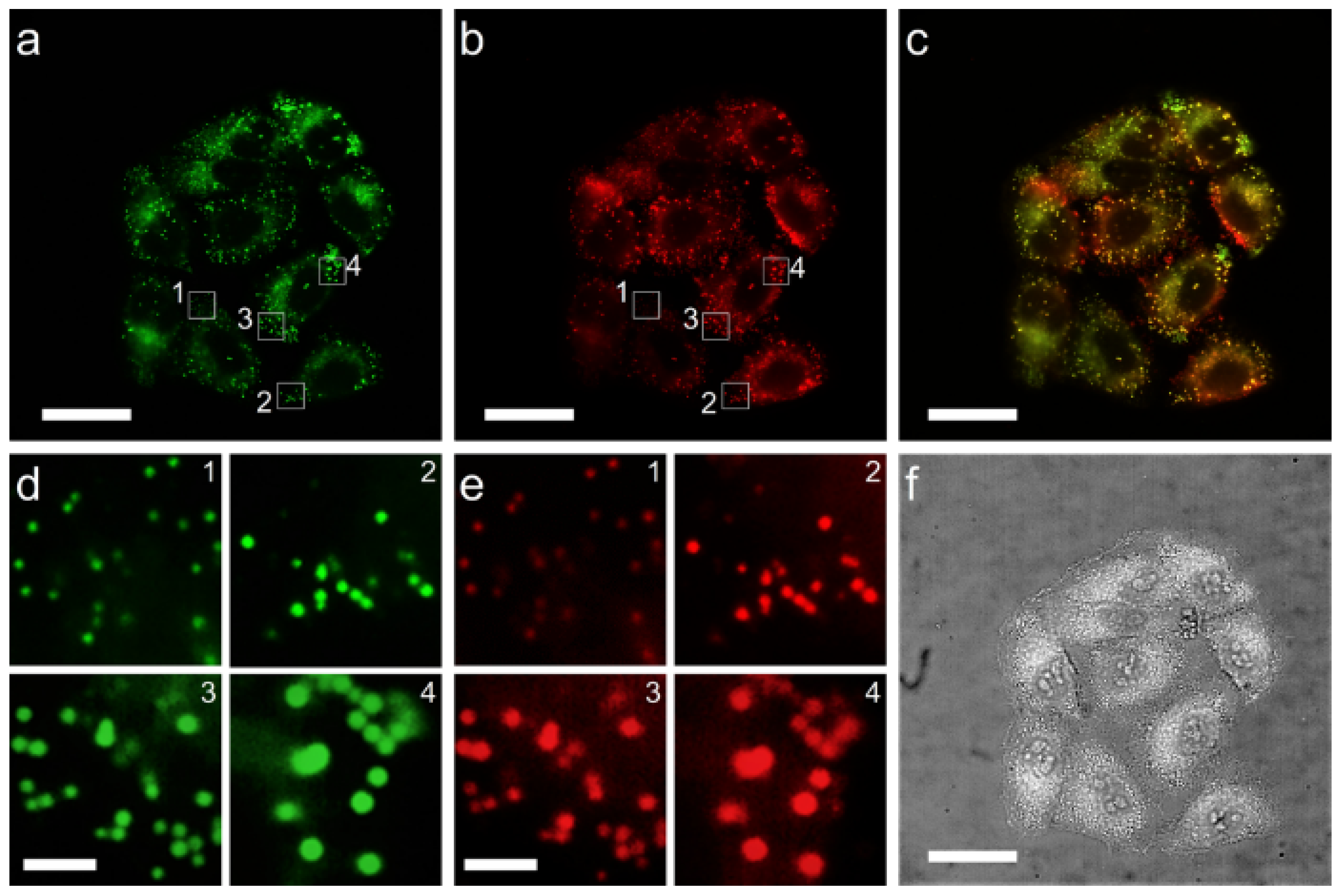
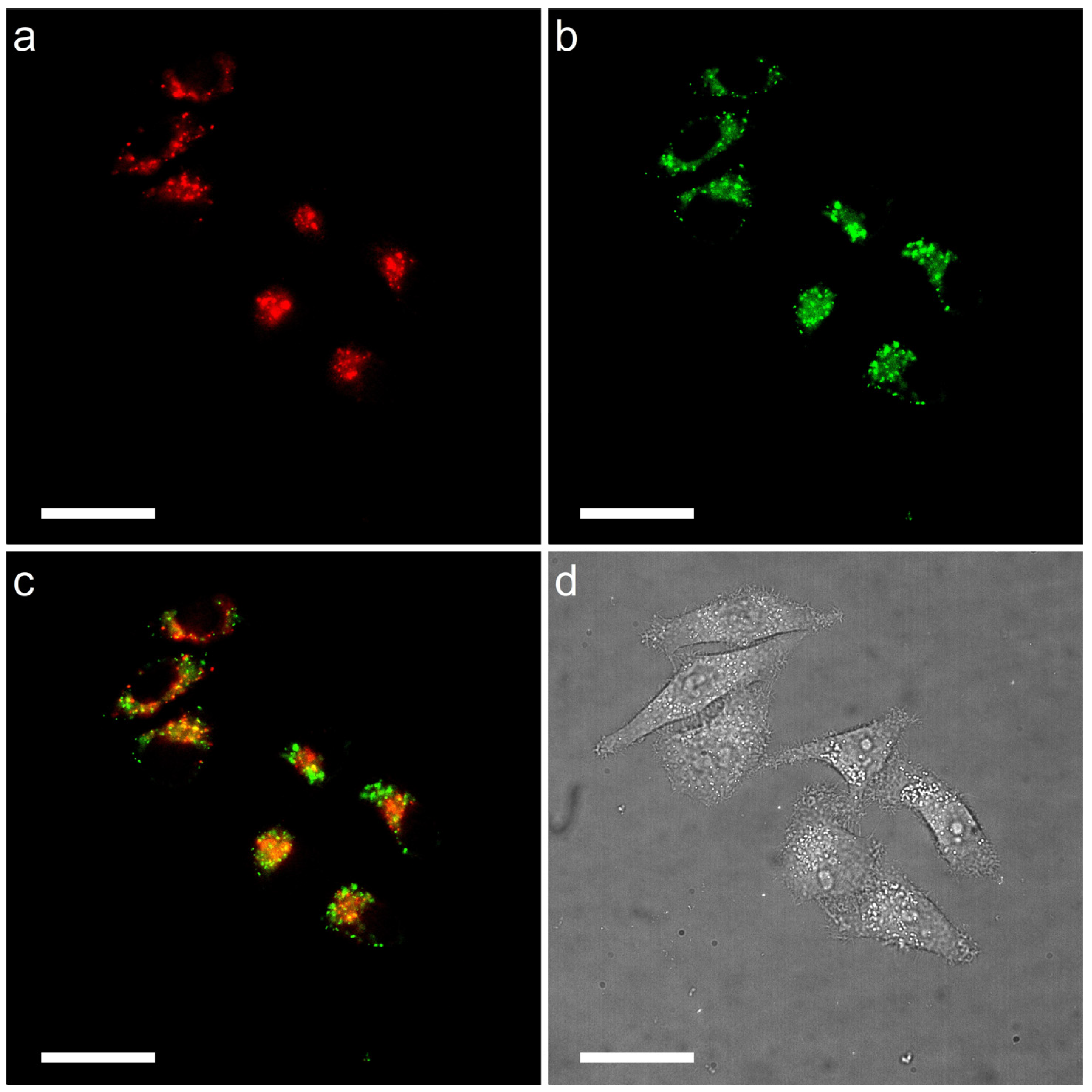
2.3. LD-Targeting and Lysosomal Co-Localization Imaging
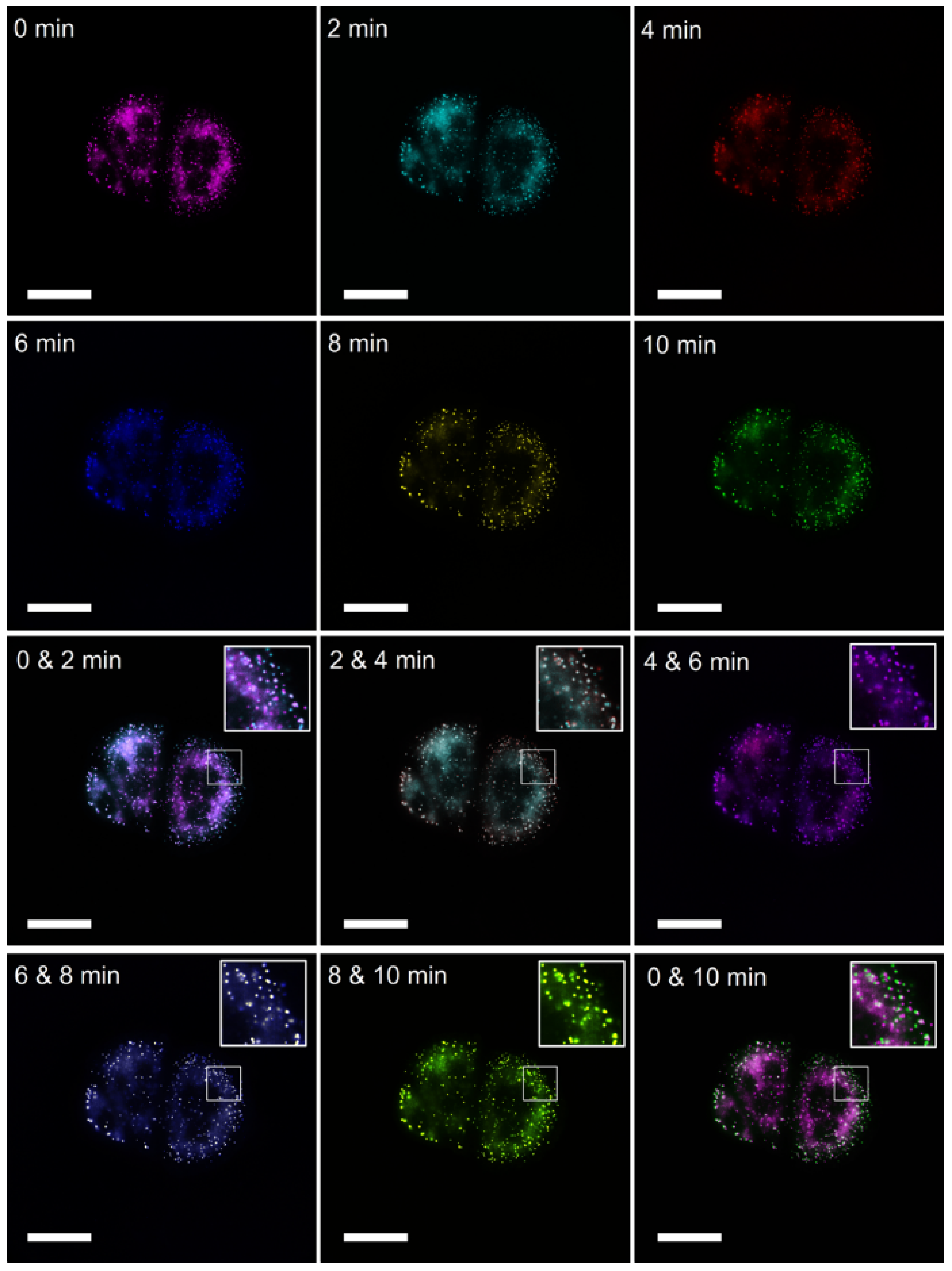
2.4. Dynamic Imaging of Intracellular LDs
3. Materials and Methods
3.1. Synthesis of TPA-CN
3.2. Acid Response Investigation of TPA-CN
3.3. In Vitro Cytotoxicity Assay
3.4. Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krahmer, N.; Farese, R.V., Jr.; Walther, T.C. Balancing the Fat: Lipid Droplets and Human Disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-Enhanced Theranostics: AIE Sparkles in Biomedical Field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- He, X.; Xiong, L.-H.; Huang, Y.; Zhao, Z.; Wang, Z.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-Based Energy Transfer Systems for Biosensing, Imaging, and Therapeutics. TrAC-Trend. Anal. Chem. 2020, 122, 115743. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens as Fluorescent Bioprobes. TrAC-Trend. Anal. Chem. 2020, 123, 115769. [Google Scholar] [CrossRef]
- Hu, J.-J.; Jiang, W.; Yuan, L.; Duan, C.; Yuan, Q.; Long, Z.; Lou, X.; Xia, F. Recent Advances in Stimuli-Responsive Theranostic Systems with Aggregation-Induced Emission Characteristics. Aggregate 2021, 2, 48–65. [Google Scholar] [CrossRef]
- Gu, J.; Yue, B.; Baryshnikov, G.V.; Li, Z.; Zhang, M.; Shen, S.; Ågren, H.; Zhu, L. Visualizing Material Processing Via Photoexcitation-Controlled Organic-Phase Aggregation-Induced Emission. Research 2021, 2021, 9862093. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, H.T.; Chen, X.Y.; Gao, P.F.; Hu, C.-H. A Multifunctional AIEgen with High Cell-Penetrating Ability for Intracellular Fluorescence Assays, Imaging and Drug Delivery. Mater. Chem. Front. 2019, 3, 1151–1158. [Google Scholar] [CrossRef]
