Recombinant Human Prolidase (rhPEPD) Induces Wound Healing in Experimental Model of Inflammation through Activation of EGFR Signalling in Fibroblasts
Abstract
1. Introduction
2. Results
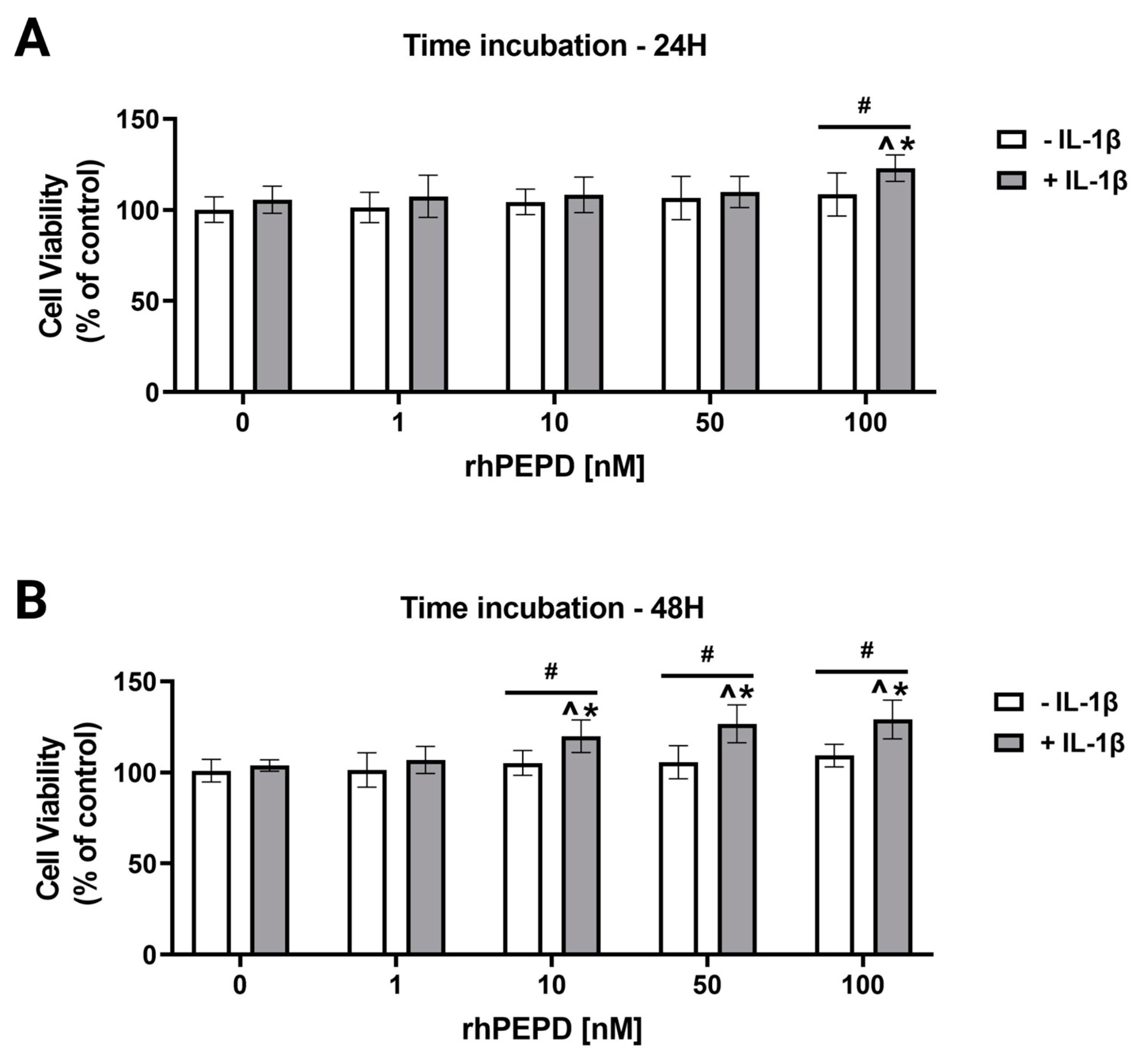
2.1. Recombinant Human Prolidase (rhPEPD) Induces Increase in Cell Viability in Experimental Model of Inflammation in Fibroblasts
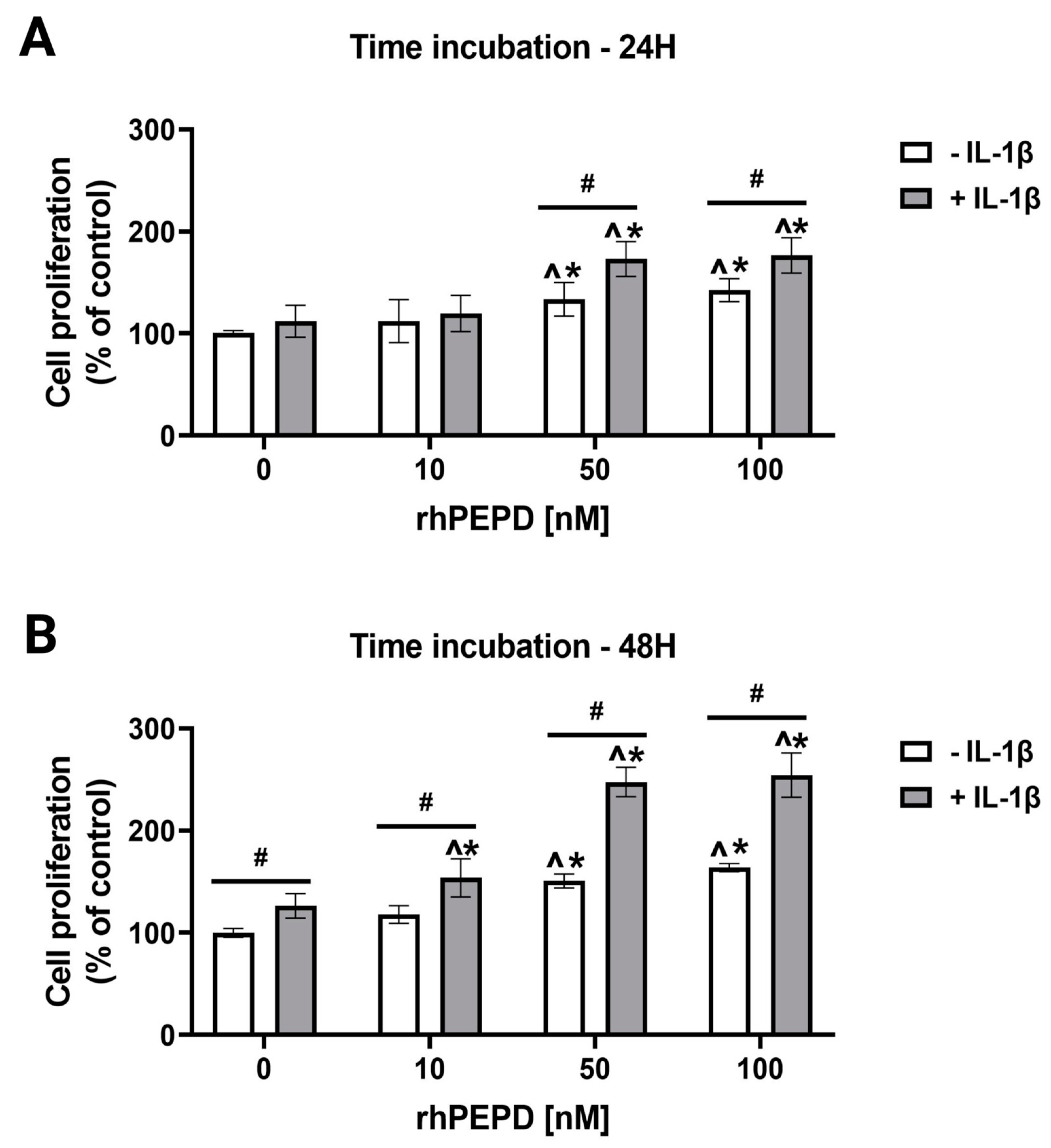
2.2. IL-1β Augments rhPEPD-Stimulated Fibroblast Proliferation
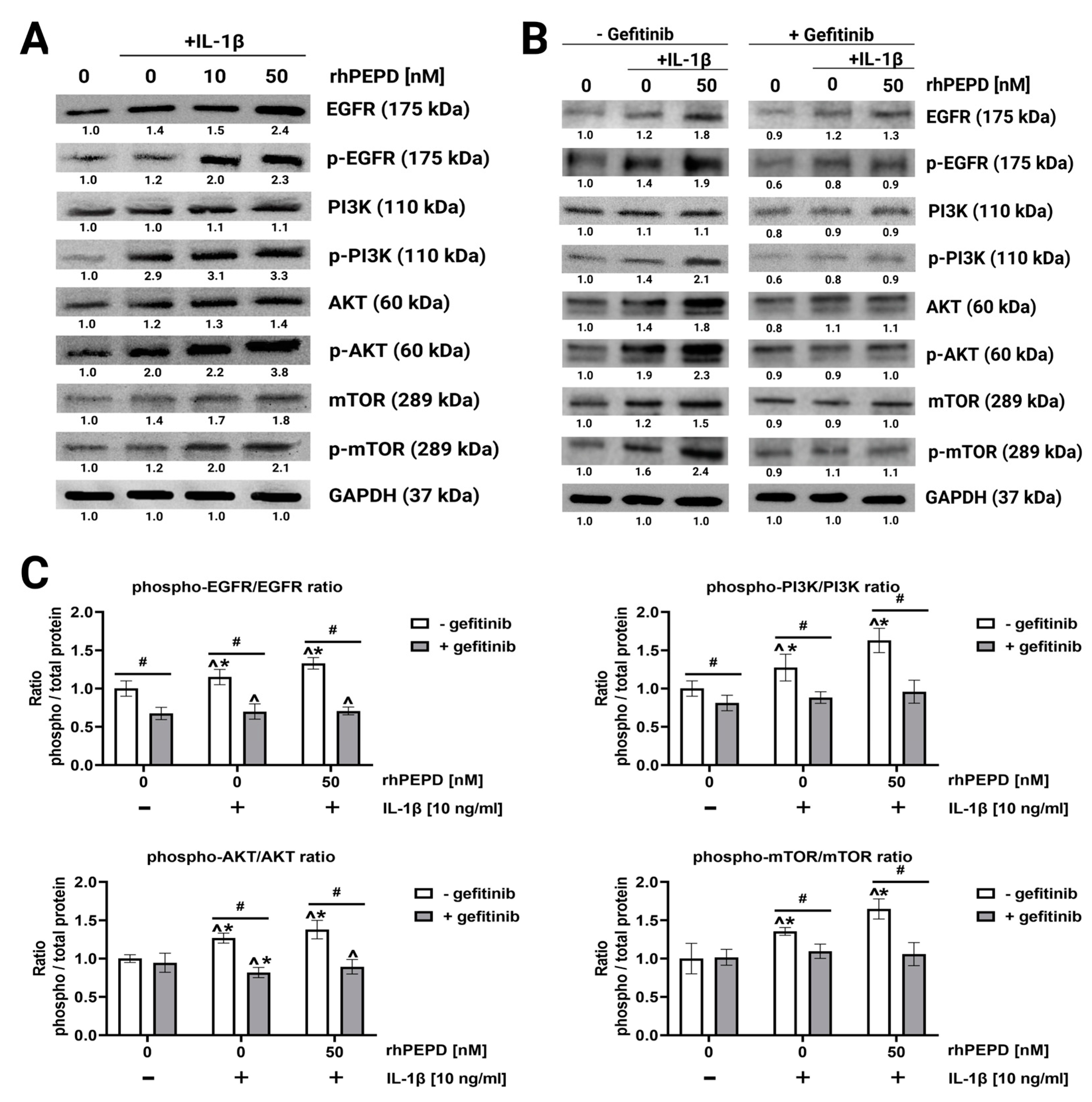
2.3. rhPEPD Augments IL-1β-Induced EGFR-Downstream Signalling in Fibroblasts
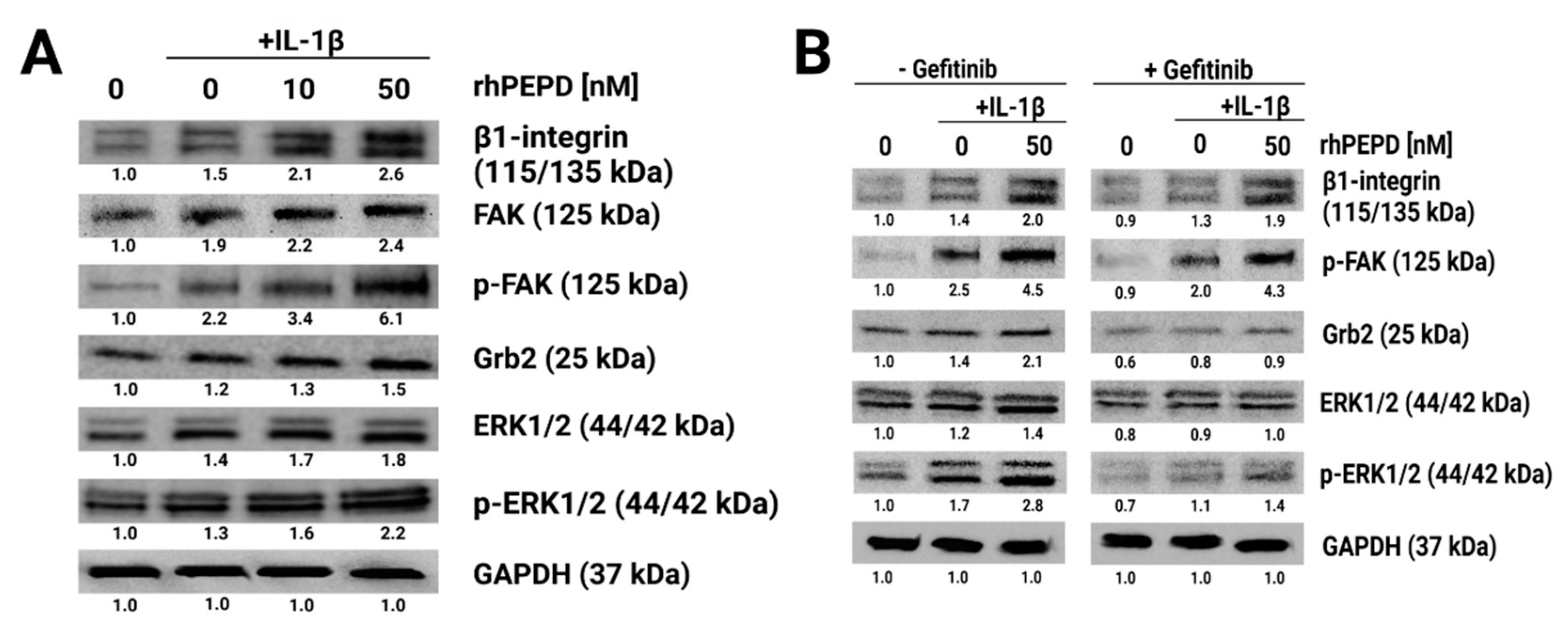
2.4. rhPEPD Augments IL-1β-Induced Expression of β1-Integrin Receptor Downstream Signaling Proteins in Fibroblasts
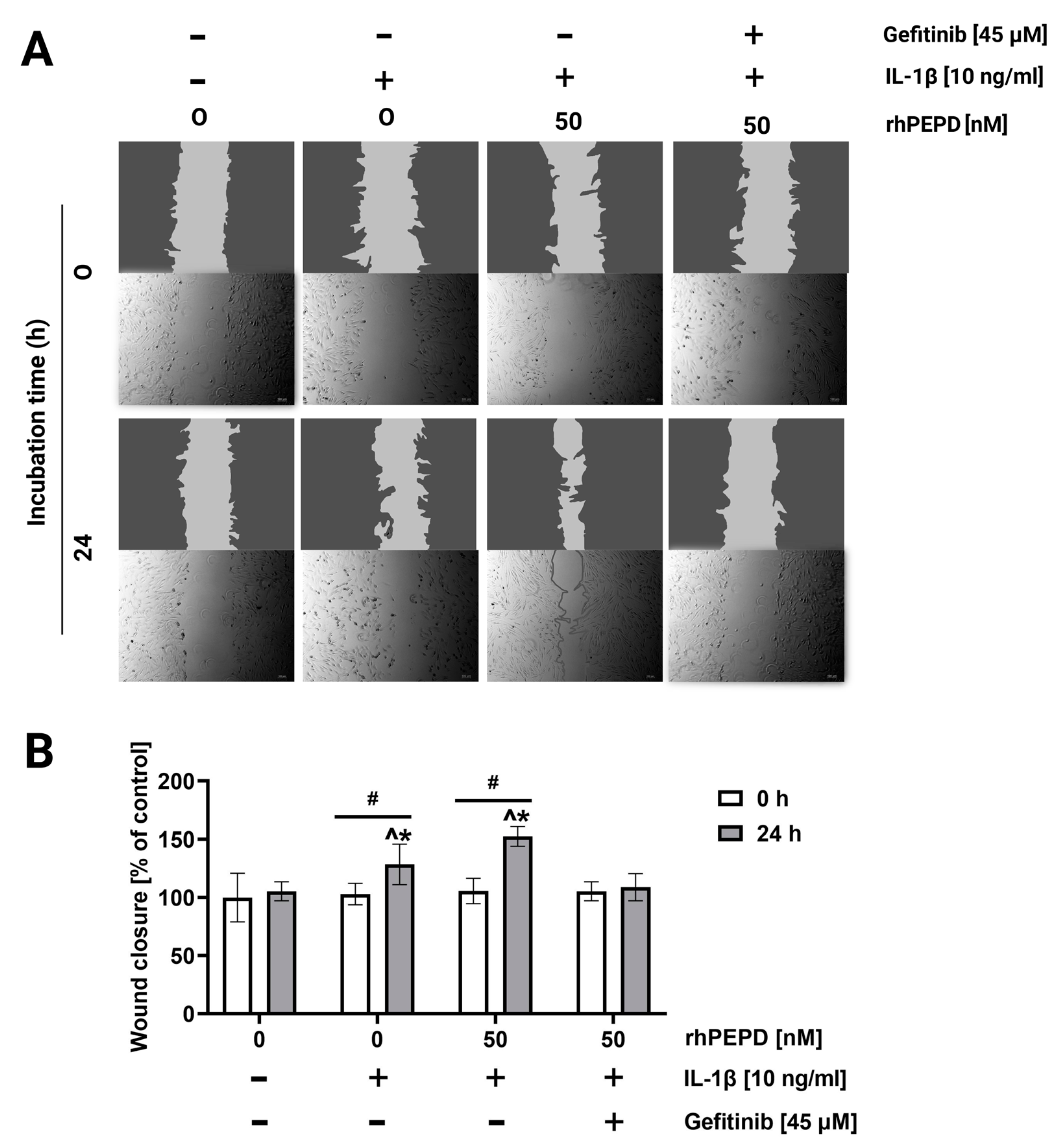
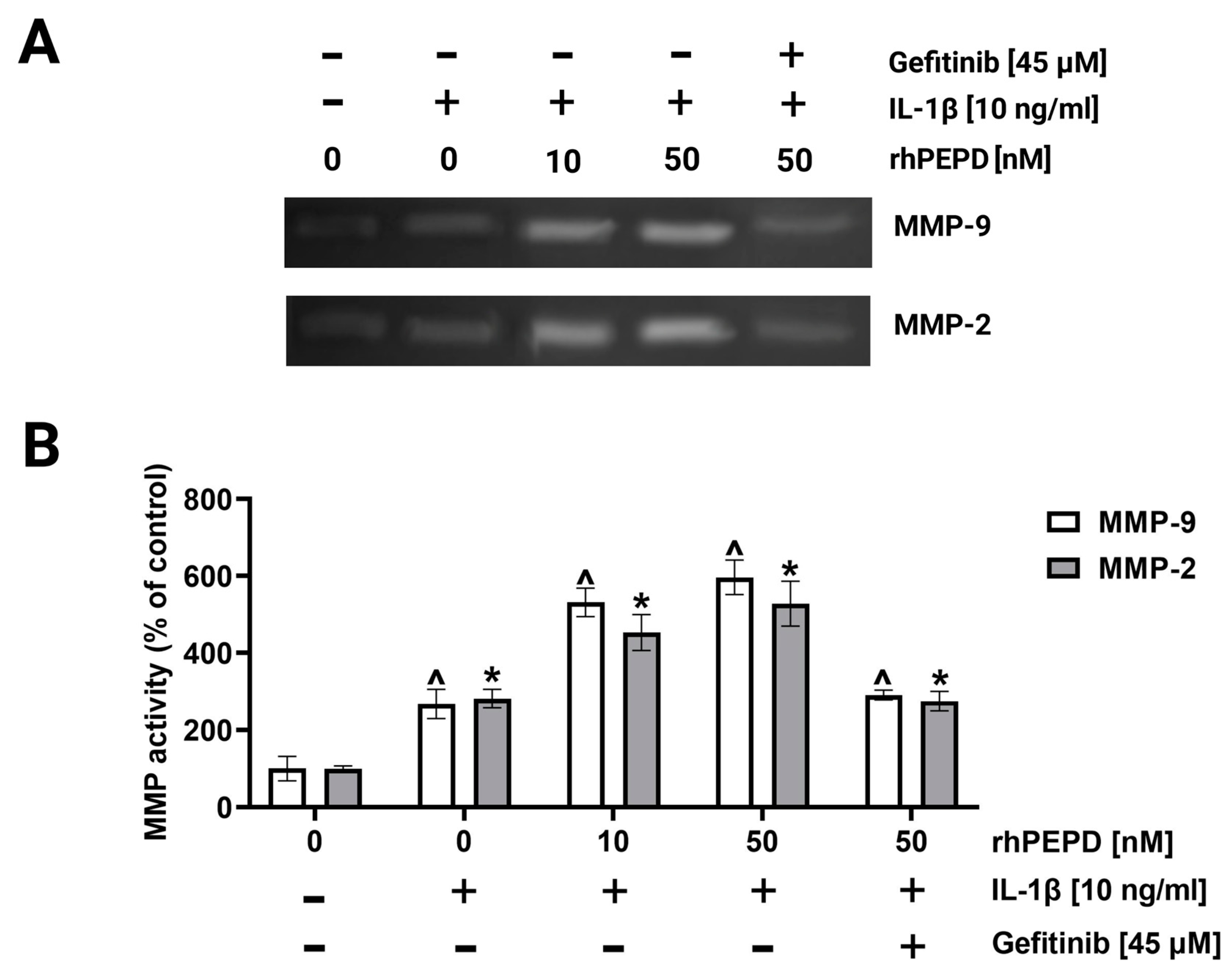
2.5. rhPEPD Promotes the Migration of Fibroblasts in a Model of Wound Healing and Activates MMP-2 and MMP-9
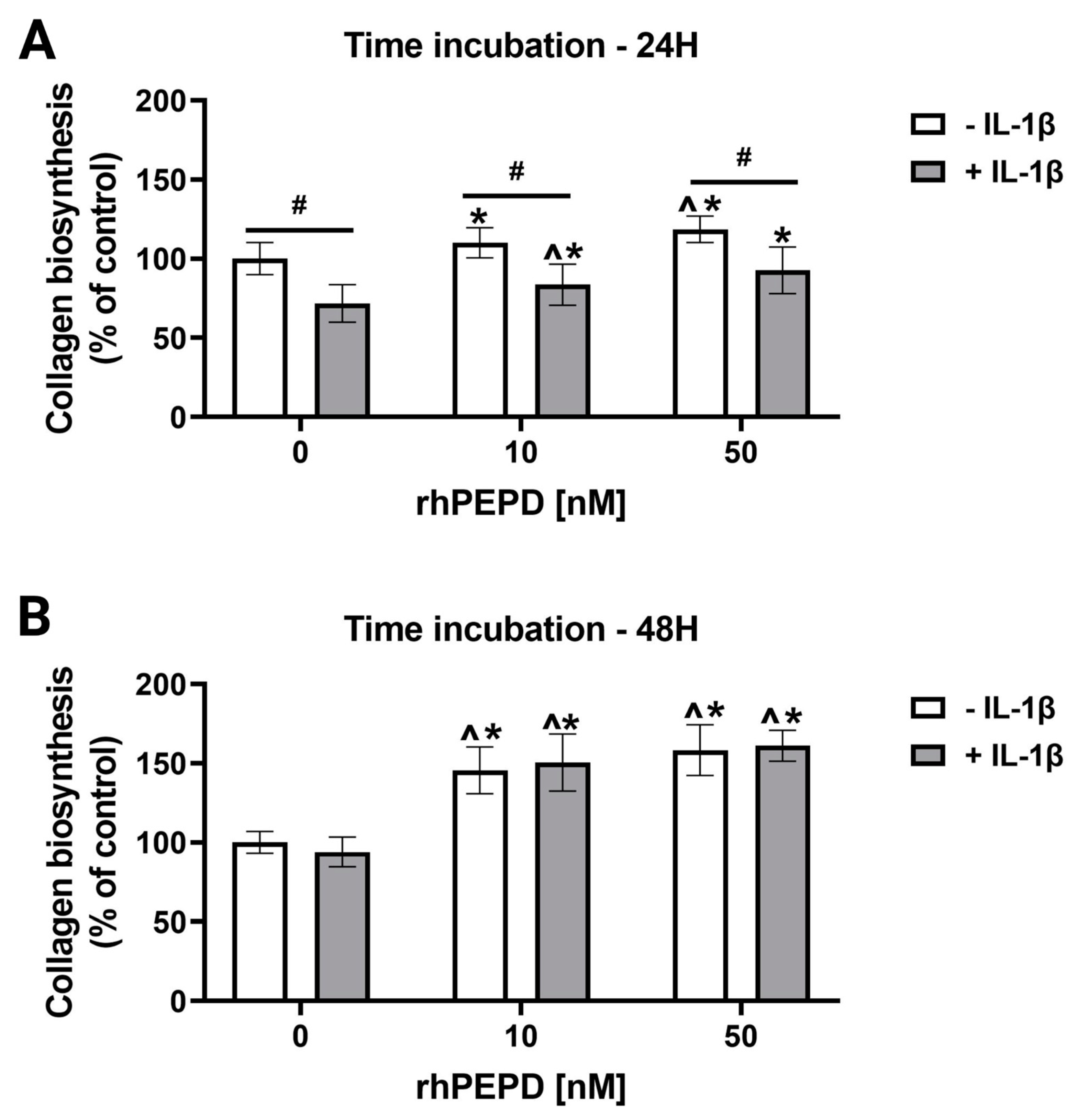
2.6. rhPEPD Augments Collagen Biosynthesis in IL-1β-Treated Fibroblasts
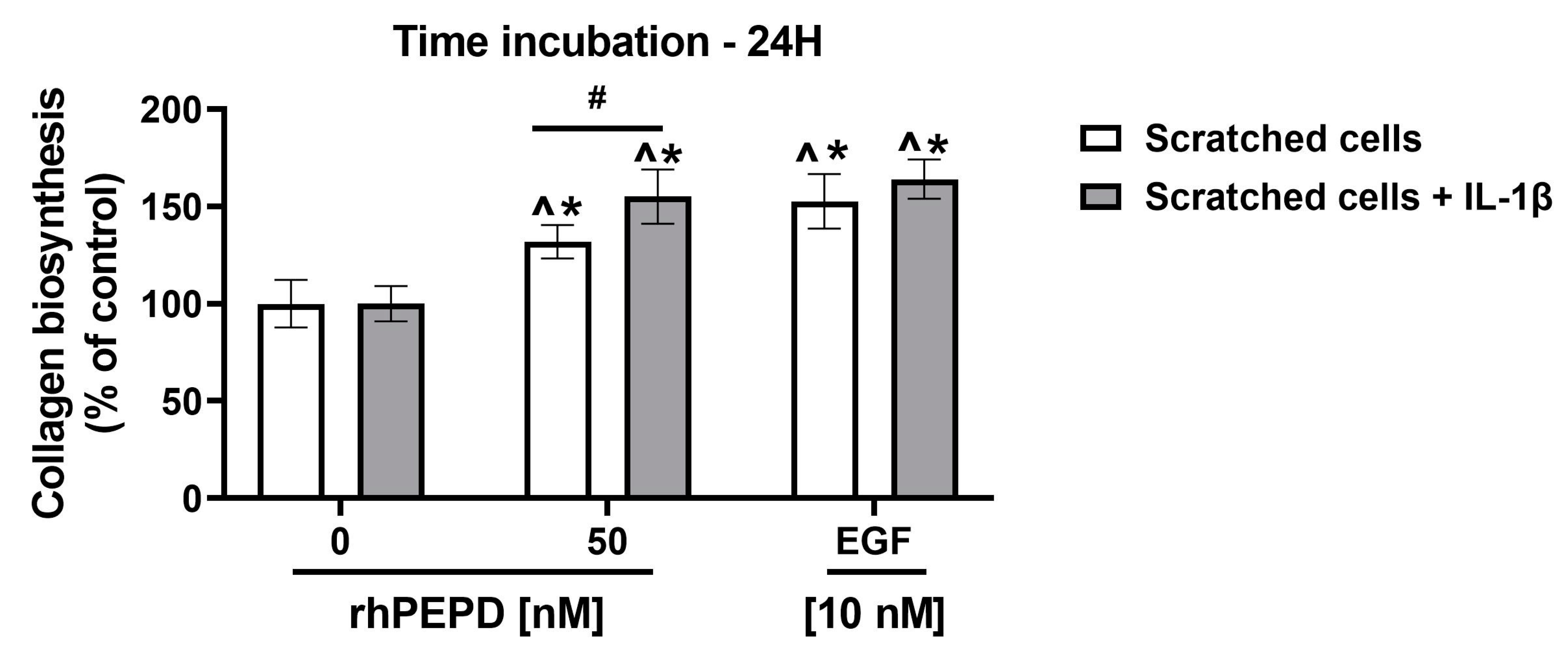
2.7. rhPEPD Stimulates Collagen Biosynthesis in IL-1β-Treated Fibroblasts in a Model of Wound Healing
3. Discussion
4. Materials and Methods
4.1. Fibroblasts Cell Cultures
4.2. Production of Recombinant Human Prolidase
4.3. Cell Viability Assay
4.4. Cell Proliferation Assay
4.5. Western Blot
4.6. Antibodies
4.7. Cell Migration Assay
4.8. Gelatin Zymography Assay
4.9. Evaluation of Collagen Biosynthesis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaron, A.; Naider, F. Proline-dependent structural and biological properties of peptides and proteins. Biochem. Mol. Biol. 1993, 28, 31–81. [Google Scholar] [CrossRef]
- Jackson, S.H.; Dennis, A.W.; Greenberg, M. Iminodipeptiduria: A genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can. Med. Assoc. J. 1975, 113, 759, 762–763. [Google Scholar]
