Amino Derivatives of Diaryl Pyrimidines and Azolopyrimidines as Protective Agents against LPS-Induced Acute Lung Injury
Abstract
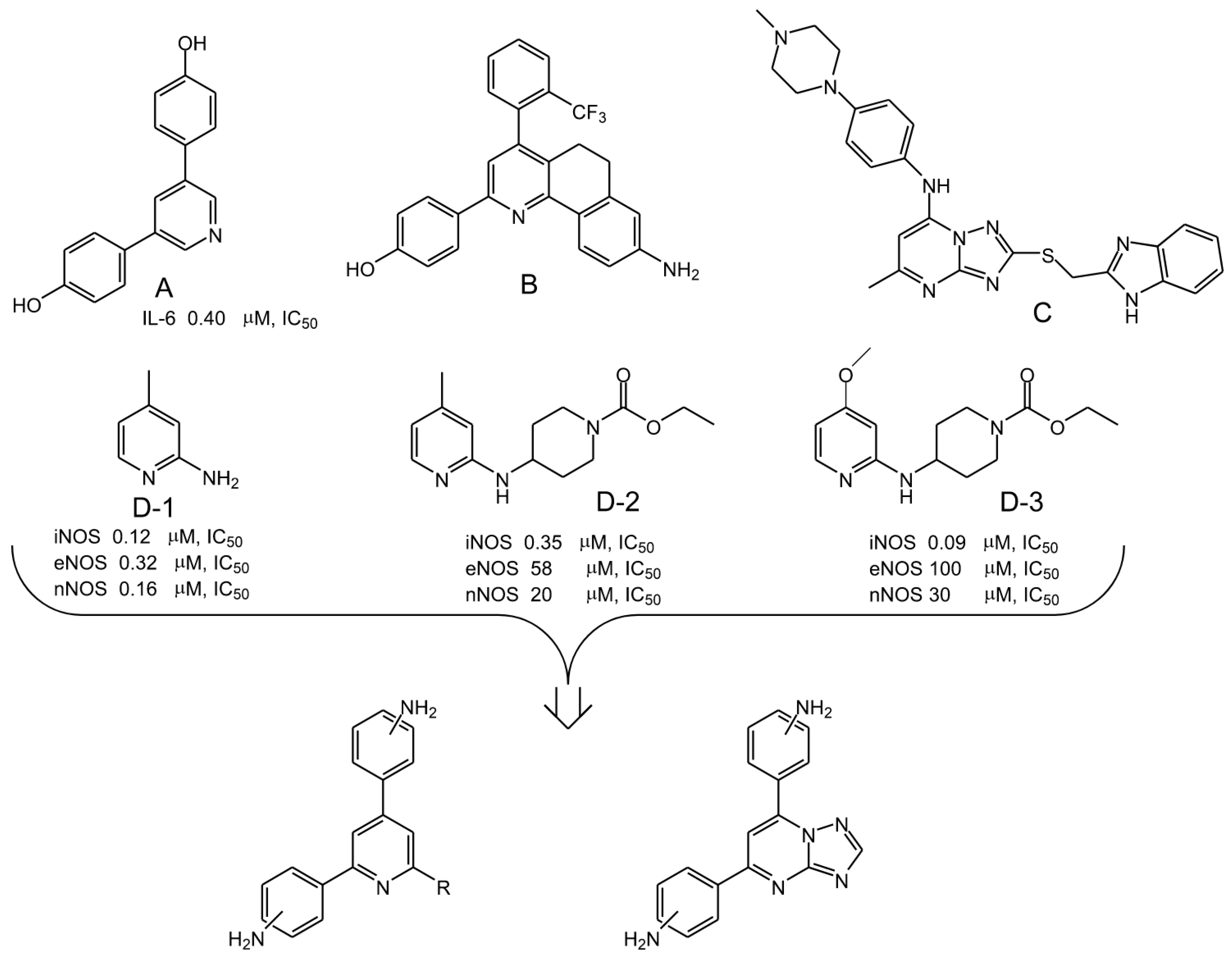
1. Introduction
2. Results
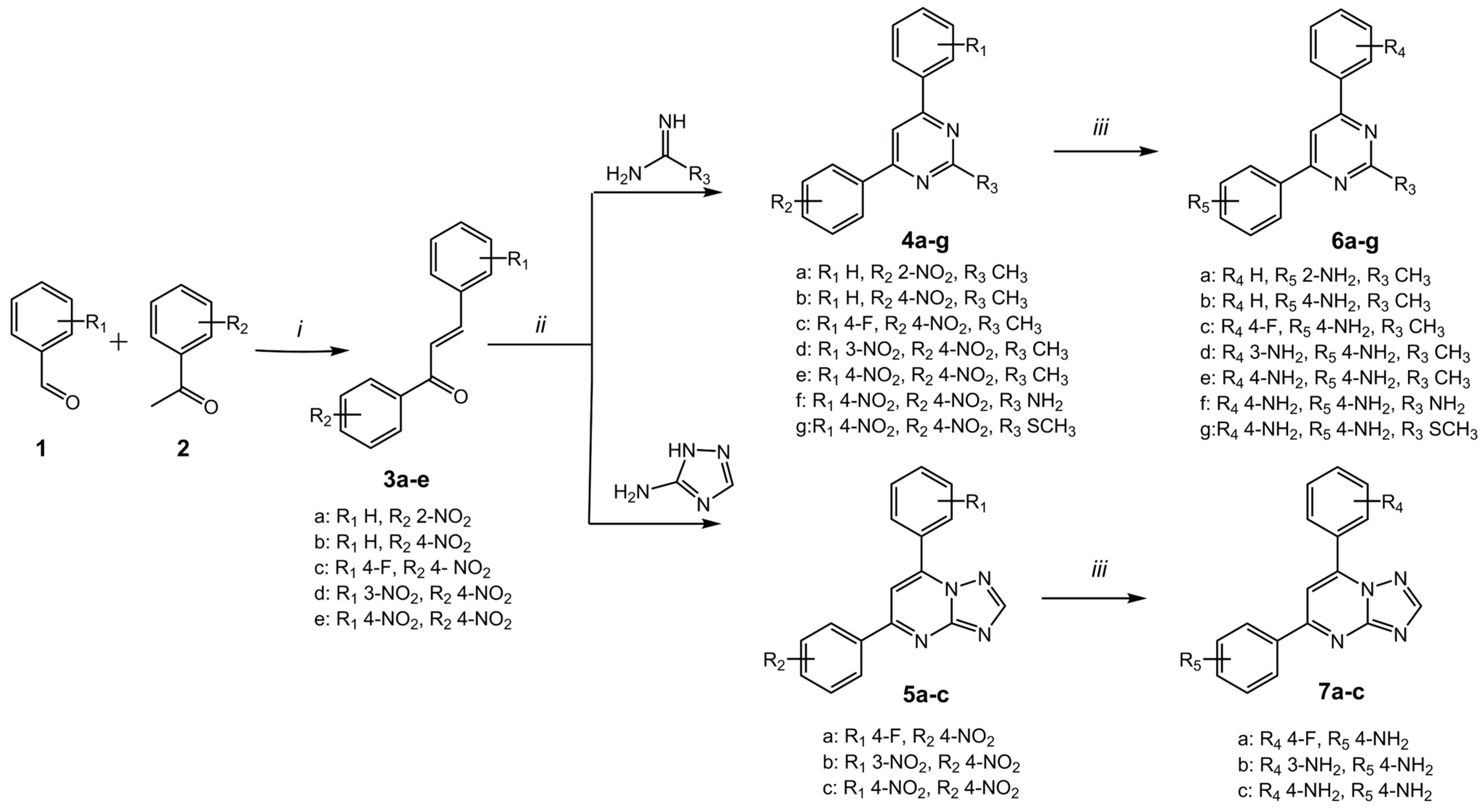
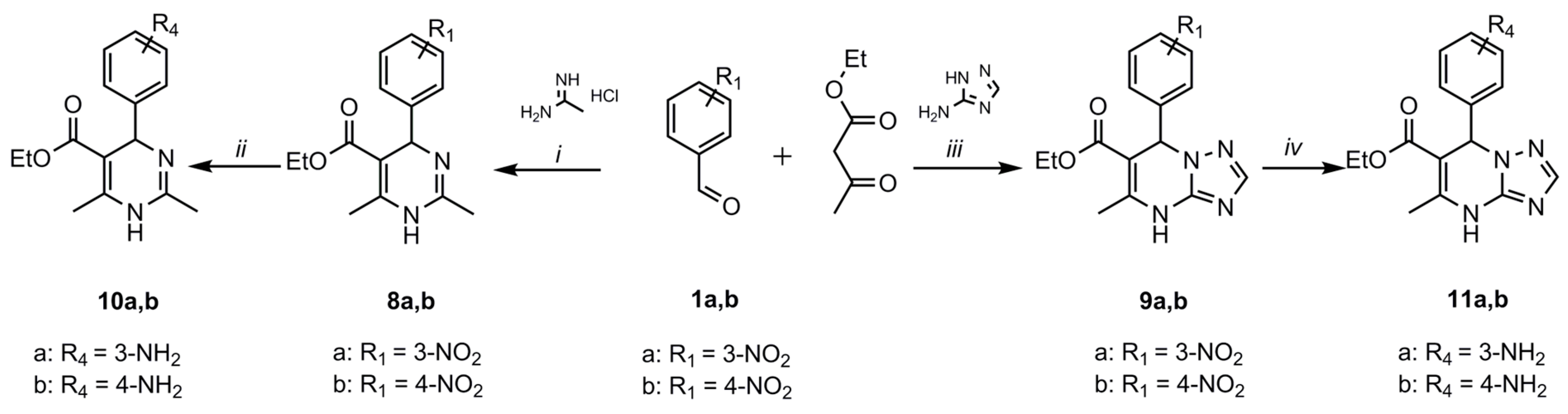
2.1. Synthesis
2.2. Target Compounds Inhibiting NO and IL-6 Release from LPS-Stimulated Macrophages In Vitro
2.3. Active Compounds Demonstrate Variable Influence on Macrophage Phagocytosis
2.4. Compound 6e Protects LPS-Induced Acute Lung Injury In Vivo
2.4.1. Open Field Test
2.4.2. Biochemical Markers of Inflammation
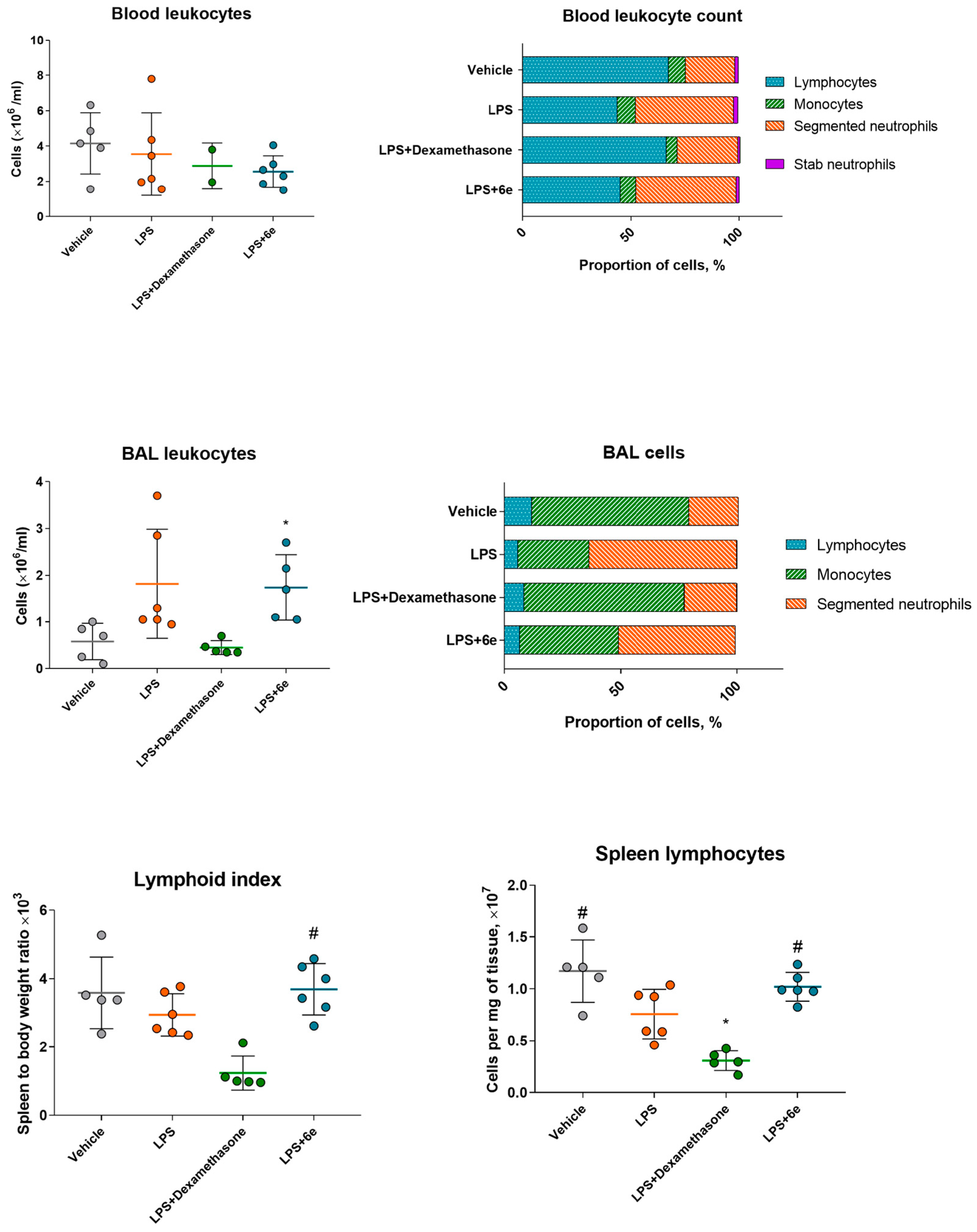
2.4.3. Leukocyte Markers
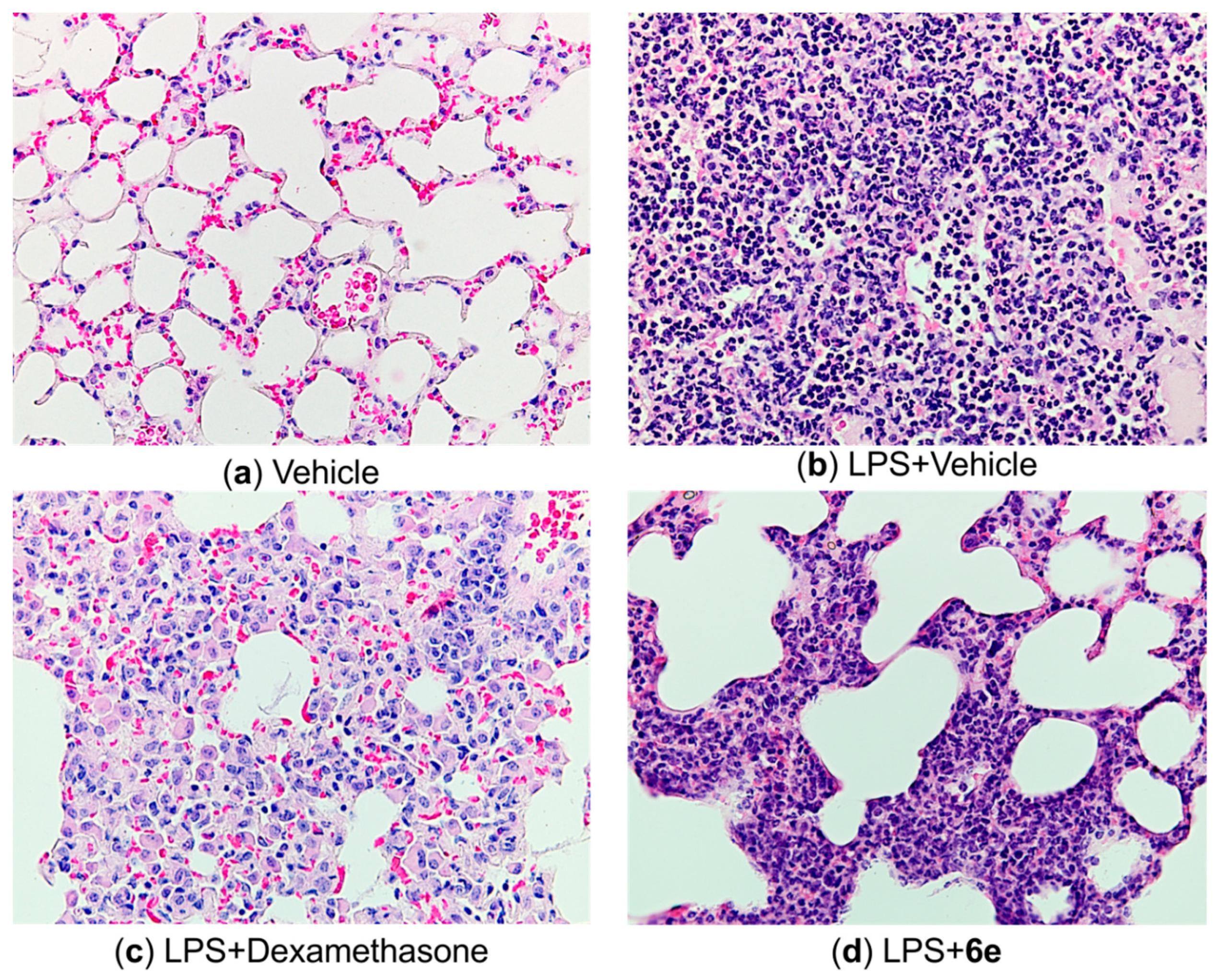
2.4.4. Histological Markers
