Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Adsorbents
2.1.1. XRD and FTIR Analysis
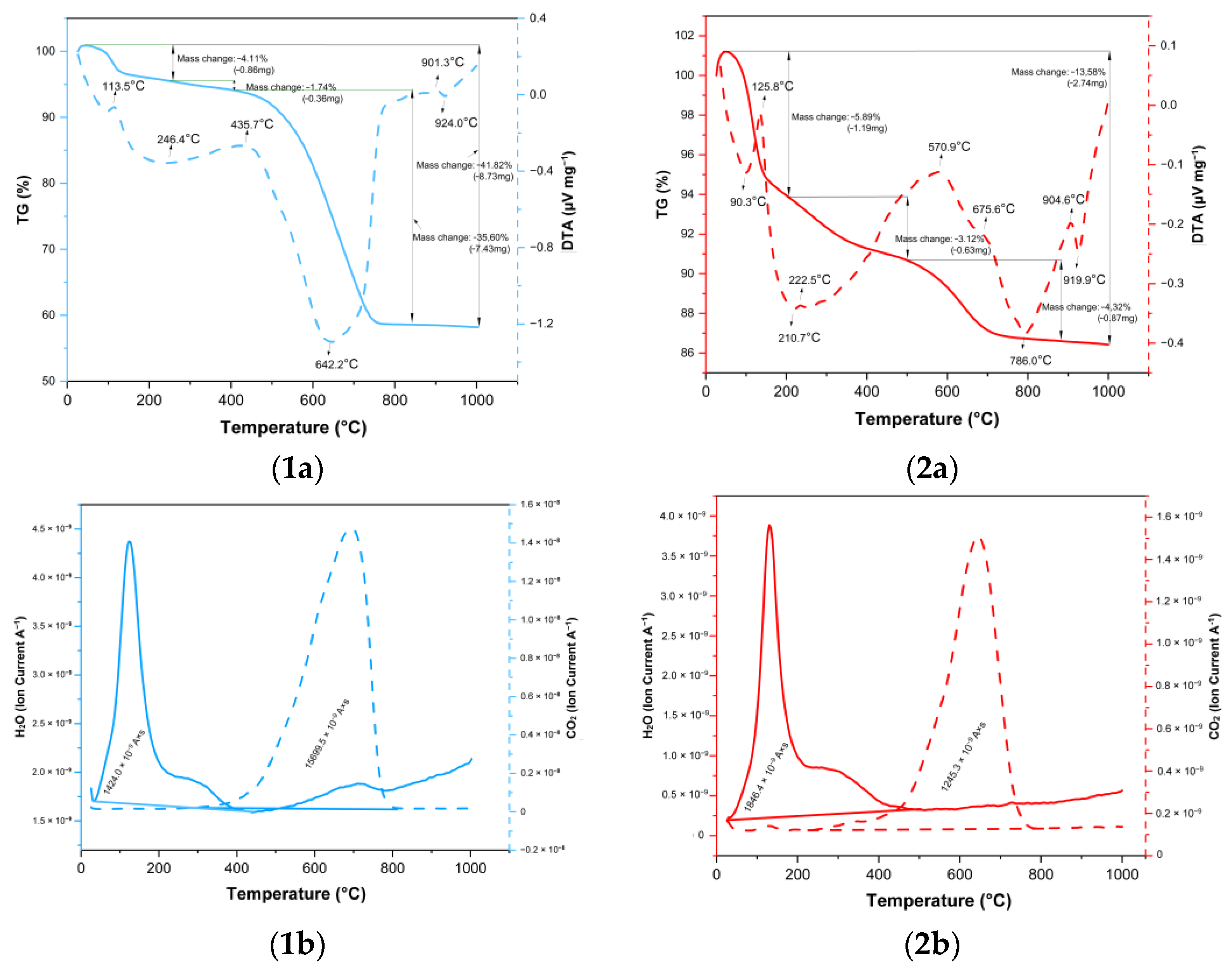
2.1.2. DTA/TG and Carbon, Hydrogen, and Nitrogen Elemental Analysis
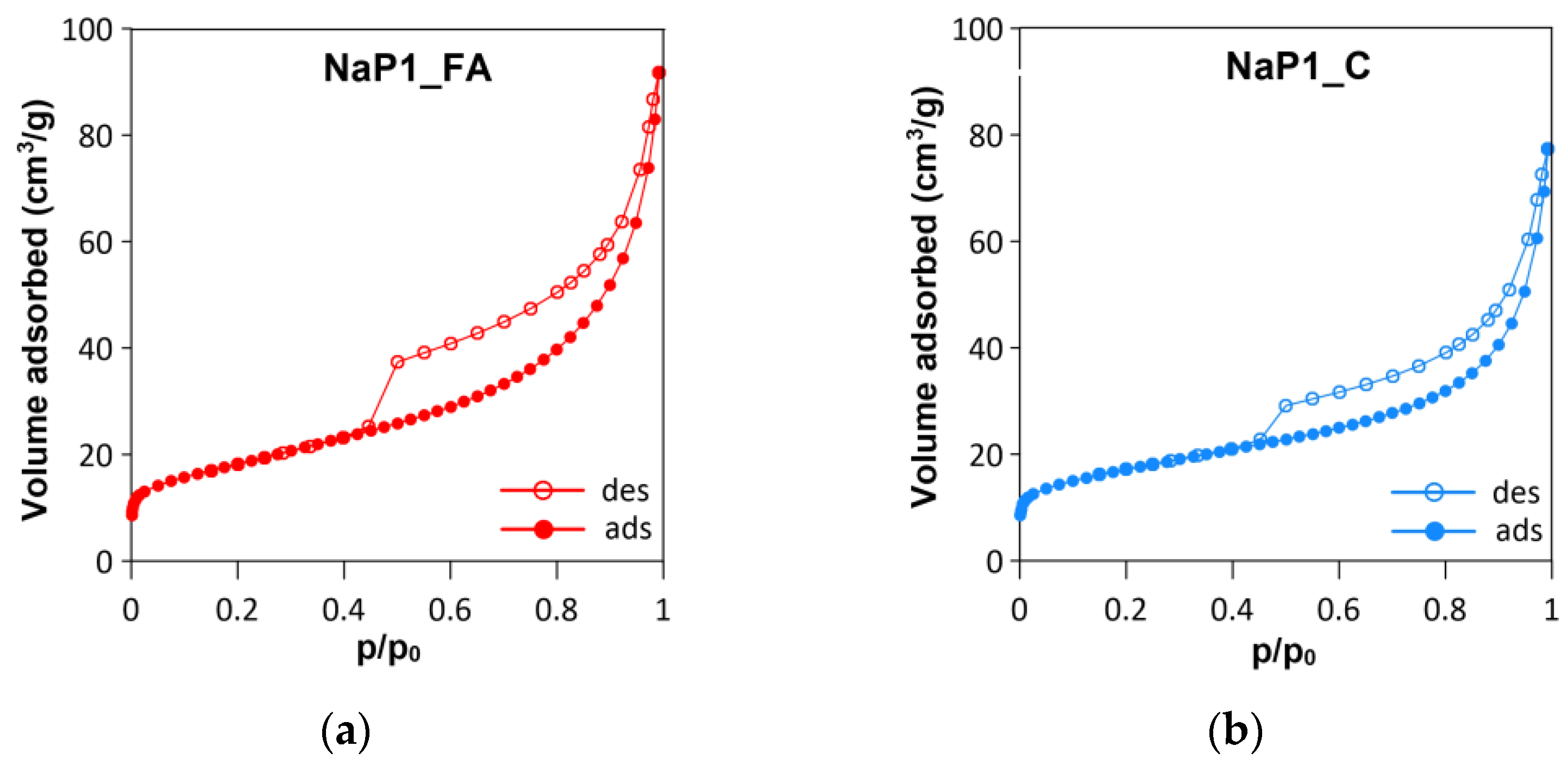
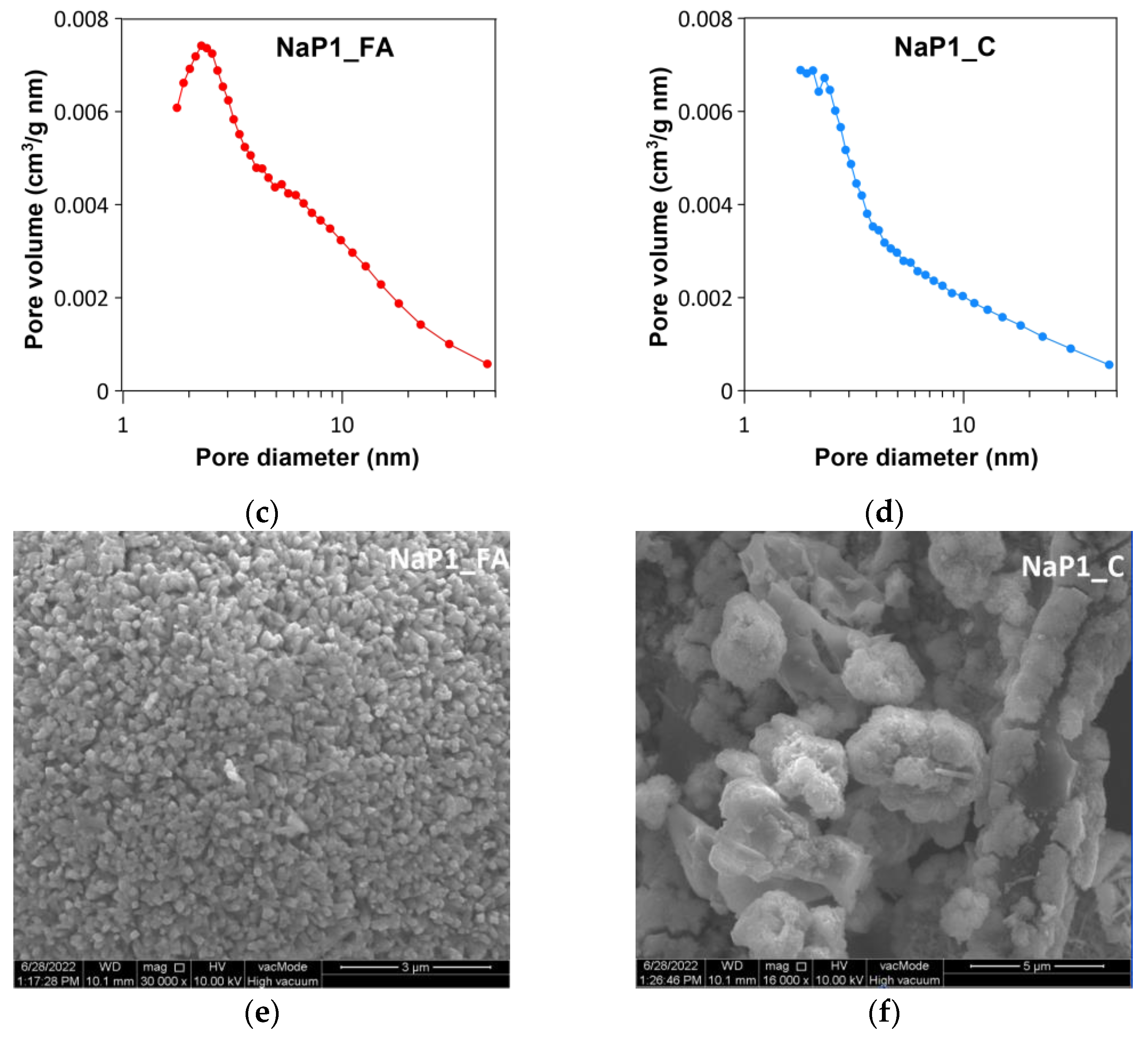
2.1.3. Texture and Morphology
2.2. EA Adsorption Experiments
2.2.1. Evaluation of Maximum Adsorption Capacity
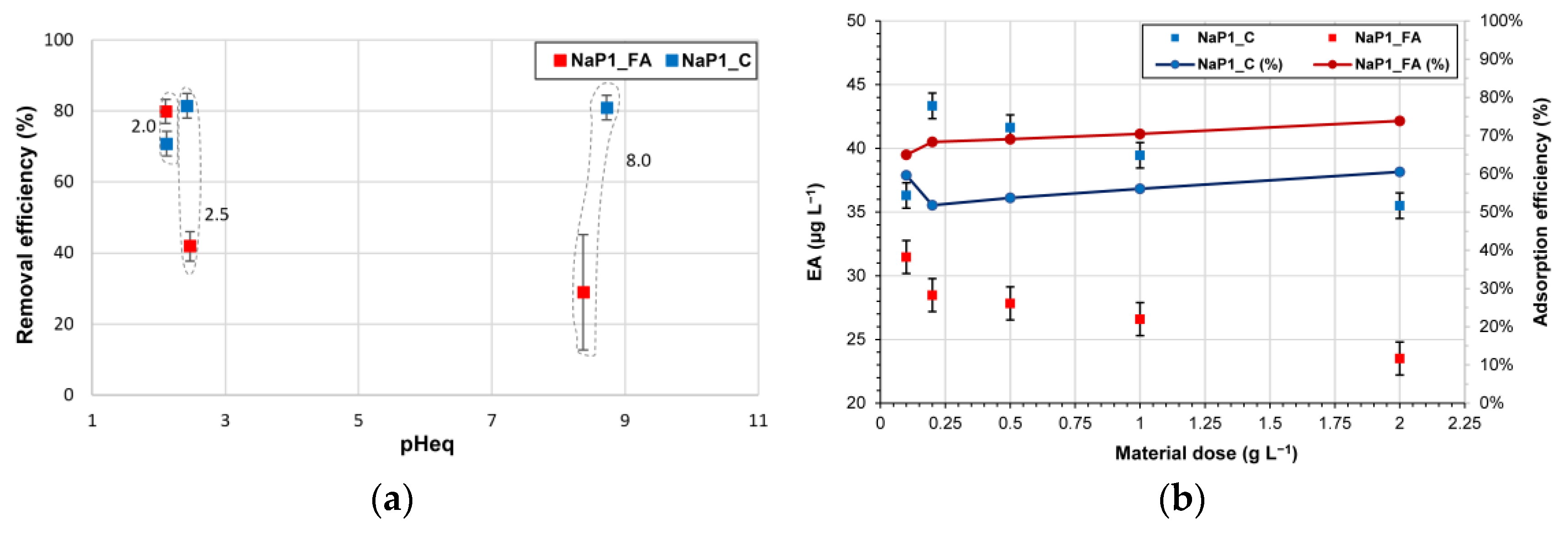
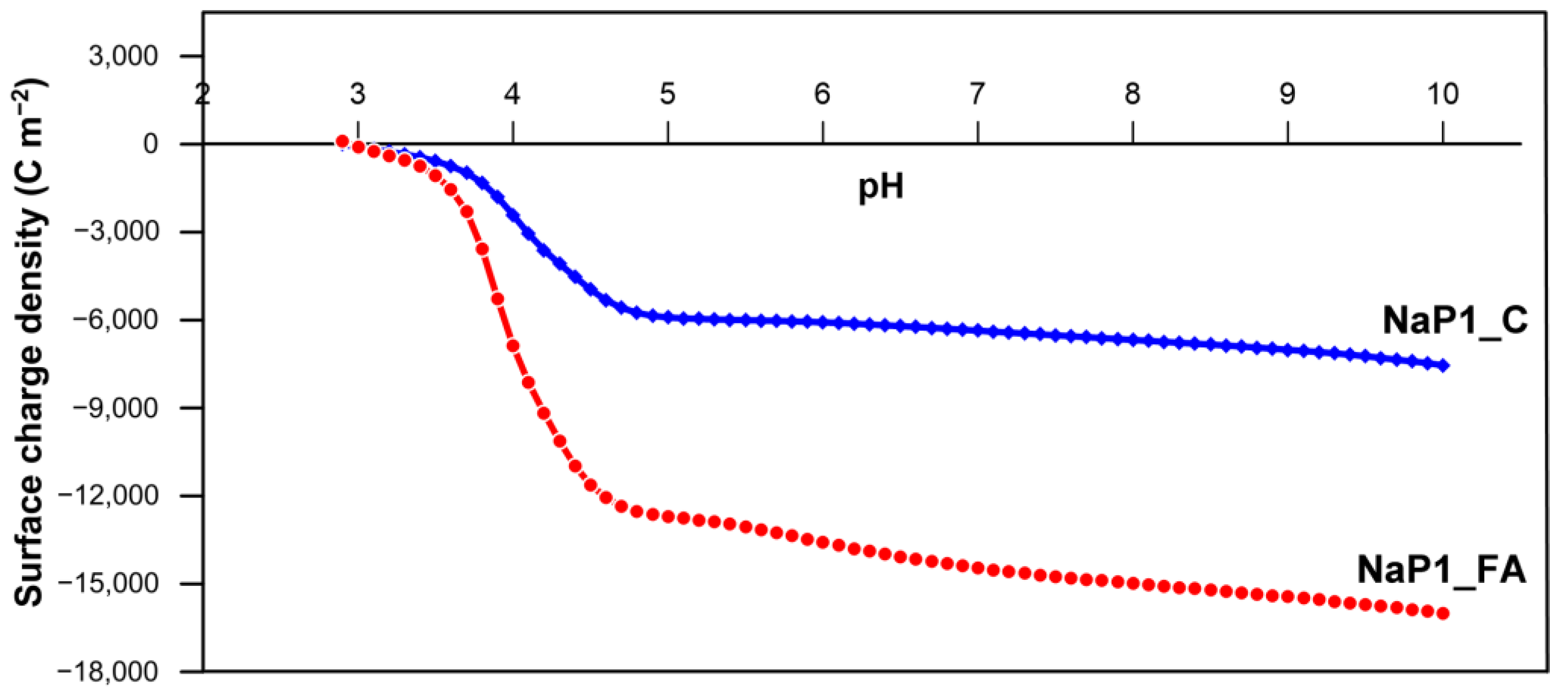
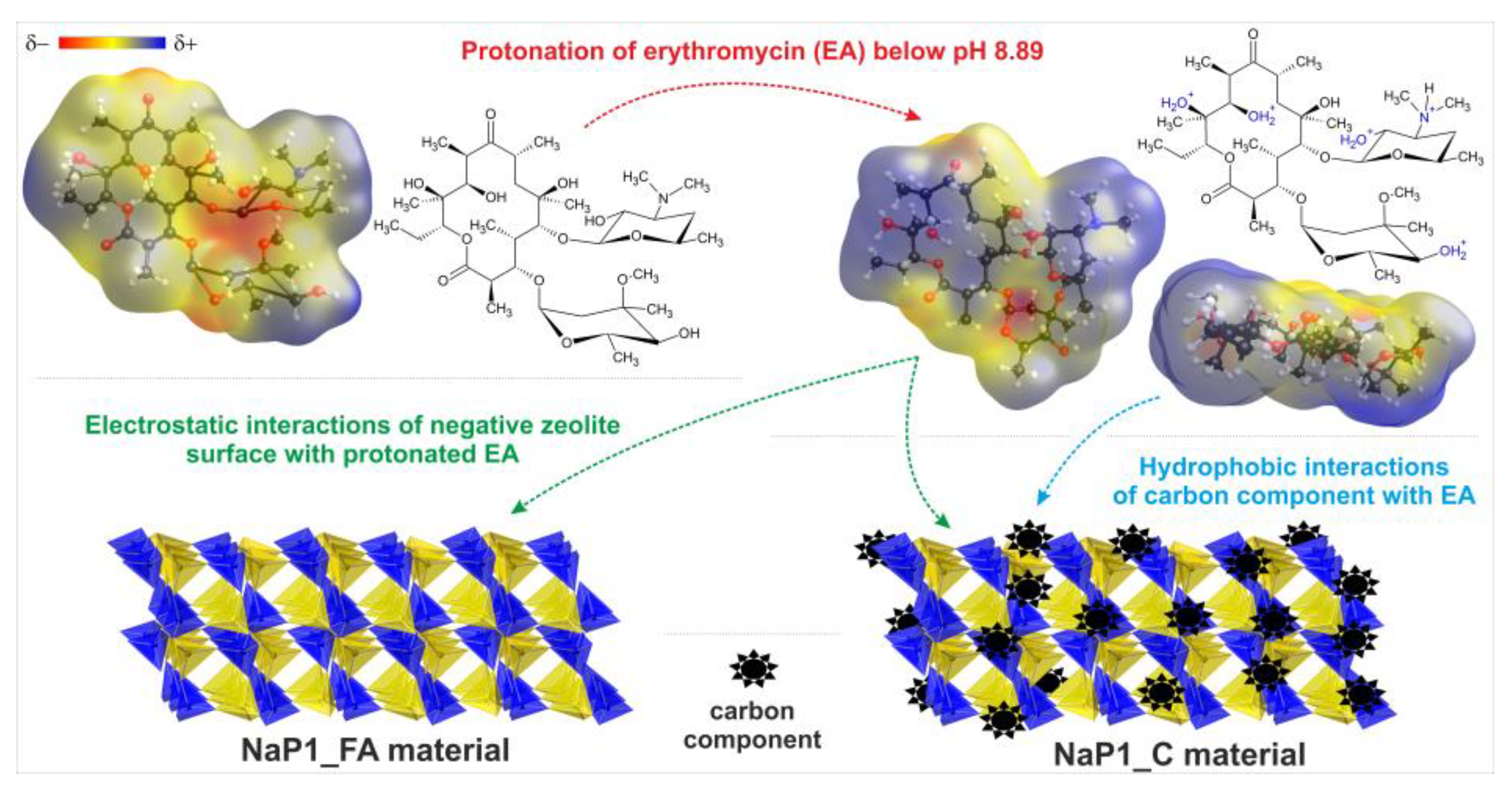
2.2.2. The Effect of pH and Dosage
2.2.3. Kinetics
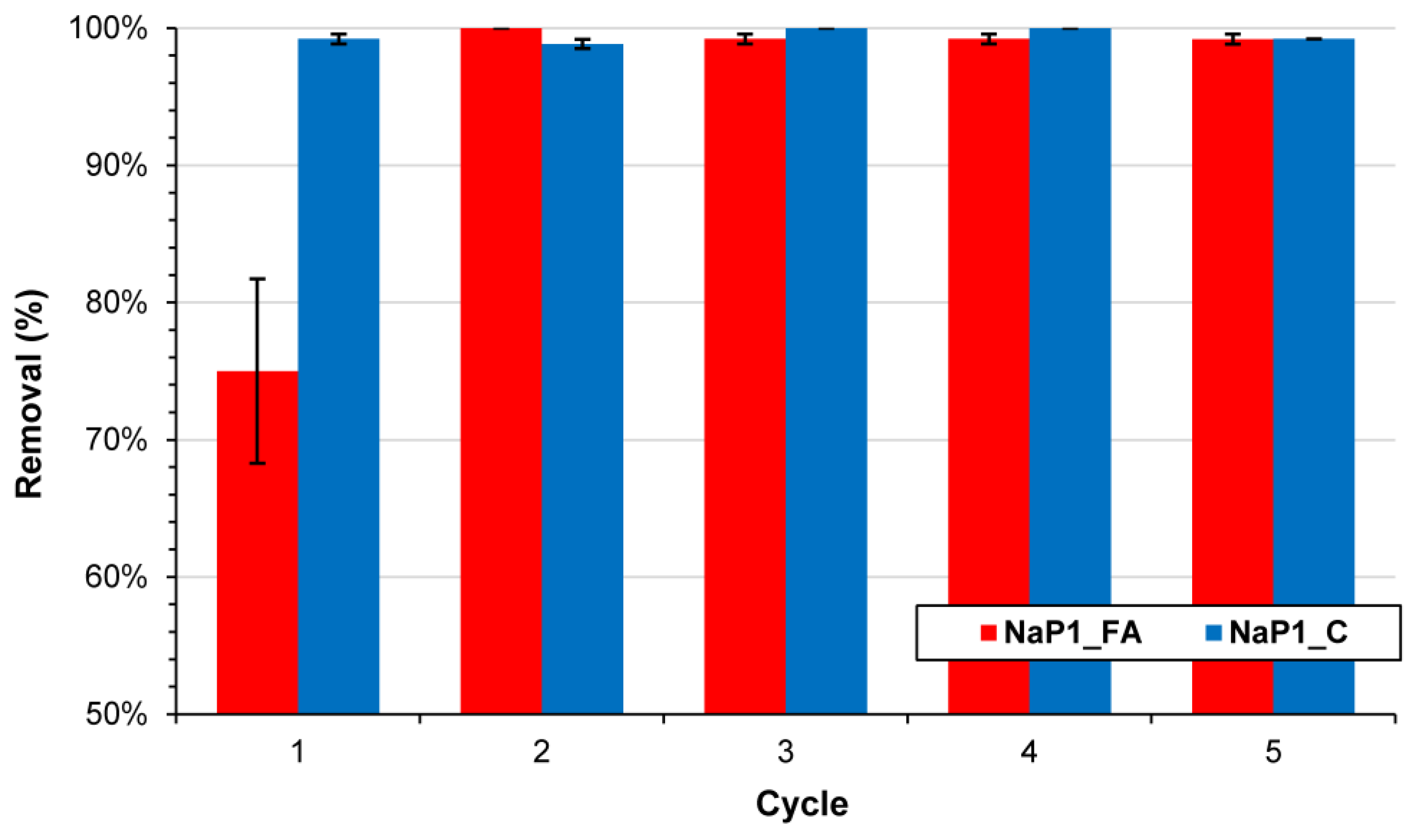
2.2.4. Regeneration Studies
2.2.5. Efficiency in the Treatment of Real Wastewater
3. Experimental Method
3.1. Materials and Chemicals
3.2. Adsorption and Desorption Experiments
3.3. Analytical Methods for Solid-State Characterization
3.4. Liquid Sample Extraction and UPLC (Ultra Performance Liquid Hromatography)–MS/MS Analysis
3.4.1. UPLC–MS/MS Determination Method
3.4.2. EA Extraction from Municipal Wastewater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Kiszkiel-Taudul, I. Determination of antihistaminic pharmaceuticals in surface water samples by SPE-LC-MS/MS method. Microchem. J. 2021, 162, 105874. [Google Scholar] [CrossRef]
- Bungau, S.; Tit, D.M.; Behl, T.; Aleya, L.; Zaha, D.C. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr. Opin. Environ. Sci. Health 2021, 19, 100224. [Google Scholar] [CrossRef]
- Bottoni, P.; Caroli, S. Presence of residues and metabolites of pharmaceuticals in environmental compartments, food commodities and workplaces: A review spanning the three-year period 2014–2016. Microchem. J. 2018, 136, 2–24. [Google Scholar] [CrossRef]
