Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA
Abstract
1. Introduction
2. Results
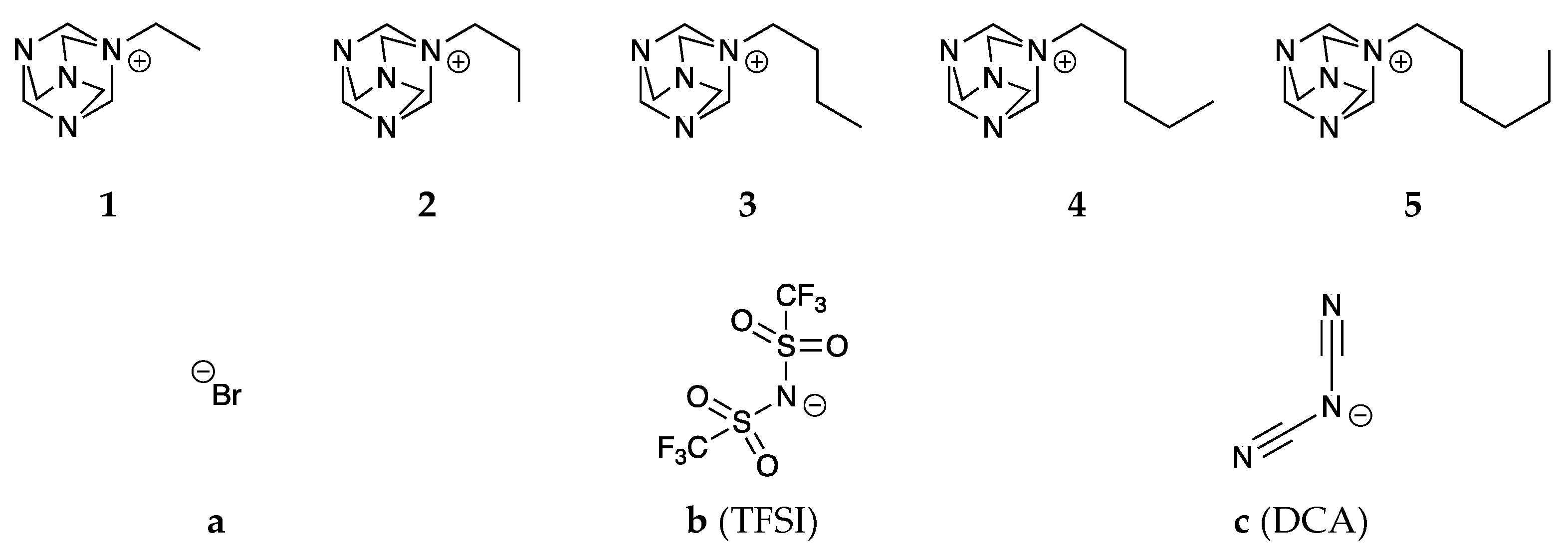
2.1. Synthesis of Ionic Liquids
2.1.1. Quaternization
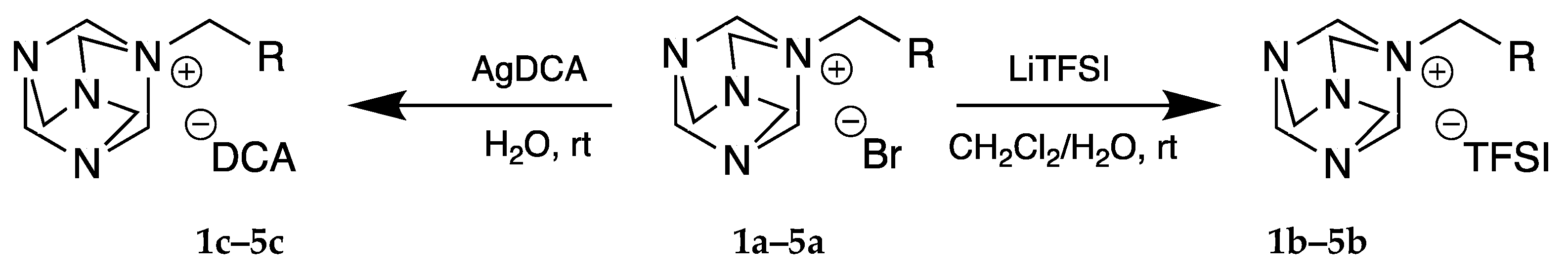
2.1.2. Metathesis Reaction
2.1.3. Thermal Properties of the Compounds
2.2. Curing Process
2.2.1. Thermal Analysis
2.2.2. Hardness of the Cured Resin
3. Discussion and Conclusions
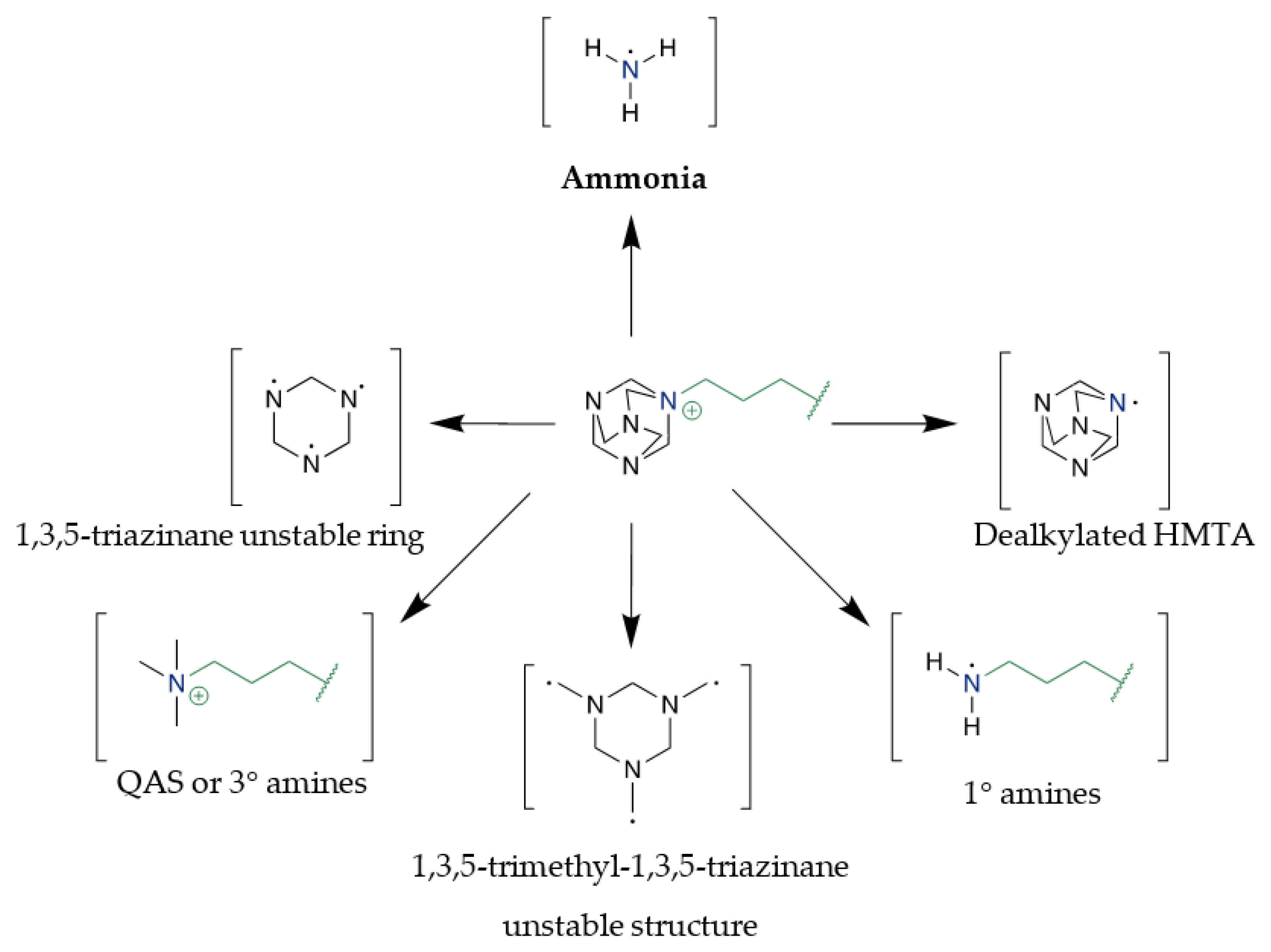
3.1. Synthesis and ILs Stability
3.2. Role of Ethylene Glycol
3.3. Curing Process and the Mechanism
3.4. Hardness of the Materials
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. Bromides
4.2.2. Bis(trifluoromethane)sulfonimides
4.2.3. Dicyanamides
4.3. Ion Chromatography
4.4. Thermal Analysis
4.4.1. Differential Scanning Calorimetry (DSC) for ILs
4.4.2. Differential Scanning Calorimetry (DSC) for Epoxy Systems
4.5. Water Content
4.6. NMR Analysis
4.7. ESI-MS Analysis
4.8. Curing Process
4.9. Hardness Measurments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Rafique, I.; Kausar, A.; Muhammad, B. Epoxy Resin Composite Reinforced with Carbon Fiber and Inorganic Filler: Overview on Preparation and Properties. Polym.-Plast. Technol. Eng. 2016, 55, 1653–1672. [Google Scholar] [CrossRef]
- Rezaei Motlagh, S.; Harun, R.; Awang Biak, D.R.; Hussain, S.A.; Wan Ab Karim Ghani, W.A.; Khezri, R.; Wilfred, C.D.; Elgharbawy, A.A.M. Screening of Suitable Ionic Liquids as Green Solvents for Extraction of Eicosapentaenoic Acid (EPA) from Microalgae Biomass Using COSMO-RS Model. Molecules 2019, 24, 713. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Tan, X.; Ooi, C.W.; Chew, K.W.; Leong, W.H.; Chai, Y.H.; Ho, S.-H.; Show, P.L. How Does Ionic Liquid Play a Role in Sustainability of Biomass Processing? J. Clean. Prod. 2021, 284, 124772. [Google Scholar] [CrossRef]
- Rieland, J.M.; Love, B.J. Ionic Liquids: A Milestone on the Pathway to Greener Recycling of Cellulose from Biomass. Resour. Conserv. Recycl. 2020, 155, 104678. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Silica-Based Supported Ionic Liquid-like Phases as Heterogeneous Catalysts. Molecules 2022, 27, 5900. [Google Scholar] [CrossRef]
- Maciejewski, H. Ionic Liquids in Catalysis. Catalysts 2021, 11, 367. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal Nanoparticles in Ionic Liquids: Synthesis and Catalytic Applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