3. Conclusions
4. Materials and Methods
4.1. Synthesis
4.2. Animals
4.3. Isolation and Treatment of Peritoneal Macrophages
4.4. Assay of Nitric Oxide (NO)
4.5. Assay of Cytokines
4.6. Cytotoxicity Study
4.7. Phagocytosis Assay
4.8. LPS-Induced Acute Lung Injury
4.9. Open Field Test
4.10. Bronchoalveolar Lavage and Plasma Preparation
4.11. Leukocyte Count in Blood and BAL
4.12. Lung Permeability Index
4.13. Histological Study
4.14. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhry, N.; Zhao, X.; Xu, D.; Zanin, M.; Chen, W.; Yang, Z.; Chen, J. Chinese Therapeutic Strategy for Fighting COVID-19 and Potential Small-Molecule Inhibitors Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Med. Chem. 2020, 63, 13205–13227. [Google Scholar] [CrossRef] [PubMed]
- Marks-Konczalik, J.; Chu, S.C.; Moss, J. Cytokine-Mediated Transcriptional Induction of the Human Inducible Nitric Oxide Synthase Gene Requires Both Activator Protein 1 and Nuclear Factor κB-Binding Sites. J. Biol. Chem. 1998, 273, 22201–22208. [Google Scholar] [CrossRef]
- Kishimoto, T.; Akira, S.; Taga, T. Interleukin-6 and Its Receptor: A Paradigm for Cytokines. Science 1992, 258, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A Panoramic Review of IL-6: Structure, Pathophysiological Roles and Inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Gonda, K.; Shibata, M.; Ohtake, T.; Matsumoto, Y.; Tachibana, K.; Abe, N.; Ohto, H.; Sakurai, K.; Takenoshita, S. Myeloid-Derived