- Karthikraj, R.; Vasu, A.K.; Balakrishna, K.; Sinha, R.K.; Kannan, K. Occurrence and fate of parabens and their metabolites in five sewage treatment plants in India. Sci. Total Environ. 2017, 593–594, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Bidshahi, H.S.; Mesdaghinia, A. An optimized SPE-LC-MS/MS method for antibiotics residue analysis in ground, surface and treated water samples by response surface methodology- central composite design. J. Environ. Health Sci. Eng. 2017, 15, 21. [Google Scholar] [CrossRef]
- Leili, M.; Fazlzadeh, M.; Bhatnagar, A. Green synthesis of nano-zerovalent iron from Nettle and Thyme leaf extracts and their application for the removal of cephalexin antibiotic from aqueous solutions. Environ. Technol. 2018, 39, 1158–1172. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhao, Y.; Cui, L.; Huang, Z.; Long, J.; Xu, J.; Deng, J.; Wu, C.; Liao, W. Decontamination of tetracycline by thiourea-dioxide–reduced magnetic graphene oxide: Effects of pH, ionic strength, and humic acid concentration. J. Colloid Interface Sci. 2017, 495, 68–77. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Barchiesi, M.; Chiavola, A.; Di Marcantonio, C.; Boni, M.R. Presence and Fate of Microplastics in theWater Sources: Focus on the Role of Wastewater and Drinking Water Treatment Plants. J. Water Process Eng. 2021, 40, 101787. [Google Scholar] [CrossRef]
- Loganathan, B.; Phillips, M.; Mowery, H.; Jones-Lepp, T.L. Contamination profiles and mass loadings of macrolide antibiotics and illicit drugs from a small urban wastewater treatment plant. Chemosphere 2009, 75, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Borova, V.; Boix, C.; Aalizadeh, R.; Bade, R.; Thomaidis, N.S.; Hernández, F. UHPLC-QTOF MS screening of pharmaceuticals and their metabolites in treated wastewater samples from Athens. J. Hazard. Mater. 