- Palka, J.A.; Miltyk, W.; Karna, E.; Wolczynski, S. Modulation of prolidase activity during in vitro aging of human skin fibroblasts the role of extracellular matrix collagen. Tokai J. Exp. Clin. Med. 1996, 21, 207–213. [Google Scholar]
- Yang, L.; Li, Y.; Ding, Y.; Choi, K.S.; Kazim, A.L.; Zhang, Y. Prolidase directly binds and activates epidermal growth factor receptor and stimulates downstream signaling. J. Biol. Chem. 2013, 288, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Scriver, C.R. Glycyl-Proline in Urine of Humans with Bone Disease. Can. J. Physiol. Pharmacol. 1964, 42, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.I.; Solomons, C.C.; Muschenheim, F.; McIntyre, C.A.; Miles, B.; O’Brien, D. A syndrome resembling lathyrism associated with iminodipeptiduria. Am. J. Med. 1968, 45, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.F.; Maniscalco, R.M. Bound hydroxyproline excretion following gelatin loading in prolidase deficiency. Metabolism 1976, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S. Studies on a patient with iminodipeptiduria. II. Lack of prolidase activity in blood cells. Physiol. Chem. Phys. 1978, 10, 279–283. [Google Scholar]
- Isemura, M.; Hanyu, T.; Gejyo, F.; Nakazawa, R.; Igarashi, R.; Matsuo, S.; Ikeda, K.; Sato, Y. Prolidase deficiency with imidodipeptiduria. A familial case with and without clinical symptoms. Clin. Chim. Acta 1979, 93, 401–407. [Google Scholar] [CrossRef]
- Freij, B.J.; Levy, H.L.; Dudin, G.; Mutasim, D.; Deeb, M.; Der Kaloustian, V.M. Clinical and biochemical characteristics of prolidase deficiency in siblings. Am. J. Med. Genet. 1984, 19, 561–571. [Google Scholar] [CrossRef]
- Pierard, G.E.; Cornil, F.; Lapiere, C.M. Pathogenesis of ulcerations in deficiency of prolidase. The role of angiopathy and of deposits of amyloid. Am. J. Dermatopathol. 1984, 6, 491–497. [Google Scholar] [CrossRef]
- Misiura, M.; Guszczyn, T.; Oscilowska, I.; Baszanowska, W.; Palka, J.; Miltyk, W. Platelet-Rich Plasma Promotes the Proliferation of Human Keratinocytes via a Progression of the Cell Cycle. A Role of Prolidase. Int. J. Mol. Sci. 2021, 22, 936. [Google Scholar] [CrossRef]
