Molecular Modelling of Ionic Liquids: Situations When Charge Scaling Seems Insufficient
Abstract
1. Introduction
2. Results and Discussions
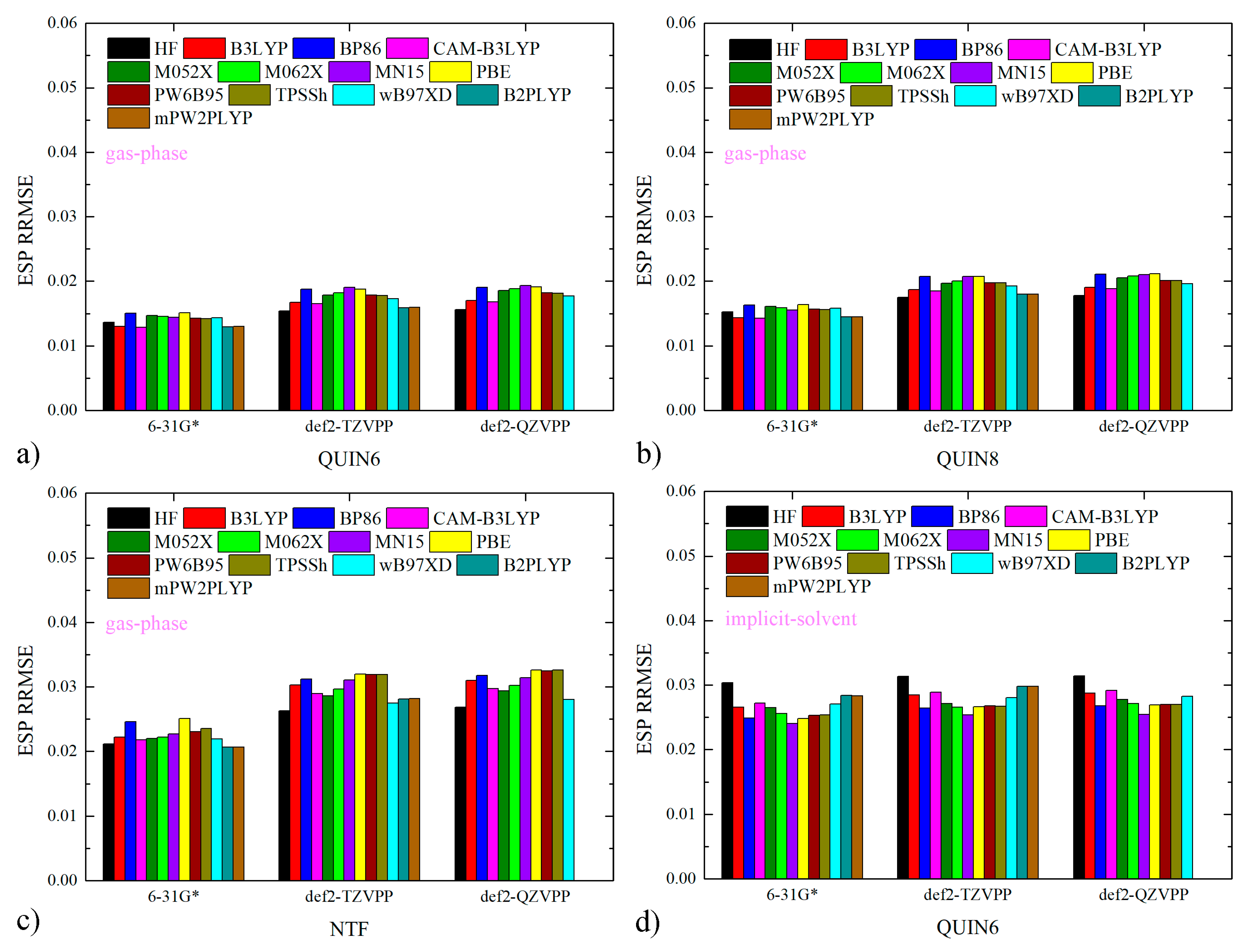
2.1. Charge Quality from ESP Analysis
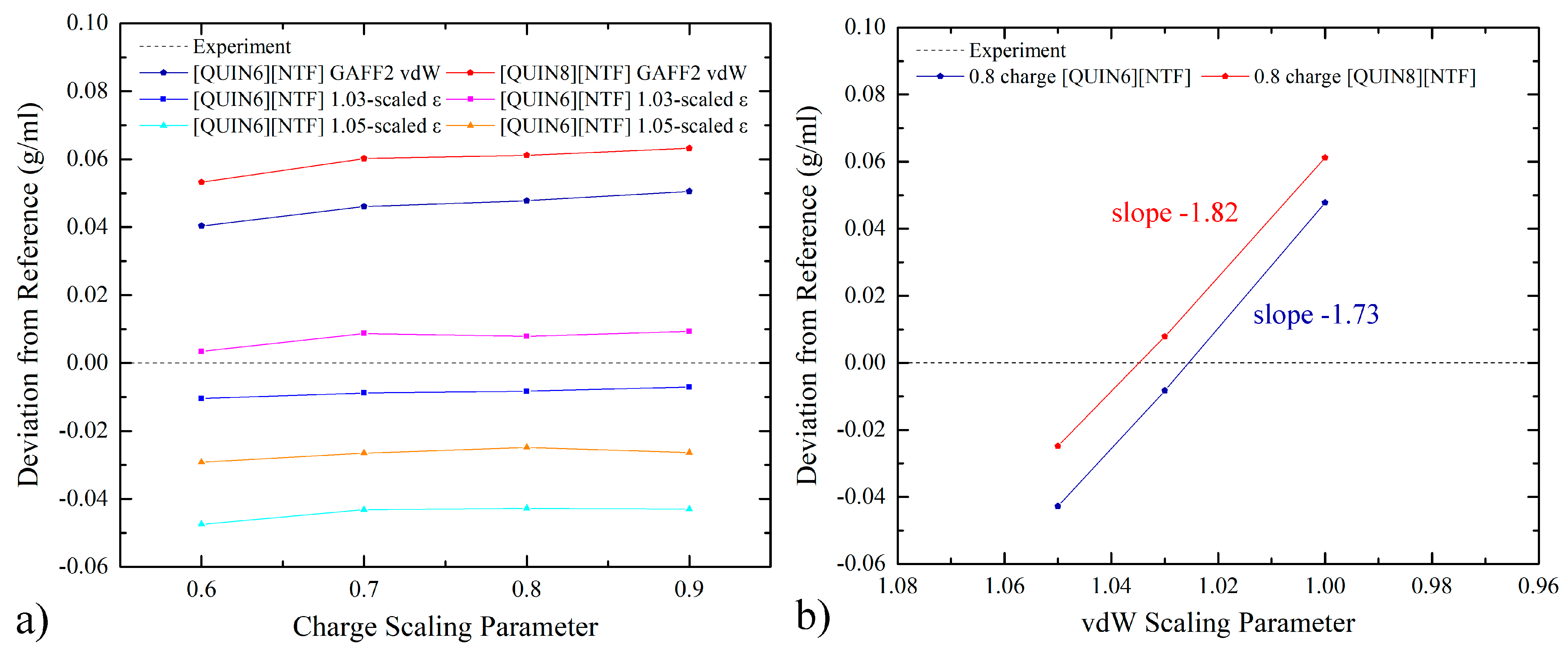
2.2. Density-Matching with Charge and vdW Scaling
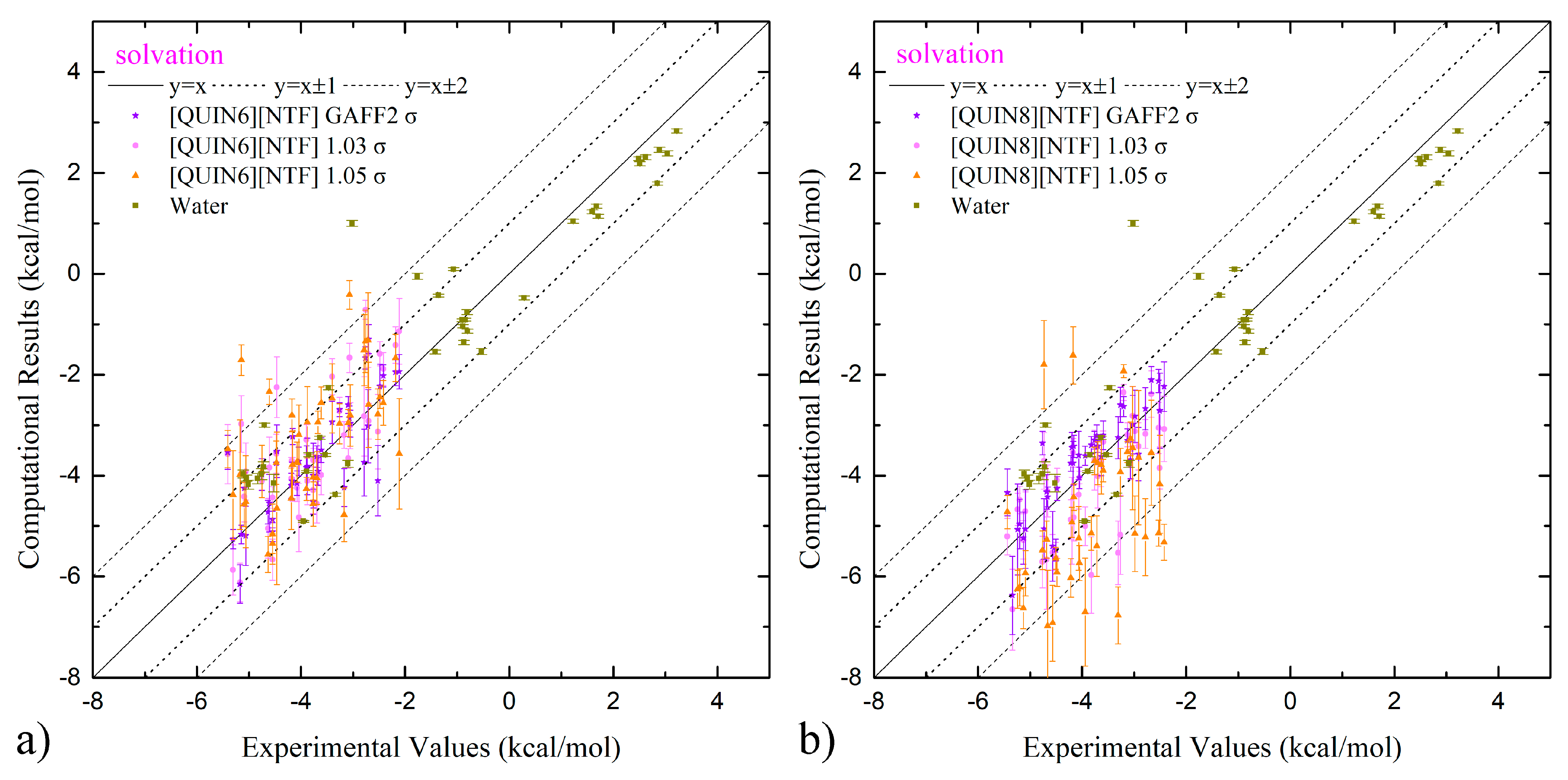
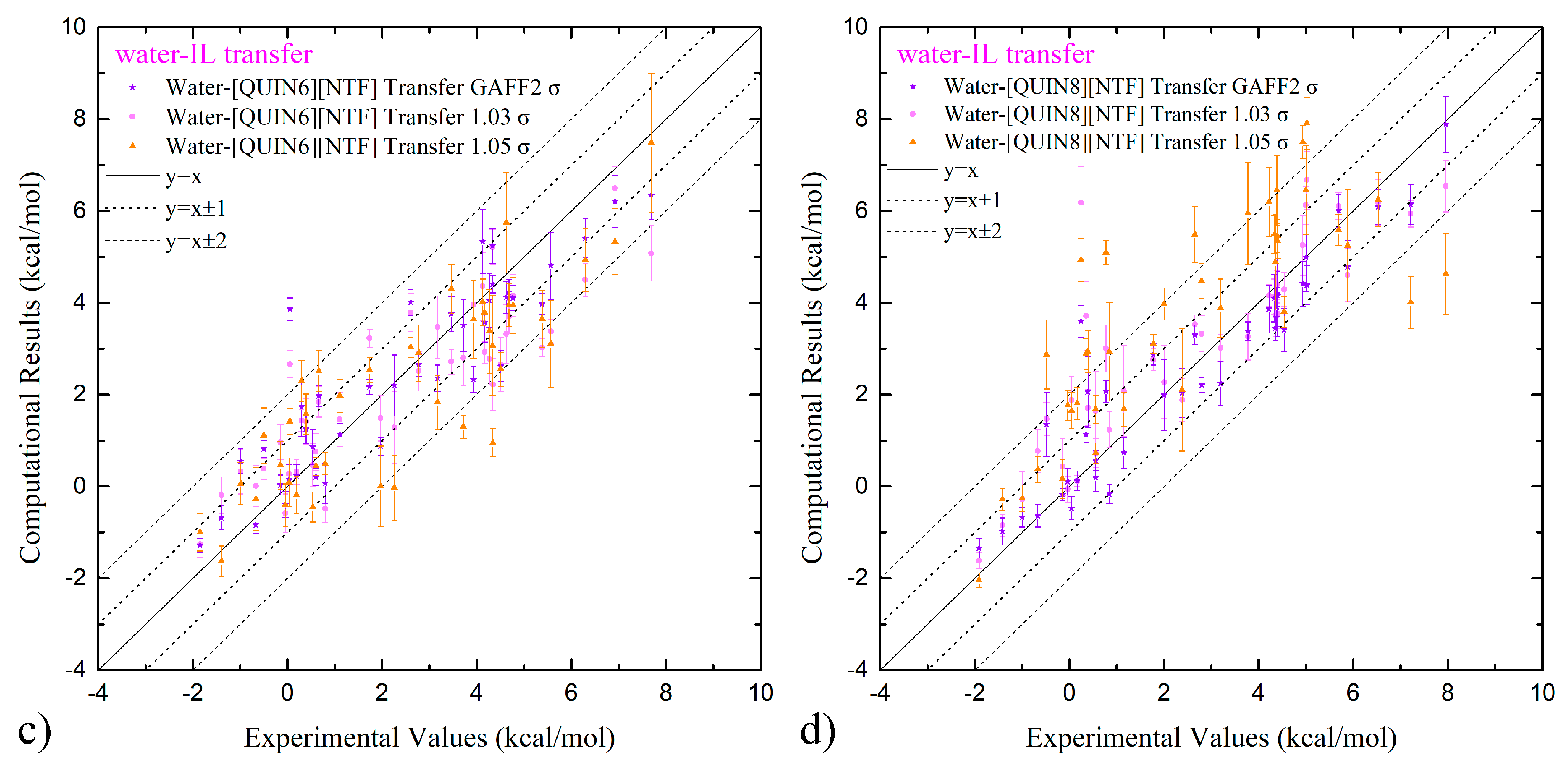
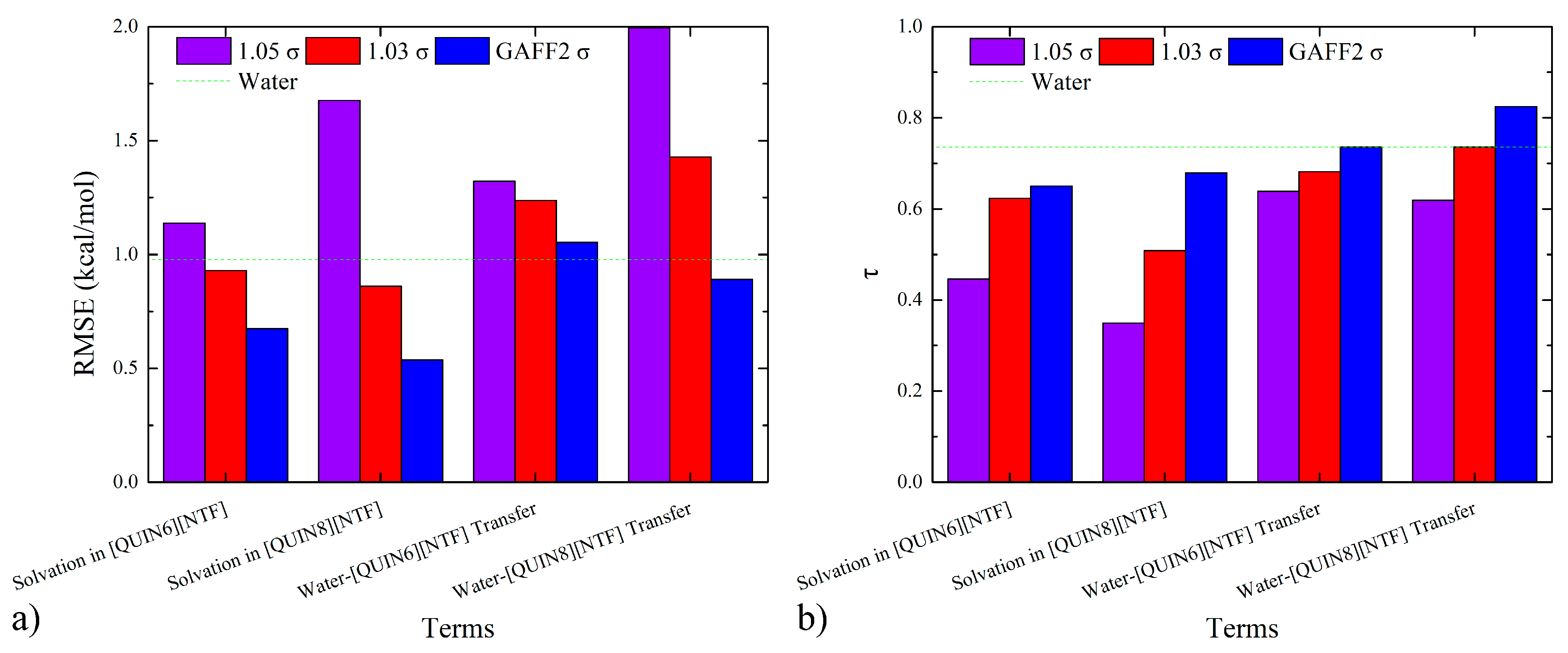
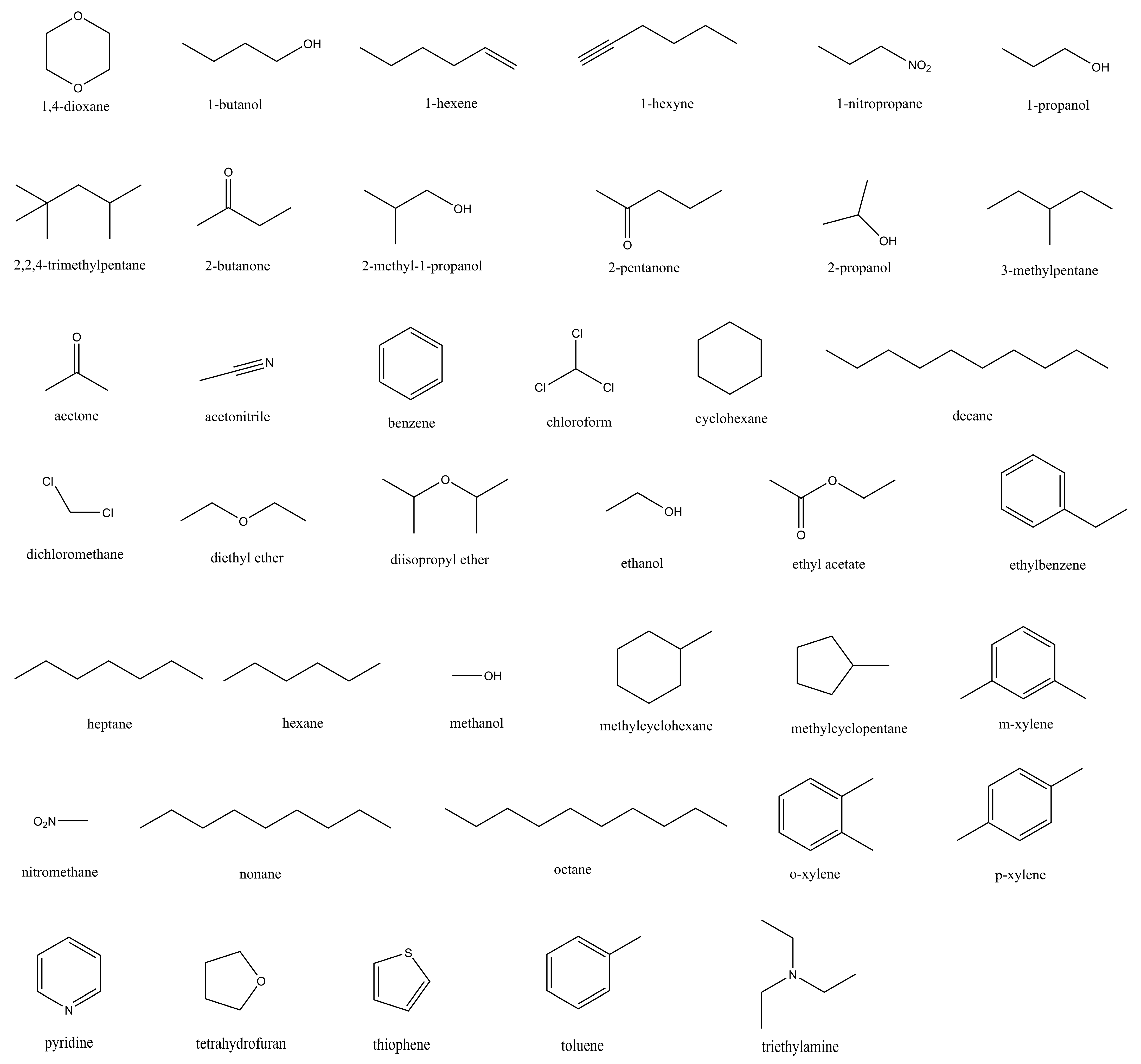
2.3. Predictive Power of Fine-Tuned Parameter Set on Solvation and Partition Thermodynamics
3. Computational Details
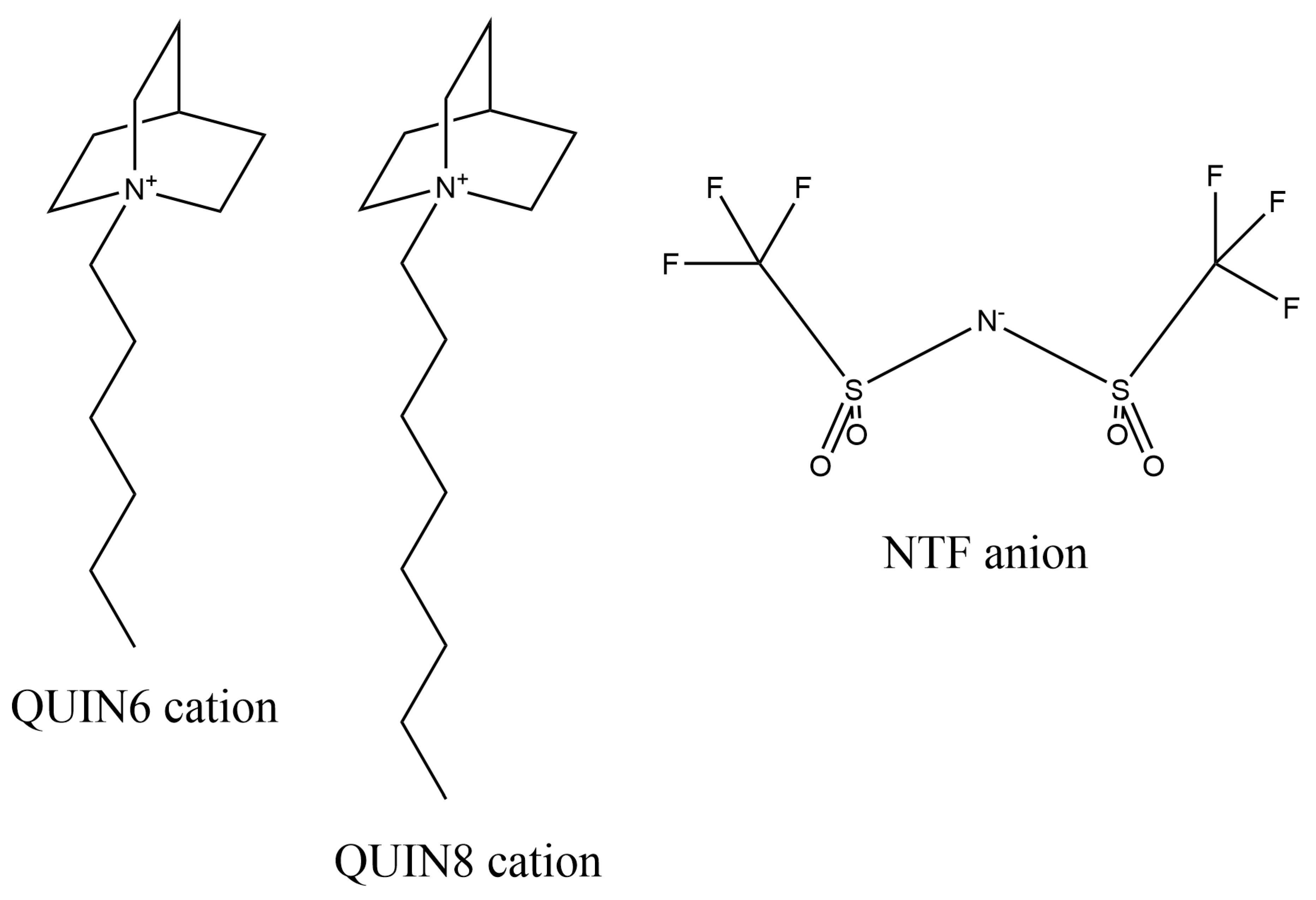
3.1. System Construction
3.2. Molecular Simulation
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Verma, R.; Mohan, M.; Goud, V.V.; Banerjee, T. Operational Strategies and Comprehensive Evaluation of Menthol Based Deep Eutectic Solvent for the Extraction of Lower Alcohols from Aqueous Media. ACS Sustain. Chem. Eng. 2018, 6, 16920–16932. [Google Scholar] [CrossRef]
- Swami, U.; Kumbhakarna, N.; Chowdhury, A. Green Hypergolic Ionic Liquids: Future Rocket Propellants. J. Ion. Liq. 2022, 2, 100039. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, H.; Singla, M. Micellization studies of selected imidazolium based ionic liquid in aqueous solution and in presence of different additives: A review. J. Ion. Liq. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Jain, P.; Antzutkin, O.N. 2-Ethylhexylsulfate Anion-based Surface-Active Ionic Liquids (SAILs) as Temperature Persistent Electrolytes for Supercapacitors. J. Ion. Liq. 2022, 2, 100034. [Google Scholar] [CrossRef]
- Hassanpour, M.; Shahavi, M.H.; Heidari, G.; Kumar, A.; Nodehi, M.; Moghaddam, F.D.; Mohammadi, M.; Nikfarjam, N.; Sharifi, E.; Makvandi, P. Ionic liquid-mediated synthesis of metal nanostructures: Potential application in cancer diagnosis and therapy. J. Ion. Liq. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Khalil, R.; Azar, M.; Malham, I.B.; Turmine, M.; Vivier, V. Electrochemical deposition of ZnO thin films in aprotic ionic liquids: Effect of the cationic alkyl-chain-length. J. Ion. Liq. 2022, 2, 100031. [Google Scholar] [CrossRef]
- Méndez-Vilas, A. Physical discoveries enabled by ionic liquid droplets. engrxiv 2022. [Google Scholar] [CrossRef]
- Łachwa, J.; Morgado, P.; Esperanca, J.M.; Guedes, H.J.; Canongia Lopes, J.N.; Rebelo, L.P.N. Fluid-phase behavior of {1-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide, [C6mim][NTf2],+ C2− C8 n-alcohol} mixtures: Liquid−liquid equilibrium and excess volumes. J. Chem. Eng. Data 2006, 51, 2215–2221. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowska, M.; Królikowski, M. Phase behaviour and physico-chemical properties of the binary systems {1-ethyl-3-methylimidazolium thiocyanate, or 1-ethyl-3-methylimidazolium tosylate+ water, or+ an alcohol}. Fluid Phase Equilib. 2010, 294, 72–83. [Google Scholar] [CrossRef]