2017, 323, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Rusko, J.; Perkons, I.; Lundanes, E.; Bartkevics, V. Determination of pharmaceutical residues in wastewater using high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2017, 133, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Zepon Tarpani, R.R.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef]
- Du, B.; Price, A.E.; Scott, W.C.; Kristofco, L.A.; Ramirez, A.J.; Chambliss, C.K.; Yelderman, J.C.; Brooks, B.W. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ. 2014, 466–467, 976–984. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef]
- Rossmann, J.; Schubert, S.; Gurke, R.; Oertel, R.; Kirch, W. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 162–170. [Google Scholar] [CrossRef]
- Cano, P.A.; Jaramillo-Baquero, M.; Zúñiga-Benítez, H.; Londoño, Y.A.; Peñuela, G.A. Use of simulated sunlight radiation and hydrogen peroxide in azithromycin removal from aqueous solutions: Optimization & mineralization analysis. Emerg. Contam. 2020, 6, 53–61. [Google Scholar]
- Basheer, A.A. Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality 2018, 30, 402–406. [Google Scholar] [CrossRef]
- Jin, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Xu, R.; Zhang, Y.; Cao, J.; Li, X.; Zhou, C. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Sci. Total Environ. 2019, 650, 408–418. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Olama, N.; Malakootian, M.; Nasiri, A. Photocatalytic degradation of metronidazole from aquatic solution by TiO2-doped Fe3+ nano-photocatalyst. Int. J. Environ. Sci. Technol. 2019, 16, 4275–4284. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zhu, C.; Chen, F.; Yang, Y.; Chen, X. Liberation and recovery of Cr from real tannery sludge by ultrasound-assisted supercritical water oxidation treatment. J. Clean. Prod. 2020, 267, 122064. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Zhang, L.; Dong, H.; Yu, H. Improved Norfloxacin degradation by urea precipitation Ti/SnO2–Sb anode under photo-electro catalysis and kinetics investigation by BP-neural-network-physical modeling. J. Clean. Prod. 2021, 280, 124412. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Al-Maadheed, S.; Goktepe, I.; Latiff, A.B.A.; Shomar, B. Antibiotics in hospital effluent and domestic wastewater treatment plants in Doha, Qatar. J. Water Process Eng. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Ondon, B.S.; Liu, Y.; Wang, H. Degradation performance and microbial community analysis of microbial electrolysis cells for erythromycin wastewater treatment. Biochem. Eng. J. 2019, 146, 1–9. [Google Scholar] [CrossRef]
- Chu, L.; Zhuan, R.; Chen, D.; Wang, J.; Shen, Y. Degradation of macrolide antibiotic erythromycin and reduction of antimicrobial activity using persulfate activated by gamma radiation in different water matrices. Chem. Eng. J. 2019, 361, 156–166. [Google Scholar] [CrossRef]
- McFarland, J.W.; Berger, C.M.; Froshauer, S.A.; Hayashi, S.F.; Hecker, S.J.; Jaynes, B.H.; Jefson, M.R.; Kamicker, B.J.; Lipinski, C.A.; Lundy, K.M.; et al. Quantitative structure-activity relationships among macrolide antibacterial agents: In vitro and in vivo potency against Pasteurella multocida. J. Med. Chem. 1997, 40, 1340–1346. [Google Scholar] [CrossRef]