- Mao, L.; Jiang, Y.; Ouyang, H.; Feng, Y.; Li, R.; Zhang, X.; Nie, Z.; Wei, Y. Revealing the Distribution of Aggregation-Induced Emission Nanoparticles Via Dual-Modality Imaging with Fluorescence and Mass Spectrometry. Research 2021, 2021, 9784053. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. Esipt-Based AIE Luminogens: Design Strategies, Applications, and Mechanisms. Aggregate 2022, 3, e160. [Google Scholar] [CrossRef]
- Hong, Y.; Geng, W.; Zhang, T.; Gong, G.; Li, C.; Zheng, C.; Liu, F.; Qian, J.; Chen, M.; Tang, B.Z. Facile Access to Far-Red Fluorescent Probes with through-Space Charge Transfer Effect for in Vivo Two-Photon Microscopy of Mouse Cerebrovascular System. Angew. Chem. Int. Ed. 2022, 61, e202209590. [Google Scholar] [CrossRef]
- Su, X.; Bao, Z.; Xie, W.; Wang, D.; Han, T.; Wang, D.; Tang, B.Z. Precise Planar-Twisted Molecular Engineering to Construct Semiconducting Polymers with Balanced Absorption and Quantum Yield for Efficient Phototheranostics. Research 2023, 6, 0194. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Ran, X.; Tang, H.; Cao, D. Recent Advance of Lipid Droplets Fluorescence Imaging with Aggregation-Induced Emission Luminogens (AIEgens). Dyes Pigment. 2022, 203, 110332. [Google Scholar] [CrossRef]
- Shi, L.; Li, K.; Li, L.-L.; Chen, S.-Y.; Li, M.-Y.; Zhou, Q.; Wang, N.; Yu, X.-Q. Novel Easily Available Purine-Based AIEgens with Colour Tunability and Applications in Lipid Droplet Imaging. Chem. Sci. 2018, 9, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xing, H.; Park, H.; Zhang, R.; Peng, C.; Sung, H.H.Y.; Williams, I.D.; Ma, C.; Wong, K.S.; Li, S.; et al. Deep-Red Aggregation-Induced Emission Luminogen Based on Dithiofuvalene-Fused Benzothiadiazole for Lipid Droplet-Specific Imaging. ACS Mater. Lett. 2022, 4, 159–164. [Google Scholar] [CrossRef]