- Misiura, M.; Baszanowska, W.; Oscilowska, I.; Palka, J.; Miltyk, W. Prolidase Stimulates Proliferation and Migration through Activation of the PI3K/Akt/mTOR Signaling Pathway in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 9243. [Google Scholar] [CrossRef]
- Baszanowska, W.; Misura, M.; Oscilowska, I.; Palka, J.; Miltyk, W. Extracellular Prolidase (PEPD) Induces Anabolic Processes through EGFR, β1-integrin, and IGF-1R Signaling Pathways in an Experimental Model of Wounded Fibroblasts. Int. J. Mol. Sci. 2021, 22, 942. [Google Scholar] [CrossRef]
- Niziol, M.; Oscilowska, I.; Baszanowska, W.; Palka, J.; Besio, R.; Forlino, A.; Miltyk, W. Recombinant Prolidase Activates EGFR-Dependent Cell Growth in an Experimental Model of Inflammation in HaCaT Keratinocytes. Implication for Wound Healing. Front. Mol. Biosci. 2022, 9, 876348. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Rose, L.F.; Chan, R.K. The Burn Wound Microenvironment. Adv. Wound Care 2016, 5, 106–118. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kim, K.Y. Inhibition of Proinflammatory Cytokines in Cutibacterium Acnes-Induced Inflammation in HaCaT Cells by Using Buddleja Davidii Aqueous Extract. Int. J. Inflam. 2020, 2020, 8063289. [Google Scholar] [CrossRef]
- Palka, J.; Adelmann-Grill, B.C.; Francz, P.I.; Bayreuther, K. Differentiation stage and cell cycle position determine the chemotactic response of fibroblasts. Folia Histochem. Cytobiol. 1996, 34, 121–127. [Google Scholar]
- DesJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and Wound Healing: An Update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Lurje, G.; Lenz, H.J. EGFR signaling and drug discovery. Oncology 2009, 77, 400–410. [Google Scholar] [CrossRef]
- Labat-Robert, J.; Robert, L. Interaction between cells and extracellular matrix: Signaling by integrins and the elastin-laminin receptor. Prog. Mol. Subcell Biol. 2000, 25, 57–70. [Google Scholar]