- Lucchetti, D.; Fabrizi, L.; Esposito, A.; Guandalini, E.; Di Pasquale, M.; Coni, E. Simple confirmatory method for the determination of erythromycin residues in trout: A fast liquid-liquid extraction followed by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 9689–9694. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Barber, J.; Morris, G.A.; Gorry, P.A. Mechanism for the degradation of erythromycin A and erythromycin A 2′-ethyl succinate in acidic aqueous solution. J. Phys. Chem. A 2007, 111, 10098–10104. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Mery-Araya, C.; Lear, G.; Perez-Garcia, O.; Astudillo-Garcia, C.; Singhal, N. Using carbon substrate as a selection pressure to enhance the potential of aerobic granular sludge microbial communities for removing contaminants of emerging concern. Bioresour. Technol. 2019, 290, 121705. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S. A combined treatment method of novel Mass Bio System and ion exchange for the removal of ammonia nitrogen from micro-polluted water bodies. Chem. Eng. J. 2019, 378, 122217. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; ALOthman, Z.A.; Al-Mohaimeed, A.M.; Alwarthan, A. Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ. Res. 2019, 170, 389–397. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Janjani, H. Antibiotics adsorption from aqueous solutions using carbon nanotubes: A systematic review. Toxin Rev. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Ocampo-Perez, R.; Sanchez-Polo, M.; Lopez-Penalver, J.J.; Gomez-Pacheco, C.V. Removal of Tetracyclines from Water by Adsorption/Bioadsorption and Advanced Oxidation Processes. A Short Review. Curr. Org. Chem. 2018, 22, 1005–1021. [Google Scholar] [CrossRef]
