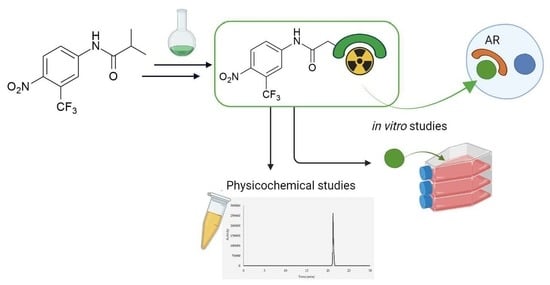
3.2. Syntheses of Flutamide Derivatives
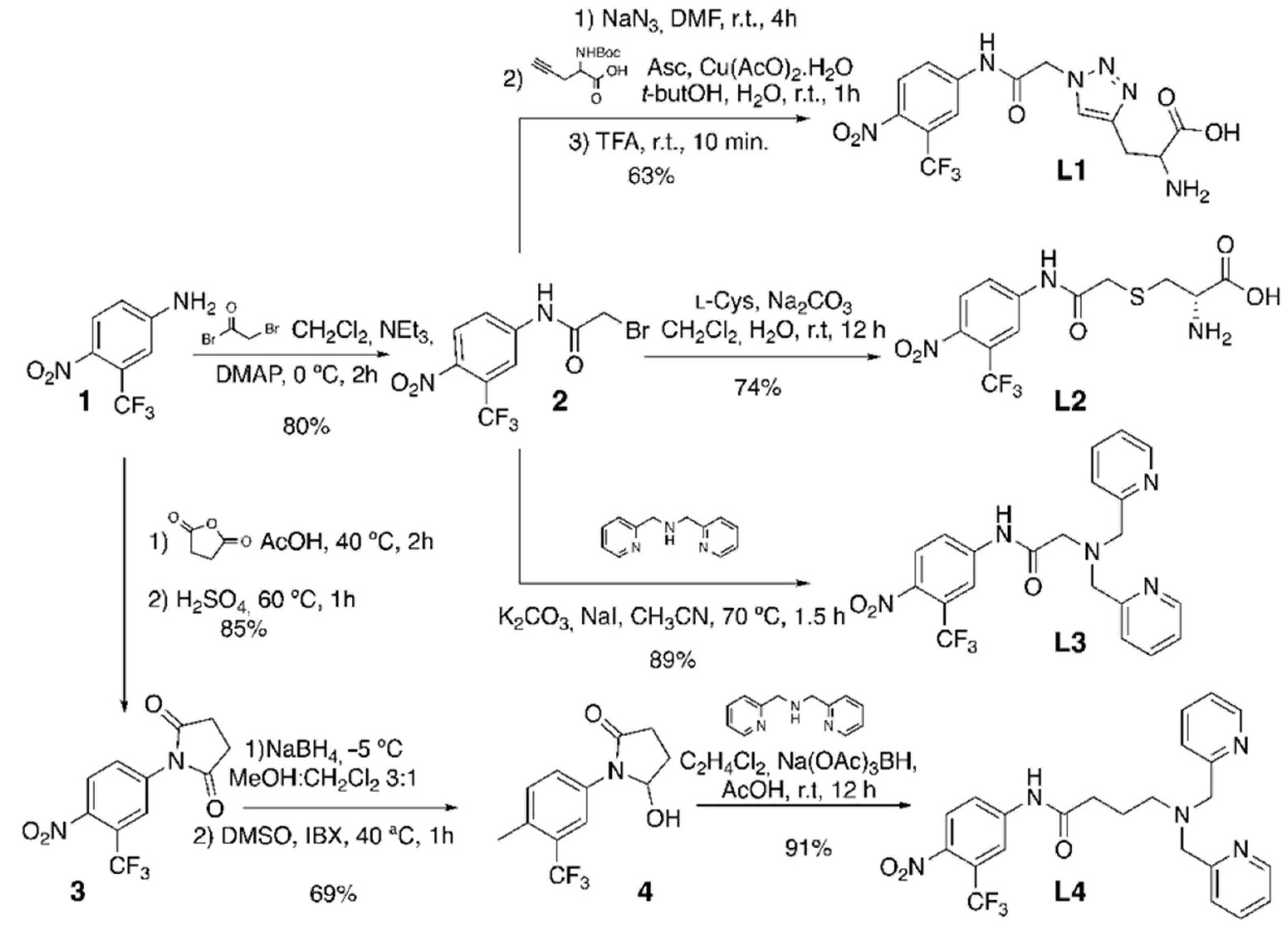
2-Bromo-N-[4-nitro-3-(trifluoromethyl)phenyl]acetamide (2). A solution of 1 (0.2 g, 0.97 mmol) in CH2Cl2 (6 mL) and triethylamine (0.74 mL, 5.15 mmol) was cooled to 0 °C in a two-neck round-bottom flask under an N2 atmosphere. Bromoacetyl bromide (0.48 g, 2.38 mmol) was added dropwise, and, finally, a catalytic amount of DMAP was added. The solution was stirred for 2 h and washed with HCl 0.5 M (3 × 10 mL) and a saturated aqueous NaCl solution (2 × 10 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and the solvent was distilled under reduced pressure. The residue was purified using flash column chromatography (CH2Cl2:Hexane 8:2), yielding 2 as a colorless oil (0.277 g, 80%).