- Palka, J.A.; Phang, J.M. Prolidase activity in fibroblasts is regulated by interaction of extracellular matrix with cell surface integrin receptors. J. Cell Biochem. 1997, 67, 166–175. [Google Scholar] [CrossRef]
- Ivaska, J.; Reunanen, H.; Westermarck, J.; Koivisto, L.; Kähäri, V.M.; Heino, J. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J. Cell Biol. 1999, 147, 401–416. [Google Scholar] [CrossRef]
- Juliano, R.L.; Haskill, S. Signal transduction from the extracellular matrix. J. Cell Biol. 1993, 120, 577–585. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Surazynski, A.; Liu, Y.; Miltyk, W.; Phang, J.M. Nitric oxide regulates prolidase activity by serine/threonine phosphorylation. J. Cell Biochem. 2005, 96, 1086–1094. [Google Scholar] [CrossRef]
- Tyler, J.A.; Bolis, S.; Dingle, J.T.; Middleson, J.F.S. Mediators of matrix catabolism. In Articular Cartilage and Osteoarthritis; Kuettner, K.E., Schleyerbach, R., Peyron, J.G., Hascall, V.C., Eds.; Raven Press: New York, NY, USA, 1992; pp. 251–264. [Google Scholar]
- Tyler, J.A. Articular cartilage cultured with catabolin (pig interleukin I) synthesized a decreased number of normal proteoglycan molecules. Biochem. J. 1985, 227, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Aydelotte, M.; Raiss, R.; Caterson, B.; Kuettner, K. Influence of interleukin-1 on the metabolism of proteoglycans and morphology of cultured bovine articular chondrocytes. Connect. Tissue Res. 1992, 8, 59–143. [Google Scholar] [CrossRef] [PubMed]
- Beekman, B.; Verzijl, N.; de Roos, J.A.; TeKoppele, J.M. Matrix degradation by chondrocytes cultured in alginate: IL-1 beta induces proteoglycan degradation and proMMP synthesis but does not result in collagen degradation. Osteoarthr. Cartil. 1998, 6, 330–340. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The Crucial Role of Protein Phosphorylation in Cell Signaling and its Use as Targeted Therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Palka, J.A.; Karna, E.; Miltyk, W. Fibroblast chemotaxis and prolidase activity modulation by insulin-like growth factor II and mannose 6-phosphate. Mol. Cell Biochem. 1997, 168, 177–183. [Google Scholar]