- Chen, R.; Li, Z.; Peng, C.; Wen, L.; Xiao, L.; Li, Y. Rational Design of Novel Lipophilic Aggregation-Induced Emission Probes for Revealing the Dynamics of Lipid Droplets During Lipophagy and Ferroptosis. Anal. Chem. 2022, 94, 13432–13439. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Chen, Z.; Liu, Q.; Li, C.; Ma, D.; Chen, J.; Zhou, L.; Li, S.; Chen, M. Nitrobenzoxadiazole Based Lipid Droplets Specific Probes for Atherosclerosis Imaging. Dyes Pigment. 2022, 205, 110518. [Google Scholar] [CrossRef]
- Kang, M.; Gu, X.; Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Li, F.; Tang, B.Z. A Near-Infrared AIEgen for Specific Imaging of Lipid Droplets. Chem. Commun. 2016, 52, 5957–5960. [Google Scholar] [CrossRef]
- Tang, Y.; Song, S.; Peng, J.; Zhang, Q.; Lin, W. An Ultrasensitive Lipid Droplet-Targeted NIR Emission Fluorescent Probe for Polarity Detection and Its Application in Liver Disease Diagnosis. J. Mater. Chem. B 2022, 10, 6974–6982. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Lin, Y.; Ling, X.; Li, S.; Qin, A.; Tang, B.Z. Photoactivatable Aggregation-Induced Emission Probes for Lipid Droplets-Specific Live Cell Imaging. Chem. Sci. 2017, 8, 1763–1768. [Google Scholar] [CrossRef]
- Park, H.; Li, S.; Niu, G.; Zhang, H.; Song, Z.; Lu, Q.; Zhang, J.; Ma, C.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Diagnosis of Fatty Liver Disease by a Multiphoton-Active and Lipid-Droplet-Specific AIEgen with Nonaromatic Rotors. Mater. Chem. Front. 2021, 5, 1853–1862. [Google Scholar] [CrossRef]