- Mota, F.A.R.; Pereira, S.A.P.; Araujo, A.R.T.S.; Saraiva, M.L.M.F.S. Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity. Molecules 2021, 26, 200. [Google Scholar] [CrossRef]
- Faísca, F.; Correia, V.; Petrovski, Ž.; Branco, L.C.; Rebelo-de-Andrade, H.; Santos, M.M. Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2. Pharmaceutics 2022, 14, 877. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Rafiq, R.; Ahmad, A.; Maulud, A.S.; Moniruzzaman, M. Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms. Molecules 2022, 27, 7775. [Google Scholar] [CrossRef]
- Zajac, A.; Szpecht, A.; Zielinski, D.; Rola, K.; Hoppe, J.; Komorowska, K.; Smiglak, M. Synthesis and Characterization of Potentially Polymerizable Amine-Derived Ionic Liquids Bearing 4-Vinylbenzyl Group. J. Mol. Liq. 2019, 283, 427–439. [Google Scholar] [CrossRef]
- Maksym, P.; Tarnacka, M.; Bielas, R.; Hachuła, B.; Zajac, A.; Szpecht, A.; Smiglak, M.; Kaminski, K.; Paluch, M. Structure-Property Relationships of Tailored Imidazolium- and Pyrrolidinium-Based Poly(Ionic Liquid)s. Solid-like vs. Gel-like Systems. Polymer 2020, 192, 122262. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Guazzelli, L.; Pomelli, C.S.; Ciccioli, A.; Brunetti, B.; Mezzetta, A.; Vecchio Ciprioti, S. Synthesis, Thermal Behavior and Kinetic Study of N-Morpholinium Dicationic Ionic Liquids by Thermogravimetry. J. Mol. Liq. 2021, 332, 115662. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation Thermodynamics of the Tetraoctylphosphonium Bis(Trifluoromethansulfonyl)Imide([P8888]NTf2) and Tetraoctylphosphonium Nonafluorobutane-1-Sulfonate ([P8888]NFBS) Ionic Liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Gond, R.; van Ekeren, W.; Mogensen, R.; Naylor, A.J.; Younesi, R. Non-Flammable Liquid Electrolytes for Safe Batteries. Mater. Horiz. 2021, 8, 2913–2928. [Google Scholar] [CrossRef] [PubMed]
- Rahmathullah, M.A.M.; Jeyarajasingam, A.; Merritt, B.; VanLandingham, M.; McKnight, S.H.; Palmese, G.R. Room Temperature Ionic Liquids as Thermally Latent Initiators for Polymerization of Epoxy Resins. Macromolecules 2009, 42, 3219–3221. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Investigating the Mechanism through Which Ionic Liquids Initiate the Polymerisation of Epoxy Resins. Polymer 2018, 139, 163–176. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Examining the Kinetics of the Thermal Polymerisation Behaviour of Epoxy Resins Initiated with a Series of 1-Ethyl-3-Methylimidazolium Based Ionic Liquids. Thermochim. Acta 2018, 663, 19–26. [Google Scholar] [CrossRef]