- Miltyk, W.; Palka, J.A. Potential role of pyrroline 5-carboxylate in regulation of collagen biosynthesis in cultured human skin fibroblasts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Rodriguez-Cuenca, S.; Rouault, C.; Figueroa-Juarez, E.; Schilbert, H.; Virtue, S.; Moreno-Navarrete, J.M.; Bidault, G.; Vázquez-Borrego, M.C.; Dias, A.R.; et al. Dysregulation of macrophage PEPD in obesity determines adipose tissue fibro-inflammation and insulin resistance. Nat. Metab. 2022, 4, 476–494. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef]
- Oscilowska, I.; Huynh, T.Y.L.; Baszanowska, W.; Prokop, I.; Surazynski, A.; Galli, M.; Zabielski, P.; Palka, J. Proline oxidase silencing inhibits p53-dependent apoptosis in MCF-7 breast cancer cells. Amino Acids 2021, 53, 1943–1956. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Yoshida, A.; Kanno, H.; Watabe, D.; Akasaka, T.; Sawai, T. The Role of Heparin-Binding EGF-like Growth Factor and Amphiregulin in the Epidermal Proliferation of Psoriasis in Cooperation with TNFα. Arch. Dermatol. Res. 2008, 300, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]
- Lee, S.H.; Zahoor, M.; Hwang, J.K.; Min, D.S.; Choi, K.Y. Valproic acid induces cutaneous wound healing in vivo and enhances keratinocyte motility. PLoS ONE 2012, 7, e48791. [Google Scholar] [CrossRef]
- Rao, T.C.; Ma, P.Y.; Blanchard, A.; Urner, T.M.; Mattheyses, A.L. EGFR activation attenuates the mechanical threshold for integrin tension and focal adhesion formation. J. Cell Sci. 2020, 133, jcs238840. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, A.; Cordes, N. Growth factor receptor and β1 integrin signaling differentially regulate basal clonogenicity and radiation survival of fibroblasts via a modulation of cell cycling. In Vitro Cell. Dev. Biol.-Anim. 2022, 58, 169–178. [Google Scholar] [CrossRef]