- Ravula, S.; Baker, S.N.; Kamath, G.; Baker, G.A. Ionic liquid-assisted exfoliation and dispersion: Stripping graphene and its two-dimensional layered inorganic counterparts of their inhibitions. Nanoscale 2015, 7, 4338–4353. [Google Scholar] [CrossRef] [PubMed]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of polyvinylpyrrolidone in a deep eutectic solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Chenard, E.; Zeller, M.; Hatab, N.; Fulvio, P.F.; Hillesheim, P.C. Examining the structure and intermolecular forces of thiazolium-based ionic liquids. J. Mol. Liq. 2021, 327, 114800. [Google Scholar] [CrossRef]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Ali, M.K.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Formation and potential application of micelles composed of biocompatible N-lauroyl-amino acid ionic liquids surfactant. J. Mol. Liq. 2020, 320, 114424. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.; Silvestre, A.J.; Freire, M.G. Ionic Liquids in Drug Delivery. Encyclopedia 2021, 1, 324–339. [Google Scholar] [CrossRef]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; De Long, H.C.; Mantz, R.A. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef] [PubMed]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; Hadj-Kali, M.K.; Bagh, F.S.G.; Alnashef, I.M. Phase equilibria of toluene/heptane with tetrabutylphosphonium bromide based deep eutectic solvents for the potential use in the separation of aromatics from naphtha. Fluid Phase Equilib. 2012, 333, 47–54. [Google Scholar] [CrossRef]
- Habibi, E.; Ghanemi, K.; Fallah-Mehrjardi, M.; Dadolahi-Sohrab, A. A novel digestion method based on a choline chloride–oxalic acid deep eutectic solvent for determining Cu, Fe, and Zn in fish samples. Anal. Chim. Acta 2013, 762, 61–67. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; AlNashef, I.M. A novel ammonium based eutectic solvent for the treatment of free fatty acid and synthesis of biodiesel fuel. Ind. Crops Prod. 2013, 46, 392–398. [Google Scholar] [CrossRef]
- García-Alvarez-Coque, M.; Ruiz-Angel, M.J.; Berthod, A.; Carda-Broch, S. On the use of ionic liquids as mobile phase additives in high-performance liquid chromatography. A review. Anal. Chim. Acta 2015, 883, 1–21. [Google Scholar] [CrossRef]
- Xiaohua, X.; Liang, Z.; Xia, L.; Shengxiang, J. Ionic liquids as additives in high performance liquid chromatography: Analysis of amines and the interaction mechanism of ionic liquids. Anal. Chim. Acta 2004, 519, 207–211. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef]
- Marszałł, M.P.; Kaliszan, R. Application of ionic liquids in liquid chromatography. Crit. Rev. Anal. Chem. 2007, 37, 127–140. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Gurau, G.; Narita, A.; Barber, P.S.; Cojocaru, O.A.; Rogers, R.D. Drug specific, tuning of an ionic liquid’s hydrophilic–lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.-H.; Scammells, P.J.; Porter, C.J. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Bridson, R.; Leeke, G. Solubilities of pharmaceutical compounds in ionic liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. [Google Scholar] [CrossRef]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, J.M. Applications of ionic liquids. Chem. Rec. 2012, 12, 329–355. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic-liquid-based paclitaxel preparation: A new potential formulation for cancer treatment. Mol. Pharm. 2018, 15, 2484–2488. