Abstract
The crystal structures of two isomeric triiodo derivatives of ortho-carborane containing substituents in the three most electron-withdrawing positions of the carborane cage, 1,2,3-I3-1,2-C2B10H9, and the three most electron-donating positions, 8,9,12-I3-1,2-C2B10H9, as well as the crystal structure of 8,9,12-Br3-1,2-C2B10H9, were determined by single-crystal X-ray diffraction. In the structure of 1,2,3-I3-1,2-C2B10H9, an iodine atom attached to the boron atom (position 3) donates its lone pairs simultaneously to the σ-holes of both iodine atoms attached to the carbon atoms (positions 1 and 2) with the I⋯I distance of 3.554(2) Å and the C-I⋯I and B-I⋯I angles of 169.2(2)° and 92.2(2)°, respectively. The structure is additionally stabilized by a few B-H⋯I-shortened contacts. In the structure of 8,9,12-I3-1,2-C2B10H9, the I⋯I contacts of type II are very weak (the I⋯I distance is 4.268(4) Å, the B8-I8⋯I12 and B12-I12⋯I8 angles are 130.2(3)° and 92.2(3)°) and can only be regarded as dihalogen bonds formally. In comparison with the latter, the structure of 8,9,12-Br3-1,2-C2B10H9 demonstrates both similarities and differences. No Br⋯Br contacts of type II are observed, while there are two Br⋯Br halogen bonds of type I.
1. Introduction
The ability of halogens to form complexes with various electron pair donors was discovered over two hundred years ago [1,2,3], and the Nobel Prize laureate Odd Hassel provided crystallographic proof for the existence of such a bond, interpreting it as a charge-transfer interaction more than fifty years ago [4,5]. However, only at the beginning of the 21st century has halogen bonding grown from a scientific curiosity to one of the most interesting and actively studied non-covalent interactions for the construction of supramolecular assemblies [6,7,8,9,10].
This progress has been largely due to a better understanding of the principles on which the strength of the halogen bond depends. The performance of the halogen bond largely depends on the degree of polarization of the halogen atom; that is, the greater the positive electrostatic potential of the σ-hole, the more efficient the halogen bond donor will be [10,11,12]. The value of the positive potential of the σ-hole depends on the ability of the halogen atom to be polarized, which decreases in the following order: I > Br > Cl >> F [13,14]. The value of the positive potential of the σ-hole can be enhanced due to the electron-withdrawing ability of the fragment to which the halogen atom is attached.
For a halogen atom to be an electron acceptor in order to form a halogen bond, it must be bonded to an electron-withdrawing atom or group. Therefore, the sp hybridization of carbon atoms bearing a halogen is favored over sp2 followed by sp3 hybridization [15,16]. The hybridization of the carbon atom can be compensated by the electron-withdrawing effect of fluorine atoms, as evidenced by the close values of the σ-hole potential of the corresponding iodine atoms in 1-iodoethynyl-4-iodobenzene and 1,4-diiodoperfluoro-benzene (172 and 169 kJ/mol, respectively) [16]. The strength of the halogen bond is highly correlated with the degree of iodobenzene fluorination [17]. Therefore, it is not surprising that 1,4-diiodoperfluorobenzene and its analogs are widely used in the design of halogen-bonded supramolecular systems [18,19,20,21,22,23,24,25,26,27,28], although arylacetylene iodides also play an important role [16,29,30,31,32,33,34,35]. In the absence of other electron density donors, the iodine atoms in these compounds are also able to play this role, which leads to the formation of I⋯I dihalogen bonds [36,37,38,39], and the number of such bonds, as a rule, increases with the number of iodine atoms in the molecule [40].
Icosahedral carboranes C2B10H12 are another class of compounds whose derivatives are promising as halogen bond donors. The predicted strength of the halogen bonds with the same electron donor (based on the σ-hole potential) is larger for C-vertex halogen-substituted carboranes than for their organic aromatic counterparts [41,42,43]. In contrast to the iodo aromatics, wherein all iodine atoms are equivalent, in the iodo derivatives of ortho-carborane iodine atoms, depending on their position, they can act preferentially as an acceptor or a donor of a halogen bond. A typical example is 1,12-diodo-ortho-carborane, in which one of the iodine atoms is bonded to the most electron-withdrawing position of the carborane cage (position 1), and the second to the most electron-donating position (position 12) (Figure 1) [44]. The first of them is an electron acceptor, and the last one is a donor, which form an ideal intermolecular I⋯I dihalogen bond of type II [45].
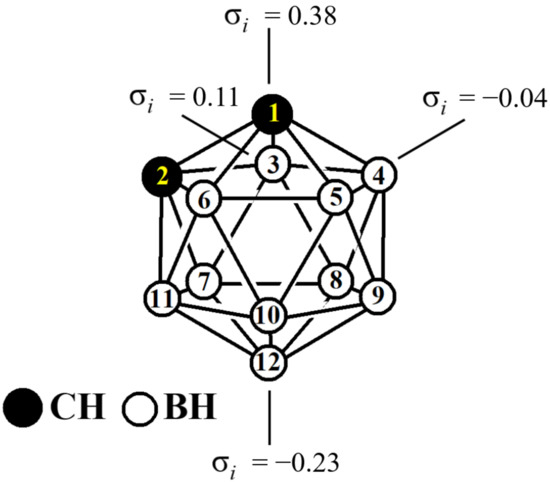
Figure 1.
Atom numbering atoms and Hammett constants σi in ortho-carborane.
In this contribution, we studied intermolecular bonding in two isomers of triiodo-ortho-carborane containing substituents in the three most electron-withdrawing positions of the carborane cage (1,2,3) and the three most electron-donating positions (8,9,12); in addition, a comparative analysis of the crystal packings of the 8,9,12-triiodo and 8,9,12-tribromo derivatives of ortho-carborane was performed.
2. Results and Discussion
To date, a number of iodo derivatives of ortho-carborane have been synthesized, and the structures of a dozen of them have been established by single-crystal X-ray diffraction. The derivatives with a high degree of substitution such as 8,9,10,12-I4-1,2-C2B10H8 [46], 4,5,7,8,9,10,11,12-I8-1,2-C2B10H4 [47], and 3,4,5,6,7,8,9,10,11,12-I10-1,2-C2B10H2 [47], as in the case of iodo-aromatics, are characterized by the formation of numerous intermolecular I⋯I dihalogen bonds varying from 3.74 to 4.05 Å. In contrast to the polyiodo derivatives, no intermolecular dihalogen bonds were found in any of the isomeric monoiodo derivatives of ortho-carborane 1-I-1,2-C2B10H11 [45], 3-I-1,2-C2B10H11 [48], 8-I-1,2-C2B10H11 [49], and 9-I-1,2-C2B10H11 [47].