- Cao, S.; Tian, X.; Cao, M.; Wang, J.; Niu, G.; Tang, B.Z. Solvatochromic near-Infrared Aggregation-Induced Emission-Active Acrylonitriles by Acceptor Modulation for Low-Power Stimulated Emission Depletion Nanoscopy. Chem. Mater. 2023, 35, 2472–2485. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Kwong, J.P.C.; Lam, J.W.Y.; Chen, C.; Wang, J.; Chen, Y.; Feng, X.; Kwok, R.T.K.; Sung, H.H.Y.; et al. Specific Two-Photon Imaging of Live Cellular and Deep-Tissue Lipid Droplets by Lipophilic AIEgens at Ultralow Concentration. Chem. Mater. 2018, 30, 4778–4787. [Google Scholar] [CrossRef]
- Wang, X.; Song, M.; Liu, Y.; Feng, X.; Redshaw, C.; Wang, D.; Zhu, K.; Li, Y.; Tang, B.Z. Lipid Droplet-Specific Red Aggregation-Induced Emission Luminogens: Fast Light-up of Gram-Positive Pathogens for Identification of Bacteria. ACS Mater. Lett. 2022, 4, 1523–1530. [Google Scholar] [CrossRef]
- Liu, H.; Yan, N.; Wong, T.Y.; Lam, H.; Lam, J.W.Y.; Kwok, R.T.K.; Sun, J.; Tang, B.Z. Fluorescent Imaging and Sorting of High-Lipid-Content Strains of Green Algae by Using an Aggregation-Induced Emission Luminogen. ACS Nano 2022, 16, 14973–14981. [Google Scholar] [CrossRef] [PubMed]
- Berardi, D.E.; Bock-Hughes, A.; Terry, A.R.; Drake, L.E.; Bozek, G.; Macleod, K.F. Lipid Droplet Turnover at the Lysosome Inhibits Growth of Hepatocellular Carcinoma in a Bnip3-Dependent Manner. Sci. Adv. 2022, 8, eabo2510. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, G.; Liu, Z.; Chau, J.H.C.; Su, H.; Lee, M.M.S.; Gu, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Single AIEgen for Multiple Tasks: Imaging of Dual Organelles and Evaluation of Cell Viability. Biomaterials 2020, 242, 119924. [Google Scholar] [CrossRef]
- Guo, L.; Tian, M.; Zhang, Z.; Lu, Q.; Liu, Z.; Niu, G.; Yu, X. Simultaneous Two-Color Visualization of Lipid Droplets and Endoplasmic Reticulum and Their Interplay by Single Fluorescent Probes in Lambda Mode. J. Am. Chem. Soc. 2021, 143, 3169–3179. [Google Scholar] [CrossRef]