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Gupta, S.; Moulik, S. Biocompatible microemulsions and their prospective uses in drug delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Kaur, R.; Kaur, R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Arora, P.; Kumar, N.; Puri, A. Development of novel ionic liquid-based microemulsion formulation for dermal delivery of 5-fluorouracil. AAPS PharmSciTech 2014, 15, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Brotzakis, Z.F.; Voets, I.K.; Bakker, H.J.; Bolhuis, P.G. Water structure and dynamics in the hydration layer of a type III anti-freeze protein. Phys. Chem. Chem. Phys. 2018, 20, 6996–7006. [Google Scholar] [CrossRef] [PubMed]
- Brotzakis, Z.F.; Gehre, M.; Voets, I.K.; Bolhuis, P.G. Stability and growth mechanism of self-assembling putative antifreeze cyclic peptides. Phys. Chem. Chem. Phys. 2017, 19, 19032–19042. [Google Scholar] [CrossRef]
- Trapl, D.; Krupička, M.; Višňovský, V.; Hozzová, J.; Ol’ha, J.; Křenek, A.; Spiwok, V. Property Map Collective Variable as a Useful Tool for a Force Field Correction. J. Chem. Inf. Model. 2022, 62, 567–576. [Google Scholar] [CrossRef]
- Abdelgawwad, A.M.A.; Monari, A.; Tuñón, I.; Francés-Monerris, A. Spatial and Temporal Resolution of the Oxygen-Independent Photoinduced DNA Interstrand Cross-Linking by a Nitroimidazole Derivative. J. Chem. Inf. Model. 2022, 62, 3239–3252. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, L.; Kai, W.; Huai, Z.; Liu, Z. Primary vs. Secondary: Directionalized Guest Coordination in β-Cyclodextrin Derivatives. Carbohydr. Polym. 2022, 297, 120050. [Google Scholar] [CrossRef]
- Sun, Z.; Huai, Z.; He, Q.; Liu, Z. A General Picture of Cucurbit [8]uril Host–Guest Binding. J. Chem. Inf. Model. 2021, 61, 6107–6134. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.Z.H. Thermodynamic Insights of Base Flipping in TNA Duplex: Force Fields, Salt Concentrations, and Free-Energy Simulation Methods. CCS Chem. 2021, 3, 1026–1039. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1173. [Google Scholar] [CrossRef]
- Köddermann, T.; Paschek, D.; Ludwig, R. Molecular Dynamic Simulations of Ionic Liquids: A Reliable Description of Structure, Thermodynamics and Dynamics. ChemPhysChem 2007, 8, 2464–2470. [Google Scholar] [CrossRef]
- Yasaka, Y.; Klein, M.L.; Nakahara, M.; Matubayasi, N. Exploring the reorientation of benzene in an ionic liquid via molecular dynamics: Effect of temperature and solvent effective charge on the slow dynamics. J. Chem. Phys. 2011, 134, 191101. [Google Scholar] [PubMed]
- Chaban, V.V.; Prezhdo, O.V. A new force field model of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid and acetonitrile mixtures. Phys. Chem. Chem. Phys. 2011, 13, 19345–19354. [Google Scholar] [CrossRef] [PubMed]
- Vahid, A.; Maginn, E.J. Monte Carlo simulation and SAFT modeling study of the solvation thermodynamics of dimethylformamide, dimethylsulfoxide, ethanol and 1-propanol in the ionic liquid trimethylbutylammonium bis(trifluoromethylsulfonyl)imide. Phys. Chem. Chem. Phys. 2015, 17, 7449–7462. [Google Scholar] [CrossRef] [PubMed]