As for the diiodo derivatives of ortho-carborane, the presence of intermolecular I⋯I dihalogen bonds inside them depends on the position of the substituents. In addition to 1,12-diiodo-ortho-carborane 1,12-I2-1,2-C2B10H10, which is characterized by the presence of strong intermolecular I⋯I dihalogen bonds (3.57 Å) [45], weak I⋯I dihalogen bonds (4.09 Å) were found in the 3,6-diiodo derivative 3,6-I2-1,2-C2B10H10 [50], while the 3,10-I2-1,2-C2B10H10 [49], 4,7-I2-1,2-C2B10H10 [51], and 9,12-I2-1,2-C2B10H10 [52] isomers do not form dihalogen bonds. Therefore, we were interested in studying the possibility of the formation of intermolecular I⋯I dihalogen bonds in triiodo-ortho-carboranes containing substituents in the three most electron-withdrawing positions of the caborane cage 1,2,3-I3-1,2-C2B10H9 and the three most electron-donating positions of 8,9,12-I3-1,2-C2B10H9.
The formation of 8,9,12-I3-1,2-C2B10H9 (1) was previously reported in the iodination of ortho-carborane with molecular iodine in acetic acid in the presence of a mixture of concentrated sulfuric and nitric acids [53]. We isolated the 8,9,12-triiodo derivative as a by-product of the reaction of ortho-carborane with iodine in dichloromethane in the presence of AlCl3 [54]. It should be noted that the unit cell parameters of 8,9,12-I3-1,2-C2B10H9 (1) have been reported [55]; however, its structure has not been yet solved.
The crystal structure of 8,9,12-I3-1,2-C2B10H9 was determined by single-crystal X-ray diffraction. A general view of 1 is presented in Figure 2. All the B-I distances in 8,9,12-I3-1,2-C2B10H9 are nearly equal (B8-I8 is 2.165(7)Å, B9-I9 is 2.160(7) Å, and B12-I12 is 2.160(7) Å) and are only slightly longer than the B-I distances in 8,9,10,12-I4-1,2-C2B10H8 (for which the average value is 2.151 Å) [46].
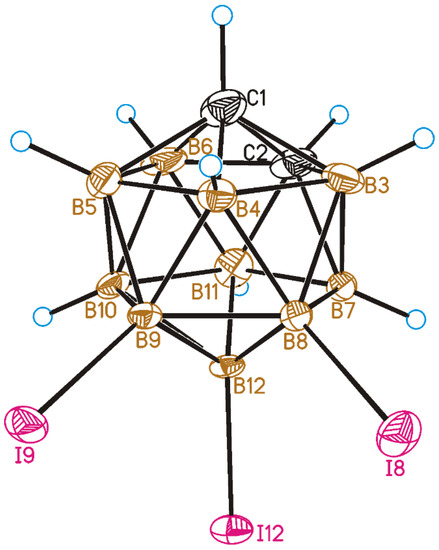
Figure 2.
General view of 8,9,12-I3-1,2-C2B10H9 (1) showing atomic numbering. Thermal ellipsoids are given at 50% probability level.
A crystal-packing fragment of 1 is depicted in Figure 3. Only weak intermolecular interactions are observed in the crystal structure. From a formal point of view, four types of intermolecular interactions are observed in the crystal of 1. Halogen atoms participate in both types (I and II) of halogen bonding, and I⋯H-C(B) hydrogen bonds as well as B-H⋯H-B contacts are formed. It should be noted that all intermolecular contacts except for one are somewhat longer than the sum of the van-der-Waals radii. For instance, the type II halogen bond is very weak (the I⋯I distance is 4.268(4) Å, the B8-I8⋯I12 angle is 130.2(3)°, and the B12-I12⋯I8 angle is 92.2(3)°) (Figure 3) and can only be regarded as a type II halogen bond formally. At the same time, the I9⋯I9 halogen bond of type I demonstrates an interhalogen distance (4.002(4) Å) shorter than the sum of the van-der-Waals radii (4.14 Å) [56]; however, halogen bonds of this type are usually relatively weak.
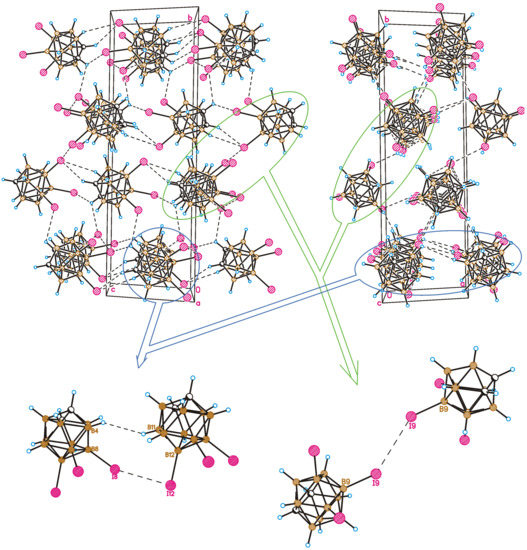
Figure 3.
Crystal-packing fragment of 8,9,12-I3-1,2-C2B10H9. Top-left: view in the bc crystallographic plane; top-right: view in the bc crystallographic plane. Detailed view of type II weak halogen bonding (bottom-left) and type I halogen bonding (bottom-right).
Therefore, it is impossible to choose one or two of the most important contacts that can be considered to be structure-forming. Interactions in the bc crystallographic plane are due to I⋯H-C(B) and H⋯H contacts, while in the crystallographic direction, a, molecules are linked mostly by I⋯I interactions. As a result, the crystal packing of 8,9,12-I3-1,2-C2B10H9 can be considered to be nearly isotropic.