- Van Wieren, E.M.; Seymour, M.D.; Peterson, J.W. Interaction of the fluoroquinolone antibiotic, ofloxacin, with titanium oxide nanoparticles in water: Adsorption and breakdown. Sci. Total Environ. 2012, 441, 1–9. [Google Scholar] [CrossRef]
- Kassotaki, E.; Buttiglieri, G.; Ferrando-Climent, L.; Rodriguez-Roda, I.; Pijuan, M. Enhanced sulfamethoxazole degradation through ammonia oxidizing bacteria co-metabolism and fate of transformation products. Water Res. 2016, 94, 111–119. [Google Scholar] [CrossRef]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP Removal from Wastewater by Powdered Activated Carbon/Ultrafiltration. Water. Air. Soil Pollut. 2016, 227, 177. [Google Scholar] [CrossRef]
- Teixeira, S.; Delerue-Matos, C.; Santos, L. Application of experimental design methodology to optimize antibiotics removal by walnut shell based activated carbon. Sci. Total Environ. 2019, 646, 168–176. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. A Review on Synthesis, Characterization and Industrial Applications of Flyash Zeolites. ChemInform 2012, 43, 65. [Google Scholar] [CrossRef]
- Martucci, A.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Dondi, F.; Alberti, A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater. 2012, 148, 174–183. [Google Scholar] [CrossRef]
- Atlas of Zeolite Framework Types—6th Edition. Available online: https://www.elsevier.com/books/atlas-of-zeolite-framework-types/baerlocher/978-0-444-53064-6 (accessed on 24 September 2022).