- Hu, R.; Chen, B.; Wang, Z.; Qin, A.; Zhao, Z.; Lou, X.; Tang, B.Z. Intriguing “Chameleon” Fluorescent Bioprobes for the Visualization of Lipid Droplet-Lysosome Interplay. Biomaterials 2019, 203, 43–51. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the Lysosome: A Control Centre for Cellular Clearance and Energy Metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Kroemer, G.; Jäättelä, M. Lysosomes and Autophagy in Cell Death Control. Nat. Rev. Cancer 2005, 5, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Lian, P.; Qian, J.; Li, X.; Wang, L.; Fu, W.; Chen, L.; Wei, X.; Li, C. Selective Imaging and Cancer Cell Death Via Ph Switchable Near-Infrared Fluorescence and Photothermal Effects. Chem. Sci. 2016, 7, 5995–6005. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine Modulates Antitumor Immune Response by Resetting Tumor-Associated Macrophages toward M1 Phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Mazi, W.; Yan, Y.; Zhang, Y.; Xia, S.; Wan, S.; Tajiri, M.; Luck, R.L.; Liu, H. A Near-Infrared Fluorescent Probe Based on a Hemicyanine Dye with an Oxazolidine Switch for Mitochondrial pH Detection. J. Mater. Chem. B 2021, 9, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.M.A.; Cardano, F.; Baker, J.D.; Giordani, S.; Raymo, F.M. Switchable Coumarins for Ratiometric pH Sensing. Front. Mater. 2021, 8, 630046. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Song, F.; Xin, L.; Zhang, Q.; Zhang, P.; Ding, C. pH-Switched Near-Infrared Fluorescent Strategy for Ratiometric Detection of Onoo– in Lysosomes and Precise Imaging of Oxidative Stress in Rheumatoid Arthritis. Anal. Chem. 2023, 95, 1301–1308. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Ma, L.; Zhang, H.; Yan, J.; Wu, J.; Liu, C. A New Near-Infrared Ratiometric Fluorescent Probe Using Conjugated Dicyanoisophorone and Quinoline with Double Vinylene Linkers for Sensing of pH and Its Application. Dyes Pigment. 2023, 219, 111629. [Google Scholar] [CrossRef]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.S.; Peña-Cabrera, E.; et al. Twisted Intramolecular Charge Transfer and Aggregation-Induced Emission of Bodipy Derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Xue, K.; Xiao, M.; Wu, K.; Lv, S.; Hao, B.; Zhu, C. Polarity-Sensitive Fluorescent Probe for Reflecting the Packing Degree of Bacterial Membrane Lipids. Anal. Chem. 2022, 94, 3303–3312. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fan, R.; Gao, P.; Hu, C.-H. A Multifunctional Aggregation-Induced Emission Luminogen with pH-Response Detachable Connector for Lipid Droplet-Specific Imaging and Tracing. Molecules 2023, 28, 7029. https://doi.org/10.3390/molecules28207029
Li Y, Fan R, Gao P, Hu C-H. A Multifunctional Aggregation-Induced Emission Luminogen with pH-Response Detachable Connector for Lipid Droplet-Specific Imaging and Tracing. Molecules. 2023; 28(20):7029. https://doi.org/10.3390/molecules28207029
Chicago/Turabian StyleLi, Yanjie, Rui Fan, Pengfei Gao, and Chang-Hua Hu. 2023. "A Multifunctional Aggregation-Induced Emission Luminogen with pH-Response Detachable Connector for Lipid Droplet-Specific Imaging and Tracing" Molecules 28, no. 20: 7029. https://doi.org/10.3390/molecules28207029
APA StyleLi, Y., Fan, R., Gao, P., & Hu, C.-H. (2023). A Multifunctional Aggregation-Induced Emission Luminogen with pH-Response Detachable Connector for Lipid Droplet-Specific Imaging and Tracing. Molecules, 28(20), 7029. https://doi.org/10.3390/molecules28207029