It would be interesting to compare the crystal packing of 8,9,12-triiodo-ortho-carborane with that of its closest analog, 8,9,12-tribromo-ortho-carborane 8,9,12-Br3-1,2-C2B10H9 (2). Despite the fact that the bromination of ortho-carborane was first described as early as the mid-1960s [57], the chemistry of the bromo-derivatives of carborane has been studied to a much lesser extent compared to its iodo-derivatives due to the difficulty in isolating pure products. Recently, we published the synthesis and characterization of the 9,12-dibromo derivative of ortho-carborane [58]. Since the 8,9,12-tribromo derivative was one of the side-products of that reaction, we decided to increase the ratio of bromine to ortho-carborane (up to 3:1) and the reaction time. This allowed us to isolate the desired compound 8,9,12-Br3-1,2-C2B10H9 (2) at a 17% yield (see Section 3.3) It should be noted that the signal of the CH carborane groups of in the 1H NMR spectrum in CDCl3, which is a convenient indicator of the CH-acidity of carboranes [59,60], for compound 2 appears in a higher field at 3.87 ppm. compared to compound 1 (4.13 ppm). This indicates a lower acidity of the CH-carborane groups in the 8,9,12-tribromo derivative compared to the 8,9,12-triiodo derivative.
The crystal structure of 8,9,12-Br3-1,2-C2B10H9 was determined by single-crystal X-ray diffraction. A general view of 2 is presented in Figure 4.
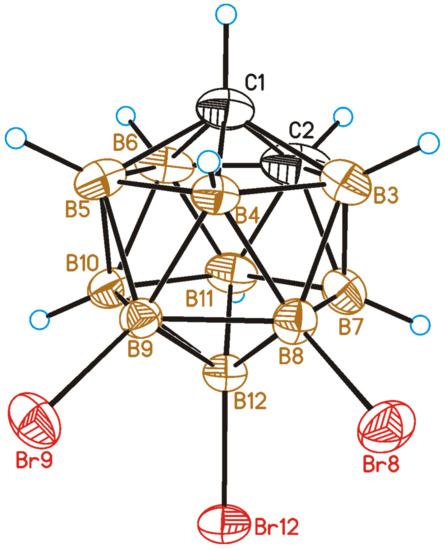
Figure 4.
General view of 8,9,12-Br3-1,2-C2B10H9 (2) showing atomic numbering. Thermal ellipsoids are given at 50% probability level.
It should be noted that the structure of 8,9,12-tibromo-ortho-carborane was determined in 1966 [61] at room temperature. The quality of that experiment was evidently low, and the experiment itself mostly concentrated on the description of the compound’s molecular geometry. Therefore, in the present study, we redetermined its structure at a low temperature (120 K), focusing on both its molecular structure and, especially, crystal-packing properties. Prior to the description of its crystal structure and comparison with that of 1, it is interesting to mention some other studied bromo- and iodo-derivatives of ortho-carborane. For instance, the crystal structures of 1,2-Me2-8,9,10,12-I4-1,2-C2B10H6 [47] and 1,2-Me2-8,9,10,12-Br4-1,2-C2B10H6 [62] are isostructural. At the same time, the crystal structures of 1,12-I2-1,2-C2B10H10 [45] and 1,12-Br2-C2B10H10 [42] do not show any similarity. Only partial similarity in terms of crystal packing was observed for 9,12-I2-1,2-C2B10H10 [52] and 9,12-Br2-1,2-C2B10H10 [58]; however, the latter appeared to be isostructural to its chloro analog 9,12-Cl2-1,2-C2B10H10 [63] (see Figure S10 in SI).
A comparison of the crystal structures of 2 and 1 studied in this work demonstrates both similarities and differences. As in compound 1, a Br9⋯Br9 halogen bond of type I is observed in the crystal structure of 8,9,12-Br3-1,2-C2B10H9 (the Br⋯Br distance is 3.586(2) Å, which is shorter than the sum of the van-der-Waals radii 3.79 Å) (Figure 5). At the same time, there are no type II halogen bonds; however, one more halogen bond of type I is found between Br8 atoms, wherein the Br⋯Br distance (3.969(2) Å) is somewhat longer than the sum of the van-der-Waals radii. As in compound 1, all the other intermolecular interactions are Br⋯H-C(B) and H⋯H. The differences in the crystal-packing properties described above result in some redistribution of the contact types (Figure 6): the contribution of Hal⋯Hal contacts increases, which leads to a decrease in the number of Hal⋯H contacts and to an increase in H⋯H ones.
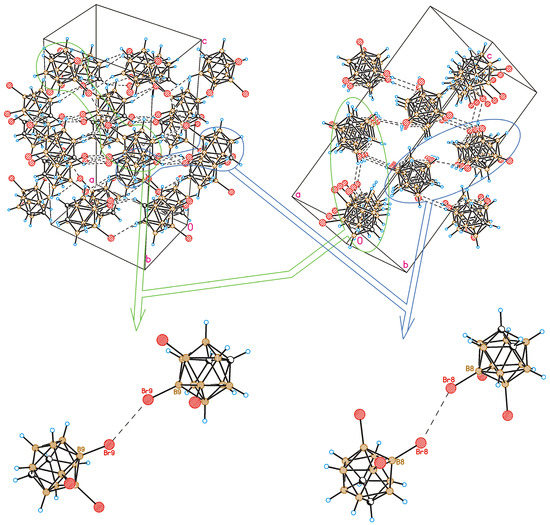
Figure 5.
Crystal-packing fragment of 8,9,12-Br3-1,2-C2B10H9. Top-left: view down [1,−1,0] direction; top-right: view down [0,1,1] direction. Bottom: detailed view of type I halogen bonding (Br9⋯Br9, left; Br8⋯Br8, right). Projections of crystal packing are chosen to be consistent with those in Figure 3 and Figure 7 (orientations of molecules are nearly the same).
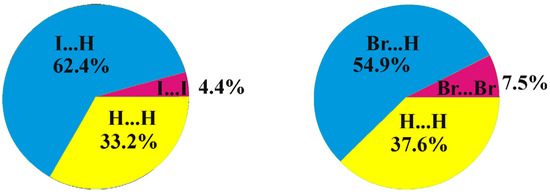
Figure 6.
Distribution of intermolecular contacts in the crystal structures of 8,9,12-I3-1,2-C2B10H9 (left) and 8,9,12-Br3-1,2-C2B10H9 (right) as obtained using the Crystal Explorer program package [64].
The observed similarities and dissimilarities in the crystal packing of 8,9,12-I3-1,2-C2B10H9 and 8,9,12-Br3-1,2-C2B10H9 can be clearly seen in Figure 7. Similar C-H⋯I(Br)-bonded chains are formed in one direction, while in the perpendicular plane, the relative orientation of molecules is somewhat different.