1H RMN (400 MHz, CDCl3) δ (ppm): 8.50 (s, 1H), 8.02 (m, 3H), and 4.07 (s, 2H). 13C RMN (101 MHz, CDCl3) δ (ppm): 164.21, 141.20, 127.22, 125.55 (q, J = 34.4 Hz), 122.59, 121.74 (q, J = 273.6 Hz), 118.61 (q, J = 5.8 Hz), and 29.03.
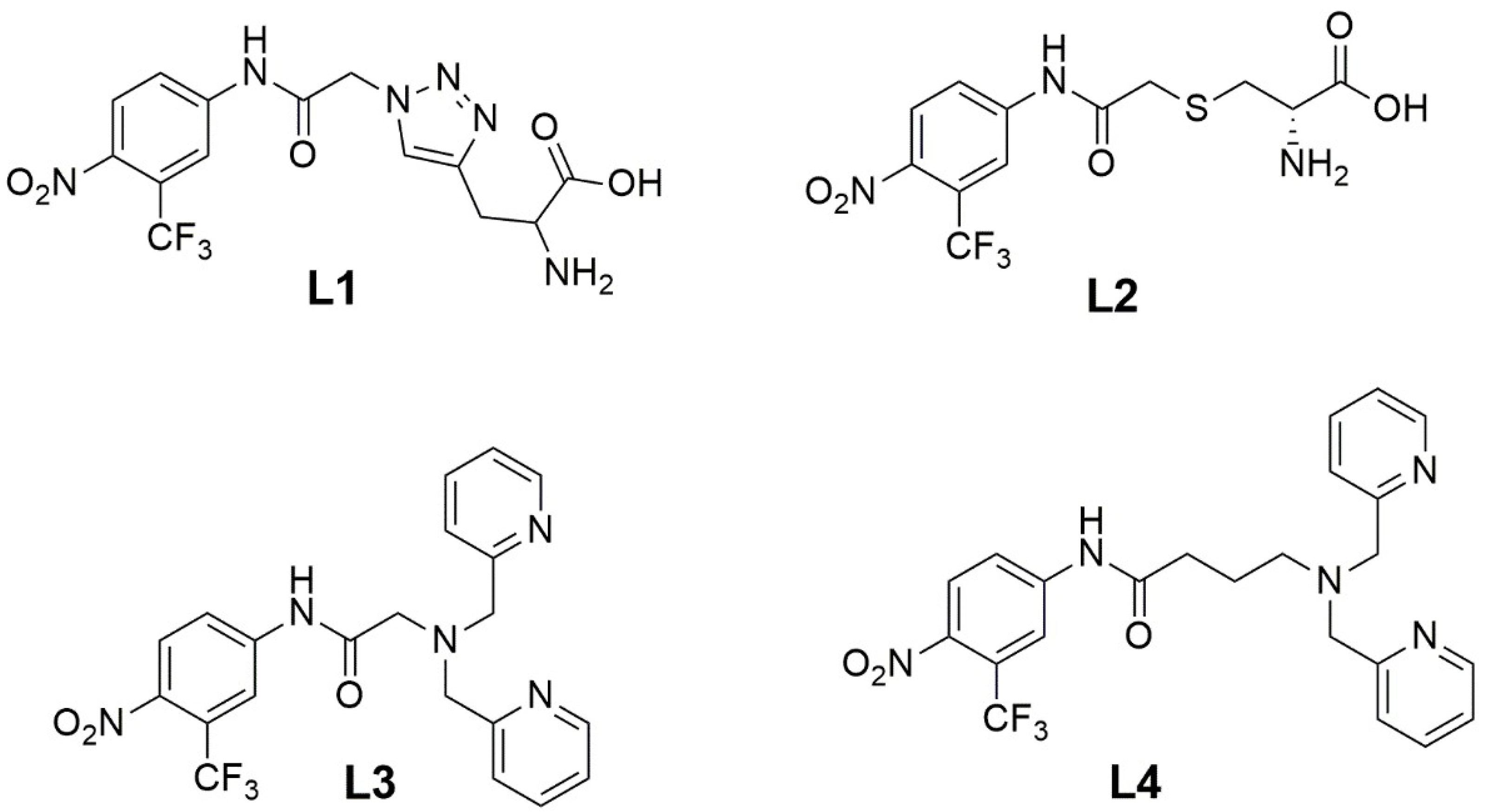
2-amino-3-(1-(2-((4-nitro-3-(trifluoromethyl)phenyl)amino)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)propanoic acid (L1).