- Zielinski, D.; Szpecht, A.; Hinc, P.; Maciejewski, H.; Smiglak, M. Mono N-Alkylated DABCO-Based Ionic Liquids and Their Application as Latent Curing Agents for Epoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 5481–5493. [Google Scholar] [CrossRef]
- Al Hokayem, K.; El Hage, R.; Svecova, L.; Otazaghine, B.; Le Moigne, N.; Sonnier, R. Flame Retardant-Functionalized Cotton Cellulose Using Phosphonate-Based Ionic Liquids. Molecules 2020, 25, 1629. [Google Scholar] [CrossRef]
- Sonnier, R.; Dumazert, L.; Livi, S.; Nguyen, T.K.L.; Duchet-Rumeau, J.; Vahabi, H.; Laheurte, P. Flame Retardancy of Phosphorus-Containing Ionic Liquid Based Epoxy Networks. Polym. Degrad. Stab. 2016, 134, 186–193. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Shi, X.-H.; Lu, J.-H.; Qi, M.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Novel Phosphorus-Containing Imidazolium as Hardener for Epoxy Resin Aiming at Controllable Latent Curing Behavior and Flame Retardancy. Compos. Part B Eng. 2020, 184, 107673. [Google Scholar] [CrossRef]
- Wei, R.; Yang, B.; He, C.; Jin, L.; Zhang, X.; Zhao, C. Versatile and Robust Poly(Ionic Liquid) Coatings with Intelligent Superhydrophilicity/Superhydrophobicity Switch in High-Efficient Oil-Water Separation. Sep. Purif. Technol. 2022, 282, 120100. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Wei, R.; Jin, L.; He, C.; Zhao, W.; Zhao, C. Design of Poly Ionic Liquids Modified Cotton Fabric with Ion Species-Triggered Bidirectional Oil-Water Separation Performance. J. Hazard. Mater. 2020, 400, 123163. [Google Scholar] [CrossRef]
- Kong, M.; Liu, C.; Tang, B.; Xu, W.; Huang, Y.; Li, G. Improved Mechanical and Thermal Properties of Trifunctional Epoxy Resins through Controlling Molecular Networks by Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 8080–8089. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.; Guo, Y.; Zhang, S.; Du, X.; Zhu, Q.; Lu, M. An Efficient Phosphonate-Based Ionic Liquid on Flame Retardancy and Mechanical Property of Epoxy Resin. J. Mater. Sci. 2017, 52, 13992–14003. [Google Scholar] [CrossRef]
- Węgrzyn, M.; Rudnik, E.; Kamocka-Bronisz, R.; Kukfisz, B. Mechanical and Thermal Properties of Biocomposites Based on Polyethylene from Renewable Resources Modified with Ionic Liquids. J. Polym. Environ. 2021, 29, 1808–1816. [Google Scholar] [CrossRef]