- Bedrov, D.; Borodin, O.; Li, Z.; Smith, G.D. Influence of polarization on structural, thermodynamic, and dynamic properties of ionic liquids obtained from molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 4984–4997. [Google Scholar] [CrossRef] [PubMed]
- Penley, D.; Wang, X.; Lee, Y.-Y.; Garaga, M.N.; Ghahremani, R.; Greenbaum, S.; Maginn, E.J.; Gurkan, B. Lithium Solvation and Mobility in Ionic Liquid Electrolytes with Asymmetric Sulfonyl-Cyano Anion. J. Chem. Eng. Data 2022, 67, 1810–1823. [Google Scholar] [CrossRef]
- Khabaz, F.; Zhang, Y.; Xue, L.; Quitevis, E.L.; Maginn, E.J.; Khare, R. Temperature Dependence of Volumetric and Dynamic Properties of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2018, 122, 2414–2424. [Google Scholar] [CrossRef]
- Bhargava, B.; Balasubramanian, S. Refined potential model for atomistic simulations of ionic liquid [bmim][PF 6]. J. Chem. Phys. 2007, 127, 114510. [Google Scholar] [CrossRef]
- Chevrot, G.; Schurhammer, R.; Wipff, G. Molecular dynamics simulations of the aqueous interface with the [BMI][PF 6] ionic liquid: Comparison of different solvent models. Phys. Chem. Chem. Phys. 2006, 8, 4166–4174. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, B.; Lall-Ramnarine, S.I.; Ramdihal, J.D.; Papacostas, K.A.; Fernandez, E.D.; Sumner, R.A.; Margulis, C.J.; Wishart, J.F.; Castner, E.W. Structural analysis of ionic liquids with symmetric and asymmetric fluorinated anions. J. Chem. Phys. 2019, 151, 074504. [Google Scholar] [CrossRef]
- Zhang, Z.; Zofchak, E.; Krajniak, J.; Ganesan, V. Influence of Polarizability on the Structure, Dynamic Characteristics, and Ion-Transport Mechanisms in Polymeric Ionic Liquids. J. Phys. Chem. B 2022, 126, 2583–2592. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, L.; Zhang, Z.-y.; Cong, Y.; Yang, J.; Wang, X. Molecular Modelling of Ionic Liquids: Perfluorinated Anionic Species with Enlarged Halogen Substitutions. Chemrxiv 2022. [Google Scholar] [CrossRef]
- Celebi, A.T.; Dawass, N.; Moultos, O.A.; Vlugt, T.J. How sensitive are physical properties of choline chloride–urea mixtures to composition changes: Molecular dynamics simulations and Kirkwood–Buff theory. J. Chem. Phys. 2021, 154, 184502. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A.; Dhabal, D.; Kashyap, H.K. Pressure-dependent morphology of trihexyl (tetradecyl) phosphonium ionic liquids: A molecular dynamics study. J. Chem. Phys. 2016, 145, 134506. [Google Scholar] [CrossRef]
- Zhang, Y.; Maginn, E.J. A Simple AIMD Approach to Derive Atomic Charges for Condensed Phase Simulation of Ionic Liquids. J. Phys. Chem. B 2012, 116, 10036–10048. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ivanov, A.S.; Margulis, C.J. A Brief Guide to the Structure of High-Temperature Molten Salts and Key Aspects Making Them Different from Their Low-Temperature Relatives, the Ionic Liquids. J. Phys. Chem. B 2021, 125, 6359–6372. [Google Scholar] [CrossRef]
- Chaban, V. Polarizability versus mobility: Atomistic force field for ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16055–16062. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, Z.; Zheng, L.; Payam, K.; Huai, Z.; Liu, Z. Molecular Modelling of Ionic Liquids: General Guidelines on Fixed-Charge Force Fields for Balanced Descriptions. J. Ion. Liq. 2022, 2, 100043. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; He, Q.; Liu, Z. Molecular Modelling of Ionic Liquids: Force-Field Validation and Thermodynamic Perspective from Large-Scale Fast-Growth Solvation Free Energy Calculations. Adv. Theory Simul. 2022, 2200274. [Google Scholar] [CrossRef]