SN2 azide substitution: To a solution of 2 (0.295 g, 0.903 mmol) in DMF (1 mL), sodium azide (0.352 mL, 5.418 mmol) was added, and the reaction was stirred at room temperature for 4 h in a two-neck round-bottom flask under a N2 atmosphere. The crude was diluted with H2O (10 mL), extracted with Et2O (3 × 10 mL), washed with an aqueous NaCl solution (2 × 10 mL), and dried over anhydrous Na2SO4. The solvent was distilled under reduced pressure, and the residue was purified using flash column chromatography (Hexane:AcOEt 7:3) (0.246 g, 94%). 1H RMN (400 MHz, CDCl3) δ (ppm): 8.50 (s, 1H), 8.02 (m, 3H), 4.24 (s, 2H), and 1.25 (s, 1H). 13C RMN (101 MHz, CDCl3) δ (ppm): 165.37, 141.08, 127.25, 125.48 (q, J = 34.2 Hz), 122.60, 121.74 (q, J = 274.7 Hz), 118.64 (q, J = 5.8 Hz), and 52.9.
Cu-catalyzed alkyne-azide cycloaddition: To a solution of the azide-containing compound (0.672 g, 2.32 mmol) in 24 mL of t-butanol, 2-((t-butoxycarbonyl)amino)pent-4-ynoic acid (0.495 g, 2.32 mmol) was added. Separately, Cu(OAc)2·H2O (0.093 g, 0.46 mmol) was added to a solution of sodium ascorbate (0.176 g, 0.93 mmol) in H2O (12 mL), and this solution was immediately poured into the reaction flask. The reaction was stirred for 1 h at room temperature until the starting material was consumed (as determined using TLC). The solvent was distilled under reduced pressure, and the residue was taken up in AcOEt (20 mL). The organic layer was washed with HCl 0.5 M (3 × 20 mL), dried over anhydrous Na2SO4, filtered, and the solvent was distilled under reduced pressure. The residue was purified using flash column chromatography (CH2Cl2:MeOH 98:2) (0.691 g, 70%). 1H NMR (400 MHz, Metanol-d4) δ (ppm): 8.24 (d, J = 2.2 Hz, 1H), 8.05 (d, J = 8.9 Hz, 1H), 7.99 (dd, J = 8.9, 2.3 Hz, 1H), 7.86 (s, 1H), 5.37 (s, 1H), 4.34 (s, 1H), 3.29–3.09 (m, 1H), and 1.41 (s, 9H). 13C NMR (101 MHz, Metanol-d4) δ (ppm): 166.80, 143.97, 128.20, 126.22, 125.66 (q, J = 33.7 Hz), 124.81 (q, J = 271.6 Hz), 123.85, 119.16 (q, J = 5.9 Hz), 80.46, 53.57, 29.55, and 28.70. Boc deprotection: TFA (0.5 mL) was added to the previously obtained triazol compound (0.025 g, 0.0622 mmol) at room temperature, and the reaction was stirred for 10 min. The TFA was distilled under reduced pressure, then a mixture of CH2Cl2:MeOH (9:1) (containing 1% of concentrated NH4OH) was added, and redistilled under reduced pressure. This procedure was repeated until L1 was obtained as a white solid (0.018g, 96%). 1H NMR (400 MHz, D2O) δ (ppm): 8.09–7.87 (m, 2H), 7.87–7.73 (m, 1H), 5.53–5.46 (m, 2H), 4.37–4.29 (m, 1H), and 3.52–3.38 (m, 2H). 13C NMR (101 MHz, D2O) δ (ppm): 171.83, 166.65, 141.60, 127.37, 126.28, 114.86, 53.33, 52.43, and 25.93.