- Surazynski, A.; Miltyk, W.; Palka, J.; Phang, J.M. Prolidase-dependent regulation of collagen biosynthesis. Amino Acids 2008, 35, 731–738. [Google Scholar] [CrossRef]
- Guszczyn, T.; Surazynski, A.; Zareba, I.; Rysiak, E.; Popko, J.; Palka, J. Differential effect of platelet-rich plasma fractions on β1-integrin signaling, collagen biosynthesis, and prolidase activity in human skin fibroblasts. Drug Des. Dev. Ther. 2017, 11, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.P.; Brisset, M.; Jourdan, C.; Bocquet, J.; Jouis, V. Effect of a mononuclear cell factor (MCF) on collagen production in cultured articular chondrocytes: Role of prostaglandin E2. Biochem. Biophys. Res. Commun. 1984, 119, 499–508. [Google Scholar] [CrossRef]
- Benton, H.P.; Tyler, J.A. Inhibition of cartilage proteoglycan synthesis by interleukin 1. Biochem. Biophys. Res. Commun. 1988, 154, 421–428. [Google Scholar] [CrossRef]
- Gowen, M.; Wood, D.D.; Ihrie, E.J.; Meats, J.E.; Russel, R.G.G. Stimulation by human interleukin-1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim. Biophys. Acta 1984, 797, 186–193. [Google Scholar] [CrossRef]
- Dingle, J.T. Mechanism of cartilage destruction and repair: The outlook. Cliniquide Rheum. 1993, 3, 1–5. [Google Scholar]
- Martel-Pelletier, J. Patophysiology of osteoarthritis. Osteoarthr. Cartil. 1998, 6, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, D. Aging and osteoarthritis: Basic mechanisms. J. Am. Geriatr. Soc. 1993, 41, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Basak, P.; Prasad Sinha, B.; Maurye, P.; Kumar Jaiswal, K.; Das, P.; Kumar Mandal, T. Anti-inflammatory effect of epidermal growth factor conjugated silk fibroin immobilized polyurethane ameliorates diabetic burn wound healing. Int. J. Biol. Macromol. 2020, 143, 1009–1032. [Google Scholar] [CrossRef]