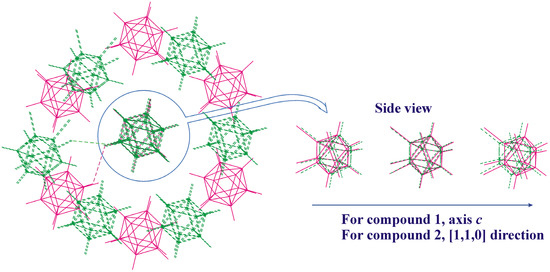
Figure 7.
Superimposition of the closest environment of the crystal structure of compounds 1 (magenta) and 2 (green). The I9⋯I9 and Br9⋯Br9 halogen bonds (of type I) are shown by dashed lines on the left-side view.
The 1,2,3-isomer 1,2,3-I3-1,2-C2B10H9 (3) was prepared by the deprotonation of 3-iodo-ortho-carborane followed by a treatment of molecular iodine (see below). The crystal structure of 1,2,3-I3-1,2-C2B10H9 was determined by single-crystal X-ray diffraction. A general view of 3 is presented in Figure 8. The molecule in the crystal occupies a special position, as it is located at the two-fold symmetry axis. The C-I distances are the same (due to symmetry) and equal to 2.103(4) Å, while the B-I bond is somewhat longer at 2.160(5) Å. These lengths are slightly shorter than the C1-I1 (2.121(2) Å) and B12-I12 (2.179(2) Å) bonds in 1,12-I2-closo-C2B10H10 [45].
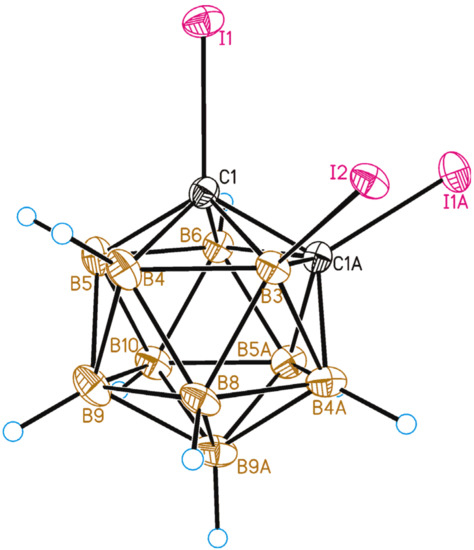
Figure 8.
General view of 1,2,3-I3-1,2-C2B10H9 (2) showing atomic numbering. Thermal ellipsoids are given at 50% probability level.
Contrary to 8,9,12-I3-1,2-C2B10H9, the crystal packing of 1,2,3-I3-1,2-C2B10H9 is formed by halogen-bonded planes parallel to the bc crystallographic plane (Figure 9). In the planes, the I2 atom (attached to the boron atom) donates its lone pairs simultaneously to the σ-holes of both iodine atoms attached to the carbon atoms (the I1⋯I2 distance is 3.554(2) Å, the C1-I1⋯I2 angle is 169.2(2)°, and the B3-I2⋯I1 angle is 92.2(2)°). Therefore, the main structure-forming unit is the trimeric halogen-bonded associate.
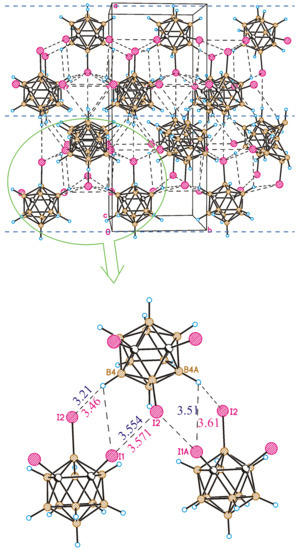
Figure 9.
(Top) Crystal-packing fragment of 1,2,3-I3-1,2-C2B10H9. Blue, dashed lines separate halogen-bonded planes. (Bottom) Halogen-bonded trimer as a structure-forming unit of 1,2,3-I3-1,2-C2B10H9. Blue and red values correspond to the experimental and calculated I⋯I and H⋯I distances, respectively (given in Å).
In our recent study, we theoretically compared the dimer formation of 1,12- and 1,3-diiodo-ortho-carboranes [45]. According to our calculations, it appeared that both dimers are stabilized by a type II halogen bond and B-H⋯I hydrogen bonds. The role of the halogen bond is more pronounced in both dimers; however, in the 1,3-isomer, the halogen bond is weaker (but only by 2.5 kJ/mol), while the hydrogen bonds are stronger (in total by 0.4 kcal/mol). This means that the probability of the formation of a type II halogen bond in a real crystal of 1,3-I2-1,2-C2B10H10 is somewhat low. Nevertheless, it is formed and is a structure-forming interaction in the crystal structure of 1,2,3-I3-1,2-C2B10H9. Indeed, there are no H⋯H shortened contacts. The structure is additionally stabilized by a few B-H⋯I shortened contacts. However, some of them are formed between molecules already linked by halogen bonds. For a better understanding of the intermolecular connection in the trimers, we optimized its structure using density functional theory (DFT) at the PBE0/def2tzvp level followed by a topological analysis of the calculated electron density in terms of the “Atoms in Molecules” theory [65]. The intermolecular interaction energies were estimated from their correlation with the potential energy density at the bond critical point [66,67] using the AIMAll program [68].
This method of investigating structural details was successfully utilized in our recent studies on noncovalent interactions [69,70,71]. Good agreement was obtained between the calculated and experimental structures. The interhalogen distances are nearly the same (Figure 9), and the calculated angles C1-I1⋯I2 (168.2°) and B3-I2⋯I1 (90.5°) also strongly agree with the experiment. The H⋯I distances are somewhat shorter, as predicted by theory. According to the calculations, the energy of the halogen bond is equal to 8.8 kJ/mol, while the energies of the H4⋯I1 and H4⋯I2 contacts are 2.5 and 2.1 kJ/mol, respectively. Therefore, the attraction energy of each two molecules in the layer is equal to (8.8 + 2.5 + 2.1) 13.4 kJ/mol, while only weak B-H⋯I contacts are observed between layers. This allows us to consider the crystal packing of compound 3 as anisotropic unlike the 8,9,12-isomer. It is interesting to note that the crystal density of the latter is somewhat higher than that of the 1,2,3-isomer. This can be explained by the increased role of the I⋯I interactions (Figure 10). The presence of relatively strong I⋯I intermolecular interactions does not allow molecules to adjust their orientations to obtain closer packing.