(2-(S-cysteinyl)-N-[4-nitro-3-(trifluoromethyl)-phenyl]-acetamide) (L2): To a solution of 2 (0.157 g, 0.482 mmol) in CH2Cl2 (8 mL), a solution of L-Cysteine (0.117 g, 0.964 mmol) in H2O (8 mL) and NaHCO3 (0.045 g, 0.530 mmol) were added. The biphasic mixture was stirred vigorously for 12 hs at room temperature. The solvent was distilled under reduced pressure, and the residue was purified using flash column chromatography (CH2Cl2:MeOH 9:1), yielding L2 (0.130 g, 74%). 1H RMN (400 MHz, DCl 0.25 M in D2O) δ (ppm): 7.97 (d, J = 9.0 Hz, 1H), 7.95 (d, J = 2.3 Hz, 1H), 7.79 (dd, J = 2.3, 8.96 Hz, 1H), 4.28 (dd, J = 7.9, 4.3 Hz, 1H), 3.54 (d, J = 15.8 Hz, 1H), 3.49 (d, J = 15.8 Hz, 1H), 3.27 (dd, J = 15.01, 4.4 Hz, 1H), and 3.12 (dd, J = 15.1, 7.9 Hz, 1H). 13C NMR (101 MHz, DCl 0.25 M in D2O) δ (ppm): 170.47, 170.13, 142.18, 142.05, 127.27, 123.96 (q, J = 33.7 Hz), 123.04, 121.64 (q, J = 273.2 Hz), 118.77 (q, J = 5.8 Hz), 52.11, 36.13, and 31.96.
2-(bis(pyridin-2-ylmethyl)amino)-N-(4-nitro-3-(trifluoromethyl)phenyl)acetamide (L3): A solution of bis(pyridin-2-ylmethyl)amine (0.040 g, 0.20 mmol), 2 (0.065 g, 0.20 mmol), K2CO3 (0.055 g, 0.40 mmol), and NaI (0.06 g, 0.40 mmol) in dried CH3CN was stirred at 70 °C for 1.5 h. The solvent was distilled under reduced pressure, and the crude was dissolved in CH2Cl2, and the solution was washed with an aqueous NaCl solution (3 × 10 mL) and dried over anhydrous Na2SO4. The residue was purified using column chromatography (CH2Cl2:MeOH 95:5/NH4OH (1%)), yielding L3 (0.085 g, 89%). 1H NMR (400 MHz, MeOD) δ (ppm): 11.83 (s, 1H), 8.63 (dt, J = 4.7, 1.5 Hz, 2H), 8.39 (dd, J = 8.9, 2.3 Hz, 1H), 8.22 (d, J = 2.3 Hz, 1H), 8.01 (d, J = 8.9 Hz, 1H), 7.65 (dt, J = 7.7, 1.8 Hz, 2H), 7.31 (m, 4H), 3.94 (s, 4H), and 3.58 (s, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm): 171.25, 157.84, 149.55, 143.37, 142.56, 137.01, 127.37, 125.16 (q, J = 33.8 Hz), 123.54, 123.02, 122.16 (q, J = 273.4 Hz), 122.04, 118.53 (q, J = 5.9 Hz), 60.20, and 58.89.
1-(4-nitro-3-(trifluoromethyl)phenyl)pyrrolidine-2,5-dione (3). Succinic anhydride (0.515 g, 4.85 mmol) was added to a solution of 1 (0.5 g, 2.45 mmol) in AcOH (2 mL). The reaction was stirred for 2 h at 40 °C, then H2SO4 cc (260 mL) was added, the temperature was raised to 60 °C, and the reaction was stirred for an additional hour. The reaction was diluted with H2O (5 mL), the organic layer was extracted with AcOEt (3 × 5 mL), washed with saturated aqueous NaCl solution (2 × 5 mL), dried over anhydrous Na2SO4, filtered, and the solvent was distilled under reduced pressure. The residue was purified using flash column chromatography (Hexane:AcOEt 6:4), yielding 3 (0.597 g, 85%). 1H NMR (400 MHz, Acetone-d6) δ (ppm): 8.26 (d, J = 8.6 Hz, 1H), 8.04 (d, J = 2.2 Hz, 1H), 7.99 (dd, J = 8.7, 2.1 Hz, 1H), and 2.95 (s, 4H). 13C NMR (101 MHz, Acetone-d6) δ (ppm): 176.68, 137.97, 132.51, 127.13, 126.72 (q, J = 5.6 Hz), 123.93 (q, J = 34.2 Hz), 122.93 (q, J = 272.7 Hz), and 29.29 (s, 4C).