- Shi, T.; Livi, S.; Duchet-Rumeau, J.; Gérard, J.-F. Enhanced Mechanical and Thermal Properties of Ionic Liquid Core/Silica Shell Microcapsules-Filled Epoxy Microcomposites. Polymer 2021, 233, 124182. [Google Scholar] [CrossRef]
- Sanaei-Rad, S.; Saeidiroshan, H.; Mirhosseini-Eshkevari, B.; Ghasemzadeh, M.A. Hexamethylenetetramine-Based Ionic Liquid Anchored onto the Metal–Organic Framework MIL-101(Cr) as a Superior and Reusable Heterogeneous Catalyst for the Preparation of Hexahydroquinolines. Res. Chem. Intermed. 2021, 47, 2143–2159. [Google Scholar] [CrossRef]
- Mirhosseini-Eshkevari, B.; Ali Ghasemzadeh, M.; Esnaashari, M.; Taghvaei Ganjali, S. Hexamethylenetetramine-Based Ionic Liquid/MIL-101(Cr) Metal–Organic Framework Composite: A Novel and Versatile Tool for the Preparation of Pyrido[2,3-D:5,6-d ′]Dipyrimidines. RSC Adv. 2021, 11, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, X.; Kong, S.; Bai, L.; He, X.; Meng, F. Synthesis and Characterization of Hexamethylenetetramine-Based Ionic Liquid Crystals. J. Mol. Struct. 2019, 1178, 135–141. [Google Scholar] [CrossRef]
- Jordan, A.; Huang, S.; Sneddon, H.F.; Nortcliffe, A. Assessing the Limits of Sustainability for the Delépine Reaction. ACS Sustain. Chem. Eng. 2020, 8, 12746–12754. [Google Scholar] [CrossRef]
- Surrey, A.R. Name Reactions in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4832-5868-3. [Google Scholar]
- Cheng, S.; Fu, X.; Liu, J.; Zhang, J.; Zhang, Z.; Wei, Y.; Han, B. Study of Ethylene Glycol/TX-100/Ionic Liquid Microemulsions. Colloids Surf. Physicochem. Eng. Asp. 2007, 302, 211–215. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, G.; Kumar, S.; Navnidhi; Kang, T.S. Thermally Stable Microemulsions Comprising Imidazolium Based Surface Active Ionic Liquids, Non-Polar Ionic Liquid and Ethylene Glycol as Polar Phase. J. Colloid Interface Sci. 2018, 511, 344–354. [Google Scholar] [CrossRef]
- Dewan, M.; Kumar, A.; Saxena, A.; De, A.; Mozumdar, S. Using Hydrophilic Ionic Liquid, [Bmim]BF4—Ethylene Glycol System as a Novel Media for the Rapid Synthesis of Copper Nanoparticles. PLoS ONE 2012, 7, e29131. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, G.; Wu, D.; Cai, P.; Pan, Y. A Novel Strategy to Utilize Ethylene Glycol-Ionic Liquids for the Selective Precipitation of Polysaccharides. J. Sep. Sci. 2019, 42, 1757–1767. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Visser, A.E.; Spear, S.K.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Rogers, R.D. Mercury(II) Partitioning from Aqueous Solutions with a New, Hydrophobic Ethylene-Glycol Functionalized Bis-Imidazolium Ionic Liquid. Green Chem. 2003, 5, 129–135. [Google Scholar] [CrossRef]