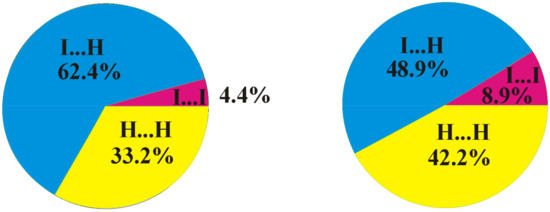
Figure 10.
Distribution of intermolecular contacts in the crystal structures of 8,9,12-I3-1,2-C2B10H9 (left) and 1,2,3-I3-1,2-C2B10H9 (right).
The same reasons can be used to explain the higher density of water in comparison to ice, and have also been used to explain the differences in the crystal-packing density of polynitro compounds [72,73].
3. Materials and Methods
3.1. General Methods
The reactions were carried under an inert atmosphere. 3-Iodo-ortho-carborane was prepared according to a procedure from the literature [74]. 1,2-Dimethoxyethane was dried using standard procedures [75]. All other chemical reagents were purchased from Sigma Aldrich, Acros Organics, and ABCR and used without purification. The reaction progress was monitored by thin-layer chromatography (Merck F254 silica gel on aluminum plates) and visualized using 0.5% PdCl2 in 1% HCl in aq. MeOH (1:10). Acros Organics silica gel (0.060–0.200 mm) was used for column chromatography. The NMR spectra at 400 MHz (1H) and 128 MHz (11B) were recorded with Varian Inova 400 spectrometer. The residual signal of the NMR solvent relative to Me4Si was taken as the internal reference for 1H spectra. 11B NMR spectra were referenced using BF3·Et2O as external standard.
3.2. Preparation of 8,9,12-Triiodo-ortho-Carborane 8,9,12-I3-1,2-C2B10H9
8,9,12-I3-ortho-C2B10H9 was isolated as a by-product from the di-iodination reaction of ortho-carborane under standard conditions [51]. Iodine (3.553 g, 14.00 mmol) and anhydrous AlCl3 (0.400 g) were added to a solution of ortho-carborane (1.009 g, 7.00 mmol) in dichloromethane (30 mL) and heated under reflux for 8 h. Then, the reaction mixture was cooled and treated with a solution of Na2S2O3·5H2O (3.000 g) in water (50 mL). The organic phase was separated, and the aqueous fraction was extracted with dichloromethane (3 × 50 mL). The organic phases were combined, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica using diethyl ether as eluent to yield 1.900 g (69%) of 9,12-I2-1,2-C2B10H10 and 0.102 g (3%) of 8,9,12-I3-1,2-C2B10H9 as white powders.
8,9,12-I3-1,2-C2B10H9: 1H NMR (CDCl3, ppm): 4.13 (2H, br s, CHcarb), 3.8−2.0 (7H, br m, BH). 11B NMR (CDCl3, ppm): δ −6.1 (1B, d, J = 157 Hz), −11.5 (4B, s + d), −13.1 (2B, d, J = 171 Hz), −14.7 (1B, d, J = 220 Hz), −16.4 (1B, d, J = 220 Hz), and −17.2 (1B, s, B(8)).
3.3. Preparation of 8,9,12-Tribromo-ortho-Carborane 8,9,12-Br3-1,2-C2B10H9
Bromine (1.08 mL, 3.356 g, and 21.00 mmol) and anhydrous aluminum chloride (0.400 g) were added to a solution of ortho-carborane (1.009 mg and 7.00 mmol) in 1,2-dichloroethane (30 mL) and heated under reflux for 40 h. Then, the reaction mixture was cooled and treated with a solution of Na2S2O3·5H2O (5.000 g) in water (50 mL). The organic phase was separated, and the aqueous fraction was extracted with dichloromethane (3 × 50 mL). The organic phases were combined, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica using chloroform as eluent to yield 0.450 g (17%) of 8,9,12-Br3-1,2-C2B10H9 as a white powder.
8,9,12-Br3-1,2-C2B10H9: 1H NMR (CDCl3, ppm): 3.87 (2H, br s, CHcarb), 3.5−1.7 (7H, br m, BH). 11B NMR (CDCl3, ppm): 0.4 (2B, s, B(9,12)), −5.3 (1B, s, B(8)), −8.4 (1B, d, J = 161 Hz, B(10)), −13.9 (2B, d, J = 176 Hz, B(4,7)), −15.6 (2B, d, J = 178 Hz, B(5,11)), −17.1 (1B, d, J = 188 Hz, B(3)), and −20.4 (1B, d, J = 185 Hz, B(6)). 13C NMR (CDCl3, ppm): 45.5 (CHcarb).
3.4. Preparation of 1,2,3-Triiodo-ortho-Carborane 1,2,3-I3-1,2-C2B10H9
A 2.25 M hexane solution of n-butyllithium (390 μL; 0.88 mmol) was added to a solution of 3-iodo-ortho-carborane (110 mg; 0.41 mmol) in 1,2-dimethoxyethane (10 mL) and stirred at room temperature for 1 h. Iodine (244 mg; 0.96 mmol) was added in one portion and the reaction mixture was stirred at room temperature overnight; then, it was treated with a solution of Na2S2O3·5H2O (250 mg) in water (50 mL). The organic phase was separated, and the aqueous fraction was extracted with dichloromethane (3 × 50 mL). The organic phases were combined, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica using diethyl ether as eluent to yield 137 mg of mixture of 3-I-1,2-C2B10H11 and 1,2,3-I3-1,2-C2B10H9.
3.5. Single-Crystal X-ray Diffraction Study
Single-crystal X-ray diffraction experiments of 1, 2, and 3 (see Supplementary Materials) were carried out using SMART APEX2 CCD diffractometer (λ(Mo-Kα) = 0.71073 Å; graphite monochromator; ω-scans) at 120 K. Collected data were processed by the SAINT and SADABS programs incorporated into the APEX2 program package [76]. The structures were determined by direct methods and refined by the full-matrix-least-squares procedure against F2 in anisotropic approximation. The refinement was carried out with the SHELXTL program [77]. The CCDC numbers (2216663 for 1, 2234154 for 2, and 2216664 for 3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
Crystallographic data for 8,9,12-I3-1,2-C2B10H9 (1): C2H9B10I3 are monoclinic; space group P21/n: a = 7.5776(6) Å, b = 24.0030(18) Å, c = 7.7535(6) Å, β = 109.487(2)°, V = 1329.46(18) Å3, and Z = 4, M = 521.89, dcryst = 2.607 g·cm−3. wR2 = 0.0794 calculated on F2hkl for all 2611 independent reflections with 2θ < 52.1° (GOF = 1.117, R = 0.0343 calculated on Fhkl for 2331 reflections with I > 2σ(I)).