- Liu, X.; Turner, C.H. Computational study of the electrostatic potential and charges of multivalent ionic liquid molecules. J. Mol. Liq. 2021, 340, 117190. [Google Scholar] [CrossRef]
- Sun, Z.; Kayal, A.; Gong, Z.; Zheng, L.; He, Q. Molecular Modelling of Ionic Liquids: Physical Properties of Species with Extremely Long Aliphatic Chains from a Near-Optimal Regime. J. Mol. Liq. 2022, 367, 120492. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Maginn, E.J. Molecular dynamics study of the ionic liquid 1-n-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ([C6C1im][FAP]): Force field development and the effect of [FAP]− isomer content on properties. J. Ion. Liq. 2022, 2, 100040. [Google Scholar] [CrossRef]
- Liu, H.; Maginn, E.; Visser, A.E.; Bridges, N.J.; Fox, E.B. Thermal and Transport Properties of Six Ionic Liquids: An Experimental and Molecular Dynamics Study. Ind. Eng. Chem. Res. 2012, 51, 7242–7254. [Google Scholar] [CrossRef]
- Zhang, Y.; Squire, H.; Gurkan, B.; Maginn, E.J. Refined Classical Force Field for Choline Chloride and Ethylene Glycol Mixtures over Wide Composition Range. J. Chem. Eng. Data 2022, 67, 1864–1871. [Google Scholar] [CrossRef]
- Barbosa, N.S.; Zhang, Y.; Lima, E.R.; Tavares, F.W.; Maginn, E.J. Development of an AMBER-compatible transferable force field for poly (ethylene glycol) ethers (glymes). J. Mol. Model. 2017, 23, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; McCready, M.J.; Maginn, E.J. Evaluation and Refinement of the General AMBER Force Field for Nineteen Pure Organic Electrolyte Solvents. J. Chem. Eng. Data 2018, 63, 3488–3502. [Google Scholar] [CrossRef]
- Grünewald-Lüke, A.; Marler, B.; Hochgräfe, M.; Gies, H. Quinuclidine derivatives as structure directing agents for the synthesis of boron containing zeolites. J. Mater. Chem. 1999, 9, 2529–2536. [Google Scholar] [CrossRef]
- Kazock, J.-Y.; Taggougui, M.; Carré, B.; Willmann, P.; Lemordant, D. Simple and efficient synthesis of N-quaternary salts of quinuclidinium derivatives. Synthesis 2007, 2007, 3776–3778. [Google Scholar] [CrossRef]
- Lan, X.; Wang, X.; Zhang, D.X.; Mu, T.; Lan, X.Z. Cation and Anion Transfer in Quinuclidinium Hexafluorophosphate Plastic Crystal: Role of Constituent Ions and the Crystalline Structure. J. Phys. Chem. C 2021, 125, 21169–21178. [Google Scholar] [CrossRef]
- Skočibušić, M.; Odžak, R.; Štefanić, Z.; Križić, I.; Krišto, L.; Jović, O.; Hrenar, T.; Primožič, I.; Jurašin, D. Structure–property relationship of quinuclidinium surfactants—Towards multifunctional biologically active molecules. Colloids Surf. B. Biointerfaces 2016, 140, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Kazock, J.-Y.; Taggougui, M.; Anouti, M.; Willman, P.; Carré, B.; Lemordant, D. Ionic liquids based on 1-aza-bicyclo [2,2,2]octane (Quinuclidine) salts: Synthesis and physicochemical properties. J. Appl. Electrochem. 2009, 39, 2461. [Google Scholar] [CrossRef]