- Liu, L.; Song, S.; Zhang, Y.P.; Wang, D.; Zhou, Z.; Chen, Y.; Jin, X.; Hu, C.F.; Shen, C.X. Amphiregulin promotes cardiac fibrosis post myocardial infarction by inducing the endothelial-mesenchymal transition via the EGFR pathway in endothelial cells. Exp. Cell Res. 2020, 390, 111950. [Google Scholar] [CrossRef]
- Overstreet, J.M.; Wang, Y.; Wang, X.; Niu, A.; Gewin, L.S.; Yao, B.; Harris, R.C.; Zhang, M.Z. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J. 2017, 31, 4407–4421. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone Promotes the Cutaneous Wound Healing Process via Activation of the PI3K/AKT, Wnt/beta-catenin and NF-kappaB Signaling Pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem. Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Kitchener, R.L.; Grunden, A.M. Prolidase function in proline metabolism and its medical and biotechnological applications. J. Appl. Microbiol. 2012, 113, 233–247. [Google Scholar] [CrossRef]
- Karaszewski, J.; Zareba, I.; Guszczyn, T.; Darewicz, B.; Palka, J. Verapamil and collagenase differentially affect collagen metabolism in experimental model of Peyronie’s disease. Mol. Cell Probes. 2020, 49, 101488. [Google Scholar] [CrossRef]
- Lupi, A.; Rossi, A.; Campari, E.; Pecora, F.; Lund, A.M.; Elcioglu, N.H.; Gultepe, M.; Di Rocco, M.; Cetta, G.; Forlino, A. Molecular characterisation of six patients with prolidase deficiency: Identification of the first small duplication in the prolidase gene and of a mutation generating symptomatic and asymptomatic outcomes within the same family. J. Med. Genet. 2006, 43, e58. [Google Scholar] [CrossRef]
- Besio, R.; Gioia, R.; Cossu, F.; Monzani, E.; Nicolis, S.; Cucca, L.; Profumo, A.; Casella, L.; Tenni, R.; Bolognesi, M.; et al. Kinetic and Structural Evidences on Human Prolidase Pathological Mutants Suggest Strategies for Enzyme Functional Rescue. PLoS ONE 2013, 8, e58792. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zareba, I.; Surazynski, A.; Chrusciel, M.; Miltyk, W.; Doroszko, M.; Rahman, N.; Palka, J. Functional Consequences of Intracellular Proline Levels Manipulation Affecting PRODH/POX-Dependent Pro-Apoptotic Pathways in a Novel in Vitro Cell Culture Model. Cell Physiol. Biochem. 2017, 43, 670–684. [Google Scholar] [CrossRef]
- Wechselberger, C.; Doppler, C.; Bernhard, D. An Inexpensive Staining Alternative for Gelatin Zymography Gels. Methods Protoc. 2019, 2, 61. [Google Scholar] [CrossRef]
- Peterkofsky, B.; Diegelmann, R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry 1971, 10, 988–994. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baszanowska, W.; Niziol, M.; Oscilowska, I.; Czyrko-Horczak, J.; Miltyk, W.; Palka, J. Recombinant Human Prolidase (rhPEPD) Induces Wound Healing in Experimental Model of Inflammation through Activation of EGFR Signalling in Fibroblasts. Molecules 2023, 28, 851. https://doi.org/10.3390/molecules28020851
Baszanowska W, Niziol M, Oscilowska I, Czyrko-Horczak J, Miltyk W, Palka J. Recombinant Human Prolidase (rhPEPD) Induces Wound Healing in Experimental Model of Inflammation through Activation of EGFR Signalling in Fibroblasts. Molecules. 2023; 28(2):851. https://doi.org/10.3390/molecules28020851
Chicago/Turabian StyleBaszanowska, Weronika, Magdalena Niziol, Ilona Oscilowska, Justyna Czyrko-Horczak, Wojciech Miltyk, and Jerzy Palka. 2023. "Recombinant Human Prolidase (rhPEPD) Induces Wound Healing in Experimental Model of Inflammation through Activation of EGFR Signalling in Fibroblasts" Molecules 28, no. 2: 851. https://doi.org/10.3390/molecules28020851
APA StyleBaszanowska, W., Niziol, M., Oscilowska, I., Czyrko-Horczak, J., Miltyk, W., & Palka, J. (2023). Recombinant Human Prolidase (rhPEPD) Induces Wound Healing in Experimental Model of Inflammation through Activation of EGFR Signalling in Fibroblasts. Molecules, 28(2), 851. https://doi.org/10.3390/molecules28020851