Crystallographic data for 8,9,12-Br3-1,2-C2B10H9 (2): C2H9B10Br3 are monoclinic; space group C2/c: a = 12.1453(6) Å, b = 8.4794(5) Å, c = 23.0632(11) Å, β = 90.089(2)°, V = 2375.2(2) Å3, Z = 8, M = 380.92, dcryst = 2.131 g·cm−3. wR2 = 0.0797 calculated on F2hkl for all 2349 independent reflections with 2θ < 52.1° (GOF = 1.024, R = 0.0347 calculated on Fhkl for 1908 reflections with I > 2σ(I)).
Crystallographic data for 1,2,3-I3-1,2-C2B10H9 for (3): C2H9B10I3 are orthorhombic; space group Pnma: a = 19.1157(8) Å, b = 8.0014(3) Å, c = 8.7287(4) Å, V = 1335.08(10) Å3, Z = 4, M = 521.89, dcryst = 2.596 g·cm−3. wR2 = 0.0580 calculated on F2hkl for all 1739 independent reflections with 2θ < 56.2° (GOF = 1.143, R = 0.0224 calculated on Fhkl for 1613 reflections with I > 2σ(I)).
3.6. Quantum Chemical Calculation
Quantum chemical optimization of halogen-bonded trimeric associate of 1,2,3-I3-1,2-C2B10H9 was carried out using the Gaussian program [78]. The initial geometry for optimization was taken from the X-ray data. Optimization was carried out using PBE0 functional and triple-zeta basis set def2tzvp. For better agreement with experimental geometry, calculation was carried out within polarizable continuum model (PCM) using SCRF keyword in the Gaussian program and highly polar water molecule. It has recently been shown that such a method of calculation results in better agreement of the geometry for noncovalent interactions [45,69].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020875/s1; Crystallographic data for compounds 1, 2, and 3.
Author Contributions
X-ray diffraction experiment and manuscript writing, K.Y.S.; Synthesis, S.A.A.; General manuscript concept and manuscript writing, I.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (Grant No. 21-13-00345).
Data Availability Statement
Crystallographic data for the structures of 8,9,12-I3-1,2-C2B10H9 (1), 8,9,12-Br3-1,2-C2B10H9 (2), and 1,2,3-I3-1,2-C2B10H9 (3) were deposited in the Cambridge Crystallographic Data Centre as supplementary publications CCDC 2216663 (for 1), 2234154 (for 2), and 2216664 (for 3). The Supplementary Information contains crystallographic data for compounds 1, 2, and 3.
Acknowledgments
The single-crystal X-ray diffraction data were obtained using equipment from the Center for Molecular Structure Studies at A.N. Nesmeyanov Institute of Organoelement Compounds, operating with financial support from the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-00697-22-00).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Colin, J.J. Sur quelques combinaisons de l’iode. Ann Chim. 1814, 91, 252–272. [Google Scholar]
- Wisniak, J. Jean-Jacques Colin. Rev. CENIC Cienc. Biol. 2017, 48, 112–120. [Google Scholar]
- Guthrie, F. On the iodide of iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef]
- Turunen, L.; Hansen, J.H.; Erdélyi, M. Halogen bonding: An Odd chemistry? Chem. Rec. 2021, 21, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. (Eds.) Halogen Bonding: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J. The halogen bond: Nature and applications. Phys. Sci. Rev. 2017, 2, 20170136. [Google Scholar] [CrossRef]
- Frontera, F.; Bauzá, A. On the importance of σ-hole interactions in crystal structures. Crystals 2021, 11, 1205. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Lommerse, J.P.M.; Stone, A.J.; Taylor, R.; Allen, F.H. The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J. Am. Chem. Soc. 1996, 118, 3108–3116. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. The fluorine atom as a halogen bond donor, viz. a positive site. CrystEngComm 2011, 13, 6593–6596. [Google Scholar] [CrossRef]
- Le Questel, Y.-J.; Laurence, C.; Graton, J. Halogen-bond interactions: A crystallographic basicity scale towards iodoorganic compounds. CrystEngComm 2013, 15, 3212–3221. [Google Scholar] [CrossRef]
- Askeröy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular hierarchy among halogen-bond donors. Chem. Eur. J. 2013, 19, 16240–16247. [Google Scholar] [CrossRef] [PubMed]
- Präsang, C.; Whitwood, A.C.; Bruce, D.W. Halogen-bonded cocrystals of 4-(N,N-dimethylamino)pyridine with fluorinated iodobenzenes. Cryst. Growth Des. 2009, 9, 5319–5326. [Google Scholar] [CrossRef]
- Roper, L.C.; Präsang, C.; Kozhevnikov, V.N.; Whitwood, A.C.; Karadakov, P.B.; Bruce, D.W. Experimental and theoretical study of halogen-bonded complexes of DMAP with di- and triiodofluorobenzenes. A complex with a very short N···I halogen bond. Cryst. Growth Des. 2010, 10, 3710–3720. [Google Scholar] [CrossRef]
- Wang, H.; Jin, W.J. Cocrystal assembled by 1,4-diiodotetrafluorobenzene and phenothiazine based on C-I···π/N/S halogen bond and other assisting interactions. Acta Cryst. B 2017, 73, 210–216. [Google Scholar] [CrossRef]
- Christopherson, J.C.; Topic, F.; Barret, C.J.; Friscic, T. Halogen-bonded cocrystals as optical materials: Next generation control over light-matter interactions. Cryst. Growth Des. 2018, 18, 1245–1259. [Google Scholar] [CrossRef]
- Bedeković, N.; Stilinović, V.; Friščić, T.; Cinčić, D. Comparison of isomeric meta- and para-diiodotetrafluorobenzene as halogen bond donors in crystal engineering. New J. Chem. 2018, 42, 10584–10591. [Google Scholar] [CrossRef]