- Rowe, R.; Lovelock, K.R.; Hunt, P.A. Bi (III) halometallate ionic liquids: Interactions and speciation. J. Chem. Phys. 2021, 155, 014501. [Google Scholar] [CrossRef]
- Fuerst, G.B.; Ley, R.T.; Paluch, A.S. Calculating the fugacity of pure, low volatile liquids via molecular simulation with application to acetanilide, acetaminophen, and phenacetin. Ind. Eng. Chem. Res. 2015, 54, 9027–9037. [Google Scholar] [CrossRef]
- Dasari, S.; Mallik, B.S. Solubility and solvation free energy of a cardiovascular drug, LASSBio-294, in ionic liquids: A computational study. J. Mol. Liq. 2020, 301, 112449. [Google Scholar] [CrossRef]
- Gong, Z.; Sun, H. Extension of TEAM force-field database to ionic liquids. J. Chem. Eng. Data 2019, 64, 3718–3730. [Google Scholar] [CrossRef]
- Biswas, S.; Mallik, B.S. Differing preferential ion binding to the peptide bond in ionic environment from classical and first principles molecular dynamics simulations. J. Mol. Liq. 2020, 318, 114257. [Google Scholar] [CrossRef]
- Dasari, S.; Mallik, B.S. Ion-induced free energy landscapes of Aβ33–42 peptide dimer in wet ionic liquids. J. Mol. Liq. 2020, 318, 114026. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, D.; Ji, Y. The role of hydrogen-bond in solubilizing drugs by ionic liquids: A molecular dynamics and density functional theory study. AIChE J. 2022, 68, e17672. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functiona. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Haoyu, S.Y.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn–Sham global-hybrid exchange–correlation density functional with broad accuracy for multi-reference and single-reference systems and noncovalent interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Design of density functionals that are broadly accurate for thermochemistry, thermochemical kinetics, and nonbonded interactions. J. Phys. Chem. A 2005, 109, 5656–5667. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 57–63. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 2006, 124, 034108. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, T.; Grimme, S. Towards chemical accuracy for the thermodynamics of large molecules: New hybrid density functionals including non-local correlation effects. Phys. Chem. Chem. Phys. 2006, 8, 4398–4401. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Ayad, A.; Mutelet, F.; Negadi, A.; Acree, W.E.; Jiang, B.; Lu, A.; Wagle, D.V.; Baker, G.A. Activity coefficients at infinite dilution for organic solutes dissolved in two 1-alkylquinuclidinium bis(trifluoromethylsulfonyl)imides bearing alkyl side chains of six and eight carbons. J. Mol. Liq. 2016, 215, 176–184. [Google Scholar] [CrossRef]
- Paduszyński, K. Extensive Databases and Group Contribution QSPRs of Ionic Liquids Properties. 1. Density. Ind. Eng. Chem. Res. 2019, 58, 5322–5338. [Google Scholar] [CrossRef]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Tunon, I.; Silla, E.; Pascual-Ahuir, J. Continuum-uniform approach calculations of the solubility of hydrocarbons in water. Chem. Phys. Lett. 1993, 203, 289–294. [Google Scholar] [CrossRef]