- Pal, A.; Kumar, B.; Singh Kang, T. Effect of Structural Alteration of Ionic Liquid on Their Bulk and Molecular Level Interactions with Ethylene Glycol. Fluid Phase Equilibria 2013, 358, 241–249. [Google Scholar] [CrossRef]
- Peng, Q.; Wei, L.; Zhang, X.; Wu, Y.; Mahmood, K.; Liu, Z.; Zhang, L. Direct Polycondensation of L-Lactic Acid in Hydrophobic Bis(Trifluoromethanesulfonyl)Imide-Anionic Ionic Liquids: A Kinetic Study. Eur. Polym. J. 2021, 158, 110692. [Google Scholar] [CrossRef]
- Zheng, Y.; Xuan, X.; Wang, J.; Fan, M. The Enhanced Dissolution of β-Cyclodextrin in Some Hydrophilic Ionic Liquids. J. Phys. Chem. A 2010, 114, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.J.; Tarnavchyk, I.; Chisholm, B.J.; Webster, D.C. Novel Bio-Based Epoxy Resins from Eugenol as an Alternative to BPA Epoxy and High Throughput Screening of the Cured Coatings. Polymer 2021, 233, 124191. [Google Scholar] [CrossRef]
- Dreyfors, J.M.; Jones, S.B.; Sayed, Y. Hexamethylenetetramine: A Review. Am. Ind. Hyg. Assoc. J. 1989, 50, 579–585. [Google Scholar] [CrossRef]
- Hartwig, A.; MAK Commission. Hexamethylenetetramine [MAK Value Documentation, 1993]. In The MAK-Collection for Occupational Health and Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 356–372. ISBN 978-3-527-60041-0. [Google Scholar]
- Paduszyński, K.; Domańska, U. Viscosity of Ionic Liquids: An Extensive Database and a New Group Contribution Model Based on a Feed-Forward Artificial Neural Network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of Ionic Liquids as More Efficient Media for Cellulose Dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Jenkins, D.H.; Ruzbacky, R.; Saunders, S. Solubilities of Ammonia and Ammonium Chloride in Ammoniated and Nonammoniated Methanol and Ethylene Glycol between 298 K and 353 K. J. Chem. Eng. Data 2012, 57, 1449–1455. [Google Scholar] [CrossRef]
- Galvão, A.C.; Francesconi, A.Z. Solubility of Methane and Carbon Dioxide in Ethylene Glycol at Pressures up to 14MPa and Temperatures Ranging from (303 to 423)K. J. Chem. Thermodyn. 2010, 42, 684–688. [Google Scholar] [CrossRef]