- Li, L.; Wu, W.X.; Liu, Z.F.; Jin, W.J. Effect of geometry factors on the priority of σ-hole···π and π-hole···π bond in phosphorescent cocrystals formed by pyrene or phenanthrene and trihaloperfluorobenzenes. New J. Chem. 2018, 42, 10633–10641. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Zhao, D.; Lu, X.; Lin, Y. Versatile supramolecular binding modes of 1,4-diiodotetrafluorobenzene for molecular cocrystal engineering. J. Mol. Struct. 2019, 1187, 132–137. [Google Scholar] [CrossRef]
- Grosu, I.G.; Pop, L.; Miclǎuş, M.; Hǎdade, N.D.; Terec, A.; Bende, A.; Socaci, C.; Barboiu, M.; Grosu, I. Halogen bonds (N···I) at work: Supramolecular catemeric architectures of 2,7-dipyridylfluorene with ortho-, meta-, or para-diiodotetrafluorobenzene isomers. Cryst. Growth Des. 2020, 20, 3429–3441. [Google Scholar] [CrossRef]
- Uran, E.; Fotović, L.; Bedeković, N.; Stilinović, V.; Cinčić, D. The amine group as halogen bond acceptor in cocrystals of aromatic diamines and perfluorinated iodobenzenes. Crystals 2021, 11, 529. [Google Scholar] [CrossRef]
- Nieland, E.; Komisarek, D.; Hohloch, S.; Wurst, K.; Vasylyeva, V.; Weingart, O.; Schmidt, B.M. Supramolecular networks by imine halogen bonding. Chem. Commun. 2022, 58, 5233–5236. [Google Scholar] [CrossRef]
- Hajjar, C.; Nag, T.; Al Sayed, H.; Ovens, J.S.; Bryce, D.L. Stoichiomorphic halogen-bonded cocrystals: A case study of 1,4-diiodotetrafluorobenzene and 3-nitropyridine. Can. J. Chem. 2022, 100, 245–251. [Google Scholar] [CrossRef]
- Yeo, C.I.; Tan, Y.S.; Kwong, H.C.; Lee, V.S.; Tiekink, E.R.T. I···N halogen bonding in 1: 1 co-crystals formed between 1,4-diiodotetrafluorobenzene and the isomeric n-pyridinealdazines (n = 2, 3 and 4): Assessment of supramolecular association and influence upon solid-state photoluminescence properties. CrystEngComm 2022, 24, 7579–7591. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J.; Đaković, M. Crystal engineering with iodoethynylnitrobenzenes: A group of highly effective halogen-bond donors. Cryst. Growth Des. 2015, 15, 3853–3861. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Welideniya, D.; Desper, J. Ethynyl hydrogen bonds and iodoethynyl halogen bonds: A case of synthon mimicry. CrystEngComm 2017, 19, 11–13. [Google Scholar] [CrossRef]
- Wijethunga, T.K.; Ðakovic, M.; Desper, J.; Aakeröy, C.B. A new tecton with parallel halogen-bond donors: A path to supramolecular rectangles. Acta Cryst. B 2017, 73, 163–167. [Google Scholar] [CrossRef]
- Fourmigue, M. Coordination chemistry of anions through halogen-bonding interactions. Acta Cryst. B 2017, 73, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Szell, P.M.J.; Gabidullin, B.; Bryce, D.L. 1,3,5-Tri(iodoethynyl)-2,4,6-trifluorobenzene: Halogen-bonded frameworks and NMR spectroscopic analysis. Acta Cryst. B 2017, 73, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Szell, P.M.J.; Siiskonen, A.; Catalano, L.; Cavallo, G.; Terraneo, G.; Priimagi, A.; Bryce, D.L.; Metrangolo, P. Halogen-bond driven self-assembly of triangular macrocycles. New J. Chem. 2018, 42, 10467–10471. [Google Scholar] [CrossRef]
- Bosch, E.; Kruse, S.J.; Groeneman, R.H. Infinite and discrete halogen bonded assemblies based upon 1,2-bis(iodoethynyl)benzene. CrystEngComm 2019, 21, 990–993. [Google Scholar] [CrossRef]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, polymorphism and mechanical properties of some hexahalogenated benzenes: The nature of halogen···halogen interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef]
- Raffo, P.A.; Suarez, S.; Fantoni, A.C.; Baggio, R.; Cukiernik, F.D. Polymorphism of a widely used building block for halogen-bonded assemblies: 1,3,5-trifluoro-2,4,6-triiodobenzene. Acta Cryst. C 2017, 73, 667–673. [Google Scholar] [CrossRef]
- Bartashevich, E.; Sobalev, S.; Matveychuk, Y.; Tsirelson, V. Variations of quantum electronic pressure under the external compression in crystals with halogen bonds assembled in Cl3-, Br3-, I3-synthons. Acta Cryst. B 2020, 76, 514–523. [Google Scholar] [CrossRef]
- Dominikowska, J.; Rybarczyk-Pirek, A.J.; Guerra, C.F. Lack of cooperativity in the triangular X3 halogen-bonded synthon? Cryst. Growth Des. 2021, 21, 597–607. [Google Scholar] [CrossRef]
- Saha, A.; Rather, S.A.; Sharada, D.; Saha, B.K. C–X···X–C vs C–H···X–C, which one is the more dominant interaction in crystal packing (X = halogen)? Cryst. Growth Des. 2018, 18, 6084–6090. [Google Scholar] [CrossRef]
- Lo, R.; Fanfrlík, J.; Lepšík, M.; Hobza, P. The properties of substituted 3D-aromatic neutral carboranes: The potential for σ-hole bonding. Phys. Chem. Chem. Phys. 2015, 17, 20814–20821. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Holub, J.; Růžičková, Z.; Řezáč, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Růžička, A.; Hobza, P. Competition between halogen, hydrogen and dihydrogen bonding in brominated carboranes. ChemPhysChem 2016, 17, 3373–3376. [Google Scholar] [CrossRef]
- Beau, M.; Lee, S.; Kim, S.; Han, W.-S.; Jeannin, O.; Fourmigué, M.; Aubert, E.; Espinosa, E.; Jeon, I.-R. Strong σ-hole activation on icosahedral carborane derivatives for a directional halide recognition. Angew. Chem. Int. Ed. 2021, 60, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.N.; Ol´shevskaya, V.A. Some aspects of the chemical behavior of icosahedral carboranes. Russ. Chem. Bull. 2008, 57, 815–836. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Anisimov, A.A.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. 1,12-Diiodo-ortho-carborane: A classic textbook example of the dihalogen bond. Crystals 2021, 11, 396. [Google Scholar] [CrossRef]