5-hydroxy-1-(4-nitro-3-(trifluoromethyl)phenyl)pyrrolidin-2-one (4). NaBH4 reduction: To a solution of 3 (0.522 g, 1.80 mmol) in a MeOH:CH2Cl2 3:1 mixture (40 mL), NaBH4 (0.135 g, 3.2 mmol) was added in three portions at −5 °C. The reaction was diluted with H2O (20 mL), the MeOH was distilled under reduced pressure, and the aqueous phase was extracted with AcOEt (3 × 20 mL). The organic layer was washed with a saturated aqueous NaCl solution (2 × 5 mL), dried over anhydrous Na2SO4, filtered, and the solvent was distilled under reduced pressure. The residue was purified using flash column chromatography (Hexane:AcOEt 4:6) (0.400 g, 76%). 1H NMR (400 MHz, Acetone-d6) δ (ppm): 9.91 (s, 1H), 8.35 (s, 1H), 8.12 (s, 2H), 3.75–3.67 (m, 1H), 3.62 (q, J = 5.7 Hz, 2H), 2.57 (t, J = 7.4 Hz, 2H), and 1.95–1.83 (m, 2H). 13C NMR (101 MHz, Acetone-d6) δ (ppm): 173.44, 144.99, 142.99, 128.26, 124.86 (q, J = 33.7 Hz), 123.33 (d, J = 272.5 Hz), 122.91, 118.31 (q, J = 6.1 Hz), 61.70, 34.58, and 29.00.
IBX oxidation: IBX (0.910 g, 3.26 mmol) was added to a solution of the obtained product (0.636 g, 2.18 mmol) in DMSO (10 mL), and the mixture was stirred for 1 h at 40 °C. The reaction was diluted with H2O (10 mL) and extracted with AcOEt (3 × 20 mL). The organic layer was washed with a saturated aqueous NaCl solution (2 × 5 mL), dried over anhydrous Na2SO4, and the solvent was distilled under reduced pressure. The crude was purified using flash column chromatography (Hexane:AcOEt 6:4 to 4:6), yielding 4 (0.577 g, 91%). 1H NMR (400 MHz, Acetone-d6) δ (ppm): 8.51 (d, J = 2.3 Hz, 1H), 8.24 (dd, J = 9.0, 2.4 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 5.98 (d, J = 5.7 Hz, 1H), 5.87 (s, 1H), 3.07–2.73 (m, 2H), 2.54 (d, J = 1.8 Hz, 1H), and 2.16–2.06 (m, 1H). 13C NMR (101 MHz, Acetone-d6) δ (ppm): 175.32, 143.87, 127.45, 124.91, 124.18 (q, J = 33.5 Hz), 123.23 (d, J = 272.5 Hz), 119.94 (q, J = 5.9 Hz), 84.86, 30.61, and 28.78.
4-(bis(pyridin-2-ylmethyl)amino)-N-(4-nitro-3-(trifluoromethyl)phenyl)butanamide (L4). To a solution of 4 (0.577 g, 1.99 mmol), Na(OAc)3BH (1.51 g, 7.16 mmol), and AcOH (237 µL, 4.17 mmol) in dichloroethane (10 mL), bis(pyridin-2-ylmethyl)amine (0.516 g, 2.58 mmol) was added. The reaction was stirred for 12 h at room temperature. The crude was diluted with H2O (10 mL), and the mixture was extracted with AcOEt (3 × 10 mL). The organic layer was washed with a saturated aqueous NaCl solution (2 × 5 mL), dried over anhydrous Na2SO4, and the solvent was distilled under reduced pressure. The crude was purified using flash column chromatography (AcOEt:MeOH 98:2), yielding L4 (0.850 g, 91%). 1H NMR (400 MHz, Acetone-d6) δ (ppm): 10.40 (s, 1H), 8.48 (ddd, J = 4.9, 1.9, 1.0 Hz, 2H), 8.30 (d, J = 2.1 Hz, 1H), 8.09 (d, J = 9.0 Hz, 1H), 8.05 (dd, J = 8.9, 2.2 Hz, 1H), 7.66 (td, J = 7.6, 1.9 Hz, 2H), 7.51 (dt, J = 7.9, 1.1 Hz, 2H), 7.18 (ddd, J = 7.6, 4.9, 1.2 Hz, 2H), 3.80 (s, 4H), 2.61 (t, J = 6.6 Hz, 2H), 2.51 (t, J = 7.0 Hz, 2H), and 2.01–1.91 (m, 2H). 13C NMR (101 MHz, Acetone-d6) δ (ppm): 173.53, 160.42, 149.72, 145.12, 142.72, 137.16, 128.09, 124.70 (q, J = 33.6 Hz), 124.04, 123.24 (q, J = 272.5 Hz), 122.86, 122.80, 118.22 (q, J = 6.1 Hz), 60.75, 54.11, and 23.52.