| Compound | Yield [%] | Purity [%] | Water Content [wt%] |
|---|---|---|---|
| 1a | 99.6 | 99.6 | 0.47 |
| 2a | 99.0 | 99.0 | 0.17 |
| 3a | 99.1 | 99.1 | 0.68 |
| 4a | 99.1 | 99.1 | 0.57 |
| 5a | 99.0 | 99.1 | 0.42 |
| Compound | Tm [°C] | Tg [°C] | Tdecomp [°C] |
|---|---|---|---|
| 1a | - | - | 168.3 a |
| 2a | - | - | 146.5 a |
| 3a | - | - | 155.4 a |
| 4a | - | - | 143.6 a |
| 5a | - | - | 152.4 a |
| 1b | - | −5.0 | 119.3 b |
| 2b | - | −16.6 | 126.8 b |
| 3b | - | −17.1 | 126.3 b |
| 4b | - | −29.1 | 121.6 b |
| 5b | - | −19.9 | 134.7 b |
| 1c | 69.1 | - | 120.3 b |
| 2c | 57.4 | - | 135.3 b |
| 3c | 72.1 | - | 101.6 b |
| 4c | 75.5 | - | 124.0 b |
| 5c | 86.6 | - | 126.8 b |
| System | Anion | Tonset [°C] | Tmax [°C] | Tend [°C] | ΔH [J/g] |
|---|---|---|---|---|---|
| 1b/EG/DGEBA | TFSI | 109.4 | 137.0 | 164.8 | 84.4 |
| 200.7 | 223.5 | 236.9 | 24.3 | ||
| 2b/EG/DGEBA | 122.2 | 148.1 | 201.5 | 26.5 | |
| 215.9 | 224.4 | 236.4 | 11.0 | ||
| 3b/EG/DGEBA | 122.1 | 154.4 | 185.6 | 19.8 | |
| 210.3 | 218.9 | 239.5 | 11.7 | ||
| 4b/EG/DGEBA | 119.1 | 147.0 | 205.7 | 29.3 | |
| 221.0 | 229.5 | 242.3 | 8.9 | ||
| 5b/EG/DGEBA | 119.8 | 149.0 | 193.2 | 28.5 | |
| 209.3 | 221.7 | 223.0 | 7.2 | ||
| 1c/EG/DGEBA | DCA | 140.8 | 170.7 | 224.0 | 49.5 |
| 2c/EG/DGEBA | 123.6 | 164.7 | 213.2 | 81.6 | |
| 3c/EG/DGEBA | 140.0 | 163.9 | 188.1 | 49.8 | |
| 4c/EG/DGEBA | 129.5 | 165.1 | 199.1 | 23.0 | |
| 5c/EG/DGEBA | 101.3 | 151.8 | 210.6 | 151.5 |
| Compound | Hardness [D] |
|---|---|
| RM1/RM2 | 74/71 |
| 1b | 78 |
| 2b | 80 |
| 3b | 76 |
| 4b | 81 |
| 5b | n/a |
| 1c | 87 |
| 2c | n/a |
| 3c | 89 |
| 4c | n/a |
| 5c | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinski, D.; Szpecht, A.; Hinc, P.; Smiglak, M. Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA. Molecules 2023, 28, 892. https://doi.org/10.3390/molecules28020892
Zielinski D, Szpecht A, Hinc P, Smiglak M. Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA. Molecules. 2023; 28(2):892. https://doi.org/10.3390/molecules28020892
Chicago/Turabian StyleZielinski, Dawid, Andrea Szpecht, Paulina Hinc, and Marcin Smiglak. 2023. "Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA" Molecules 28, no. 2: 892. https://doi.org/10.3390/molecules28020892
APA StyleZielinski, D., Szpecht, A., Hinc, P., & Smiglak, M. (2023). Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA. Molecules, 28(2), 892. https://doi.org/10.3390/molecules28020892