- Scott, J.; Guang, D.; Naeramitmarnsuk, K.; Thabuot, M.; Amal, R. Zeolite synthesis from coal fly ash for the removal of lead ions from aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 63–69. [Google Scholar] [CrossRef]
- Zide, D.; Fatoki, O.; Oputu, O.; Opeolu, B.; Nelana, S.; Olatunji, O. Zeolite ‘adsorption’ capacities in aqueous acidic media; The role of acid choice and quantification method on ciprofloxacin removal. Microporous Mesoporous Mater. 2018, 255, 226–241. [Google Scholar] [CrossRef]
- Huo, P.; Lu, Z.; Wang, H.; Pan, J.; Li, H.; Wu, X.; Huang, W.; Yan, Y. Enhanced photodegradation of antibiotics solution under visible light with Fe 2+/Fe 3+ immobilized on TiO 2/fly-ash cenospheres by using ions imprinting technology. Chem. Eng. J. 2011, 172, 615–622. [Google Scholar] [CrossRef]
- Swarcewicz, M.K.; Sobczak, J.; Paździoch, W. Removal of carbamazepine from aqueous solution by adsorption on fly ash-amended soil. Water Sci. Technol. 2013, 67, 1396–1402. [Google Scholar] [CrossRef]
- Nachiappan, S.; Gopinath, K.P. Treatment of pharmaceutical effluent using novel heterogeneous fly ash activated persulfate system. J. Environ. Chem. Eng. 2015, 3, 2229–2235. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Koteja, A.; Franus, W. Influence of the reaction time on the crystal structure of Na-P1 zeolite obtained from coal fly ash microspheres. Microporous Mesoporous Mater. 2018, 266, 102–108. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Wdowin, M.; Franus, W. Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash. Int. J. Miner. Process. 2017, 166, 69–78. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel 2016, 185, 181–189. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Chen, Y.; Pu, X.; Jiang, W.; Yao, L. A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution. Bioresour. Technol. 2018, 268, 726–732. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative study of the reactivity of fly ash in geopolymerization by ftir. J. Sustain. Cem. Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Sharma, P.; Song, J.S.; Han, M.H.; Cho, C.H. GIS-NaP1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci. Rep. 2016, 6, 22734. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Bohra, S.; Kundu, D.; Naskar, M.K. Synthesis of cashew nut-like zeolite NaP powders using agro-waste material as silica source. Mater. Lett. 2013, 106, 182–185. [Google Scholar] [CrossRef]
- Woszuk, A.; Zofka, A.; Bandura, L.; Franus, W. Effect of zeolite properties on asphalt foaming. Constr. Build. Mater. 2017, 139, 247–255. [Google Scholar] [CrossRef]
- Janusz, W. Electrical double layer at the metal oxide/electrolyte interface in interfacial forces and fields: Theory and applications. Surfactant Sci. Ser. 1994, 85, 135–206. [Google Scholar]
- Zhou, Q.; Xuguang, J.; Qui, Q.; Zhao, Y.; Long, L. Synthesis of high-quality Na-P1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity. Sci. Total Environ. 2023, 855, 158741. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Micropore Analysis. In Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ghobarkar, H.; Schäf, O. Synthesis of gismondine-type zeolites by the hydrothermal method. Mater. Res. Bull. 1999, 34, 517–525. [Google Scholar] [CrossRef]
- Danalıoğlu, S.T.; Bayazit, Ş.S.; Kerkez Kuyumcu, Ö.; Salam, M.A. Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J. Mol. Liq. 2017, 240, 589–596. [Google Scholar] [CrossRef]
- Jodeh, S.; Erman, I.; Hamed, O.; Massad, Y.; Hanbali, G.; Samhan, S.; Dagdag, O.; Kaya, S.; Serdaroğlu, G. Zeolite/Cellulose Acetate (ZCA) in Blend Fiber for Adsorption of Erythromycin Residue From Pharmaceutical Wastewater: Experimental and Theoretical Study. Front. Chem. 2021, 9, 709600. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Li, X.; Lei, X.; Li, C.; Yin, D.; Fan, X.; Zhang, Q. Synthesis of BSA/Fe3O4 magnetic composite microspheres for adsorption of antibiotics. Mater. Sci. Eng. C 2013, 33, 4401–4408. [Google Scholar] [CrossRef]
- Mostafapour, F.K.; Dashtizade, M.; Balarak, D. Adsorption Thermodynamics, Kinetics and Mechanism for the Adsorption of Erythromycin onto Multi-Walled Carbon Nanotubes. J. Pharm. Res. Int. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Bahmei, F.; Bahramifar, N.; Younesi, H.; Tolstoy, V. Synthesis of porous graphene nanocomposite and its excellent adsorption behavior for erythromycin antibiotic. Nanosyst. Phys. Chem. Math. 2020, 11, 214–222. [Google Scholar] [CrossRef]
- Hou, L.; Han, X.; Wang, N. High performance of molecularly imprinted polymer for the selective adsorption of erythromycin in water. Colloid Polym. Sci. 2020, 298, 1023–1033. [Google Scholar] [CrossRef]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM optimized adsorptive removal of erythromycin using magnetic activated carbon: Adsorption isotherm, kinetic modeling and thermodynamic studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Tian, Y.; Chowdhury, I. Adsorption of selected pharmaceutical and personal care products with molybdenum disulfide and tungsten disulfide nanomaterials. Environ. Eng. Sci. 2019, 36, 305–315. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Ghanbary, F. A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater. 2007, 148, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Goswami, A.; Jiang, J.Q.; Petri, M. Treatability of five micro-pollutants using modified Fenton reaction catalysed by zero-valent iron powder (Fe(0)). J. Environ. Chem. Eng. 2021, 9, 105393. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, E.A.; Castillo-Suárez, L.A.; Teutli-Sequeira, E.A.; Martínez-Miranda, V.; Vázquez Mejía, G.; Linares-Hernández, I.; Santoyo-Tepole, F.; Benavides, A. Electro-oxidation and solar electro-oxidation of commercial carbamazepine: Effect of the support electrolyte. Sep. Sci. Technol. 2022, 57, 465–483. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Mansha, A.; Bhatti, I.A.; Mustafa, G.; Nasir, S.; Yaseen, M. MnFe2O4/coal fly ash nanocomposite: A novel sunlight-active magnetic photocatalyst for dye degradation. Int. J. Environ. Sci. Technol. 2020, 17, 4233–4248. [Google Scholar] [CrossRef]