- Gallicchio, E.; Zhang, L.Y.; Levy, R.M. The SGB/NP hydration free energy model based on the surface generalized born solvent reaction field and novel nonpolar hydration free energy estimators. J. Comput. Chem. 2002, 23, 517–529. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on the generalized born approximation with asymmetric descreening. J. Chem. Theory Comput. 2009, 5, 2447–2464. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.G. A New Measure of Rank Correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Pearlman, D.A.; Charifson, P.S. Are Free Energy Calculations Useful in Practice? A Comparison with Rapid Scoring Functions for the p38 MAP Kinase Protein System. J. Med. Chem. 2001, 44, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1992, 97, 10269–10280. [Google Scholar] [CrossRef]
- Hertwig, R.H.; Koch, W. On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem. Phys. Lett. 1997, 268, 345–351. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Price, D.J.; Brooks III, C.L. A Modified TIP3P Water Potential for Simulation with Ewald Summation. J. Chem. Phys. 2004, 121, 10096–10103. [Google Scholar] [CrossRef]
- Munoz, M.; Cardenas, C. How predictive could alchemical derivatives be? Phys. Chem. Chem. Phys. 2017, 19, 16003–16012. [Google Scholar] [CrossRef]
- Sun, Z.; He, Q. Seeding the Multi-dimensional Nonequilibrium Pulling for Hamiltonian Variation: Indirect Nonequilibrium Free Energy Simulations at QM levels. Phys. Chem. Chem. Phys. 2022, 24, 8800–8819. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, J.Z.H. BAR-based Optimum Adaptive Sampling Regime for Variance Minimization in Alchemical Transformation. Phys. Chem. Chem. Phys. 2017, 19, 15005–15020. [Google Scholar] [CrossRef]
- Giovannelli, E.; Cioni, M.; Procacci, P.; Cardini, G.; Pagliai, M.; Volkov, V.; Chelli, R. Binding free energies of host–guest systems by nonequilibrium alchemical simulations with constrained dynamics: Illustrative calculations and numerical validation. J. Chem. Theory Comput. 2017, 13, 5887–5899. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Wang, W.; Pang, L.; Zhang, Q.; Liu, X. Mutation-Induced Impacts on the Switch Transformations of the GDP- and GTP-Bound K-Ras: Insights from Multiple Replica Gaussian Accelerated Molecular Dynamics and Free Energy Analysis. J. Chem. Inf. Model. 2021, 61, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.I.; Shirts, M.R. Converging free energies of binding in cucurbit [7]uril and octa-acid host–guest systems from SAMPL4 using expanded ensemble simulations. J. Comput.-Aided Mol. Des. 2014, 28, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chodera, J.D.; Yang, Y.; Shirts, M.R. Identifying ligand binding sites and poses using GPU-accelerated Hamiltonian replica exchange molecular dynamics. J. Comput.-Aided Mol. Des. 2013, 27, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Macchiagodena, M.; Karrenbrock, M.; Pagliai, M.; Procacci, P. Virtual Double-System Single-Box for Absolute Dissociation Free Energy Calculations in GROMACS. J. Chem. Inf. Model. 2021, 61, 5320–5326. [Google Scholar] [CrossRef]
- Procacci, P. Hybrid MPI/OpenMP Implementation of the ORAC Molecular Dynamics Program for Generalized Ensemble and Fast Switching Alchemical Simulations. J. Chem. Inf. Model. 2016, 56, 1117–1121. [Google Scholar] [CrossRef]