- Vaca, A.; Teixidor, F.; Kivekäs, R.; Sillanpää, R.; Viñas, C. A solvent-free regioselective iodination route of ortho-carboranes. Dalton Trans. 2006, 4884–4885. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.V.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Iodinated ortho-carboranes as versatile building blocks to design intermolecular interactions in crystal lattices. Chem. Eur. J. 2009, 15, 9764–9772. [Google Scholar] [CrossRef]
- Barberà, G.; Viñas, C.; Teixidor, F.; Rosair, G.M.; Welch, A.J. Self-assembly of carborane molecules via C–H···I hydrogen bonding: The molecular and crystal structures of 3-I-1,2-closo-C2B10H11. J. Chem. Soc. Dalton Trans. 2002, 3647–3648. [Google Scholar] [CrossRef]
- Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Kennedy, R.D.; Barnes, C.L.; Hawthorne, M.F. Unfairly forgotten member of the iodocarborane family: Synthesis and structural characterization of 8-iodo-1,2-dicarba-closo-dodecaborane, its precursors, and derivatives. Inorg. Chem. 2012, 51, 2629–2637. [Google Scholar] [CrossRef]
- Barbera, G.; Vaca, A.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Designed synthesis of new ortho-carborane derivatives: From mono- to polysubstituted frameworks. Inorg. Chem. 2008, 47, 7309–7316. [Google Scholar] [CrossRef]
- Ramachandran, B.M.; Knobler, C.B.; Hawthorne, M.F. Synthesis and structural characterization of symmetrical closo-4,7-I2-1,2-C2B10H10 and [(CH3)3NH][nido-2,4-I2-7,8-C2B9H10]. Inorg. Chem. 2006, 45, 336–340. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Fox, M.A.; Howard, J.A.K.; Hughes, A.K.; Johnson, A.L.; Martindale, S.J. 9,12-Diiodo-1,2-dicarba-closo-dodecaborane(12). Acta Cryst. A 2003, 59, O74–O76. [Google Scholar] [CrossRef] [PubMed]
- Rudakov, D.A.; Kurman, P.V.; Potkin, V.I. Synthesis and deborination of polyhalo-substituted ortho-carboranes. Russ. J. Gen. Chem. 2011, 81, 1137–1142. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, W.; Zinn, A.A.; Knobler, C.B.; Hawthorne, M.F. Facile electrophilic iodination of icosahedral carboranes. Synthesis of carborane derivatives with boron-carbon bonds via the palladium-catalyzed reaction of diiodocarboranes with Grignard reagents. Inorg. Chem. 1995, 34, 2095–2100. [Google Scholar] [CrossRef]
- Struchkov, Y.T.; Stanko, V.I.; Klimova, A.I.; Kon’kova, G.S. X-ray data on some derivatives of barene and neobarene. J. Struct. Chem. 1965, 8, 888–890. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorky, P.M. New applications of van der Waals radii in chemistry. Russ. Chem. Rev. 1995, 64, 415–428. [Google Scholar] [CrossRef]
- Smith, H.D.; Knowles, T.A.; Schroeder, H. Chemistry of decaborane-phosphorus compounds. V. Bromocarboranes and their phosphination. Inorg. Chem. 1965, 4, 107–111. [Google Scholar] [CrossRef]
- Zhidkova, O.B.; Druzina, A.A.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis and crystal structure of 9,12-dibromo-ortho-carborane. Molbank 2022, 2022, M1347. [Google Scholar] [CrossRef]
- Puga, A.V.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Arca, M.; Barbera, G.; Viñas, C. From mono- to poly-substituted frameworks: A way of tuning the acidic character of Cc-H in o-carborane derivatives. Chem. Eur. J. 2009, 15, 9755–9763. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Anufriev, S.A.; Shmalko, A.V. How substituents at boron atoms affect the CH-acidity and the electron-withdrawing effect of the ortho-carborane cage: A close look on the 1H NMR spectra. Inorg. Chim. Acta 2023, 547, 121339. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N. Molecular structure of carboranes. Molecular and crystal structure of o-B10Br3H7C2H2. Inorg. Chem. 1966, 5, 1478–1482. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N. Molecular structure of carboranes. Molecular and crystal structure of o-B10Br4H6C2(CH3)2. Inorg. Chem. 1966, 5, 1483–1488. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Zhidkova, O.B.; Suponitsky, K.Y.; Sivaev, I.B. Synthesis, crystal structure, and some transformations of 9,12-dichloro-ortho-carborane. Crystals 2022, 12, 1251. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, WA, Australia, 2017. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 15.05.18; TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Suponitsky, K.Y.; Burakov, N.I.; Kanibolotsky, A.L.; Mikhailov, V.A. Multiple noncovalent bonding in halogen complexes with oxygen organics. I. Tertiary amides. J. Phys. Chem. A 2016, 120, 4179–4190. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]-. Some evidences of BH···X hydride-halogen bonds in 9- XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The influence of halogen type on structural features of compounds containing α-halo-α,α-dinitroethyl moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]
- Gidaspov, A.A.; Zalomlenkov, V.A.; Bakharev, V.V.; Parfenov, V.E.; Yurtaev, E.V.; Struchkova, M.I.; Palysaeva, N.V.; Suponitsky, K.Y.; Lempertd, D.B.; Sheremetev, A.B. Novel trinitroethanol derivatives: High energetic 2-(2,2,2-trinitroethoxy)-1,3,5-triazines. RSC Adv. 2016, 6, 34921–34934. [Google Scholar] [CrossRef]
- Palysaeva, N.V.; Gladyshkin, A.G.; Vatsadze, I.A.; Suponitsky, K.Y.; Dmitriev, D.E.; Sheremetev, A.B. N-(2-Fluoro-2,2-dinitroethyl)azoles: Novel assembly of diverse explosophoric building block-s for energetic compounds design. Org. Chem. Front. 2019, 6, 249–255. [Google Scholar] [CrossRef]
- Zhao, D.; Xie, Z. [3-N2-o-C2B10H11][BF4]: A useful synthon for multiple cage boron functionalizations of o-carborane. Chem. Sci. 2016, 7, 5635–5639. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).