- Jodeh, S.; Hamed, O.; Melhem, A.; Salghi, R.; Jodeh, D.; Azzaoui, K.; Benmassaoud, Y.; Murtada, K. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 22060–22074. [Google Scholar] [CrossRef]
- Szabová, P.; Plekancová, M.; Gróf, N.; Bodík, I. Slovak natural zeolites as a suitable medium for antibiotics elimination from wastewater. Acta Chim. Slovaca 2020, 12, 163–167. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The conversion technology of fly ash into zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Tong, L.; Li, P.; Wang, Y.; Zhu, K. Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere 2009, 74, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, L.; Van Loco, J.; Mansour, H.B.; Reyns, T. Occurrence of antibiotics in pharmaceutical industrial wastewater, wastewater treatment plant and sea waters in Tunisia. J. Water Health 2016, 14, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, C.; Hong, Y.; Lee, W.; Lee, S.; Chung, H.; Kim, H.; Jeong, D.H. Determination of pharmaceuticals in solid samples in municipal wastewater treatment plants by online SPE LC–MS/MS using QuEChERS extraction. Environ. Monit. Assess. 2021, 193, 279. [Google Scholar] [CrossRef]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef]
- Semreen, M.H.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.; Acharya, K. Simultaneous Determination of Pharmaceuticals by Solid-phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry: A case study from sharjah sewage treatment plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef]



| Therapeutic Class | Chemical Structure | pKa | Reference |
|---|---|---|---|
| Antibacterial agent |  | 8.89 | [29] |
| Material | SBET m2 g−1 | Vtot0.99 cm3 g−1 | VmicDR cm3 g−1 | VmesBJH cm3 g−1 |
|---|---|---|---|---|
| NaP1_FA | 64.7 | 0.139 | 0.024 | 0.101 |
| NaP1_C | 61.0 | 0.115 | 0.023 | 0.078 |
| Model | Equation | Symbol Explanation |
|---|---|---|
| Langmuir | qeq—amount adsorbed by zeolite at equilibrium, mg g−1 Ceq—the equilibrium concentration in solution, mg L−1 qmax—monolayer capacity of the adsorbent, mg g−1 KL—the Langmuir adsorption constant, L mg−1 | |
| RL | RL—dimensionless C0—initial concentration in solution, mg L−1 KL—the Langmuir adsorption constant, L mg−1 | |
| Freundlich | qeq—amount adsorbed at equilibrium, mg g−1 Ceq—the equilibrium concentration in solution, mg L−1 KF—the Freundlich constant, mg1−n 1/n—heterogeneity factor, Ln g−1 |
| Adsorption Model | ||||||
|---|---|---|---|---|---|---|
| Sample | Langmuir | Freundlich | ||||
| qmax mg g−1 | KL | R2 | qmax mg g−1 | KF | R2 | |
| NaP1_C | 5.66 | 2.19 | 0.31 | 7.14 | 1.91 | 0.74 |
| NaP1_FA | 1.19 | 1.03 | 0.29 | 5.72 | 1.66 | 0.91 |
| Adsorbent | EA Concentration mg L−1 | Adsorption Capacity mg g−1 | Dosage g L−1 | Reference |
|---|---|---|---|---|
| NaP1_C | 1.0 | 17.75 | 2.0 | This work |
| NaP1_FA | 1.0 | 11.75 | 2.0 | This work |
| MAC | 65.0 | 248.91 | 1.55 | [73] |
| MACC | 60.0 | 178.52 | 0.01 | [67] |
| BSA/Fe3O4 | 144.51 | [69] | ||
| MWCNT | 100 | 104.8 | 0.8 | [70] |
| MoS2 | 4.0 | 57.10 | 1.0 | [74] |
| WS2 | 4.0 | 190.31 | 1.0 | [74] |
| PMG | 200.0 | 286.0 | 0.35 | [71] |
| ERY@MIP | 8000 | 44.03 | [72] | |
| ZCA | 20 | 17.76 | [68] |
| Kinetic Model | Adsorbent | |
|---|---|---|
| NaP1_C | NaP1_FA | |
| pseudo-zero-order model | 0.0008 | 0.1023 |
| pseudo-first-order model | 0.0082 | 0.0859 |
| pseudo-second-order model | 0.0073 | 0.062 |
| BMG model | 1 | 1 |
| Parameter or Component | Method of Determination | Unit | Result ± Uncertainty |
|---|---|---|---|
| pH | potentiometric | - | 7.6 ± 0.1 |
| Electrical conductivity | conductometric | μS cm−1 | 1730 ± 87 |
| Salinity | (at a reference temperature of 20 °C) | mg NaCl L−1 | 870 ± 44 |
| Turbidity | conductometric | FAU | 99 ± 10 |
| Color | (at a reference temperature of 20 °C) | mg Pt L−1 | 77 ± 15 |
| COD(Cr) | nephelometric | mg O2 L−1 | 390 ± 59 |
| TOC | spectrophotometric | mg L−1 | 183 ± 27 |
| Ammoniacal nitrogen | spectrophotometric | mg L−1 | 83 ± 8 |
| Total phosphorus | spectrophotometric | mg L−1 | 10 ± 1 |
| Phosphate | spectrophotometric | mg L−1 | 7.0 ± 0.7 |
| Chlorides | spectrophotometric | mg L−1 | 160 ± 16 |
| Sulphates (VI) | spectrophotometric | mg L−1 | 125 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grela, A.; Kuc, J.; Klimek, A.; Matusik, J.; Pamuła, J.; Franus, W.; Urbański, K.; Bajda, T. Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash. Molecules 2023, 28, 798. https://doi.org/10.3390/molecules28020798
Grela A, Kuc J, Klimek A, Matusik J, Pamuła J, Franus W, Urbański K, Bajda T. Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash. Molecules. 2023; 28(2):798. https://doi.org/10.3390/molecules28020798
Chicago/Turabian StyleGrela, Agnieszka, Joanna Kuc, Agnieszka Klimek, Jakub Matusik, Justyna Pamuła, Wojciech Franus, Kamil Urbański, and Tomasz Bajda. 2023. "Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash" Molecules 28, no. 2: 798. https://doi.org/10.3390/molecules28020798
APA StyleGrela, A., Kuc, J., Klimek, A., Matusik, J., Pamuła, J., Franus, W., Urbański, K., & Bajda, T. (2023). Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash. Molecules, 28(2), 798. https://doi.org/10.3390/molecules28020798