- Bergazin, T.D.; Ben-Shalom, I.Y.; Lim, N.M.; Gill, S.C.; Gilson, M.K.; Mobley, D.L. Enhancing water sampling of buried binding sites using nonequilibrium candidate Monte Carlo. J. Comput.-Aided Mol. Des. 2021, 35, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.M.; Gapsys, V.; de Groot, B.L.; Mobley, D.L. Challenges encountered applying equilibrium and nonequilibrium binding free energy calculations. J. Phys. Chem. B 2021, 125, 4241–4261. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z. BAR-Based Multi-Dimensional Nonequilibrium Pulling for Indirect Construction of QM/MM Free Energy Landscapes: Varying the QM Region. Adv. Theory Simul. 2021, 4, 2100185. [Google Scholar] [CrossRef]
- Zhuang, Y.; Thota, N.; Quirk, S.; Hernandez, R. Implementation of Telescoping Boxes in Adaptive Steered Molecular Dynamics. J. Chem. Theory Comput. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Bureau, H.R.; Lopez, C.; Bucher, R.; Quirk, S.; Hernandez, R. Energetics and structure of alanine-rich α-helices via adaptive steered molecular dynamics. Biophys. J. 2021, 120, 2009–2018. [Google Scholar] [CrossRef]
- Jarzynski, C. A Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Lett. 1997, 78, 2690–2693. [Google Scholar] [CrossRef]
- Jarzynski, C. Equilibrium free-energy differences from nonequilibrium measurements: A master-equation approach. Phys. Rev. E 1997, 56, 5018–5035. [Google Scholar] [CrossRef]
- Crooks, G.E. Nonequilibrium Measurements of Free Energy Differences for Microscopically Reversible Markovian Systems. J. Stat. Phys. 1998, 90, 1481–1487. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z. A Theoretical Interpretation of Variance-based Convergence Citeria in Perturbation-based Theories. arXiv 2018, arXiv:1803.03123. [Google Scholar]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 2384. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S.; Klein, M.L. Constant pressure molecular dynamics for molecular systems. Molecular Physics 1983, 50, 1055–1076. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zheng, L.; Zhang, Z.-Y.; Cong, Y.; Wang, M.; Wang, X.; Yang, J.; Liu, Z.; Huai, Z. Molecular Modelling of Ionic Liquids: Situations When Charge Scaling Seems Insufficient. Molecules 2023, 28, 800. https://doi.org/10.3390/molecules28020800
Sun Z, Zheng L, Zhang Z-Y, Cong Y, Wang M, Wang X, Yang J, Liu Z, Huai Z. Molecular Modelling of Ionic Liquids: Situations When Charge Scaling Seems Insufficient. Molecules. 2023; 28(2):800. https://doi.org/10.3390/molecules28020800
Chicago/Turabian StyleSun, Zhaoxi, Lei Zheng, Zuo-Yuan Zhang, Yalong Cong, Mao Wang, Xiaohui Wang, Jingjing Yang, Zhirong Liu, and Zhe Huai. 2023. "Molecular Modelling of Ionic Liquids: Situations When Charge Scaling Seems Insufficient" Molecules 28, no. 2: 800. https://doi.org/10.3390/molecules28020800
APA StyleSun, Z., Zheng, L., Zhang, Z.-Y., Cong, Y., Wang, M., Wang, X., Yang, J., Liu, Z., & Huai, Z. (2023). Molecular Modelling of Ionic Liquids: Situations When Charge Scaling Seems Insufficient. Molecules, 28(2), 800. https://doi.org/10.3390/molecules28020800