Suppressor Cells Are Increased and Correlated with Type 2 Immune Responses, Malnutrition, Inflammation, and Poor Prognosis in Patients with Breast Cancer. Oncol. Lett. 2017, 14, 1766–1774. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, G.; Meng, X.; Zhang, X. Recent Developments of Small Molecules with Anti-Inflammatory Activities for the Treatment of Acute Lung Injury. Eur. J. Med. Chem. 2020, 207, 112660. [Google Scholar] [CrossRef]
- Tagat, J.R.; McCombie, S.W.; Barton, B.E.; Jackson, J.; Shortall, J. Synthetic Inhibitors of Interleukin-6 II: 3,5-Diaryl Pyridines and Meta-Terphenyls. Bioorg. Med. Chem. Lett. 1995, 5, 2143–2146. [Google Scholar] [CrossRef]
- Kunwar, S.; Hwang, S.-Y.; Katila, P.; Park, S.; Jeon, K.-H.; Kim, D.; Kadayat, T.M.; Kwon, Y.; Lee, E.-S. Discovery of a 2,4-Diphenyl-5,6-Dihydrobenzo(H)quinolin-8-Amine Derivative as a Novel DNA Intercalating Topoisomerase IIα Poison. Eur. J. Med. Chem. 2021, 226, 113860. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. One-pot and Two-pot Methods for Chalcone Derived Pyrimidines Synthesis and Applications. J. Heterocyclic Chem. 2021, 58, 1209–1224. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pinheiro, E.M.C.; Muri, E.M.F.; Pessôa, J.C.; Cadorini, M.A.; Greco, S.J. Biological Activities of [1,2,4]triazolo[1,5-a]pyrimidines and Analogs. Med. Chem. Res. 2020, 29, 1751–1776. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.-R.; Suo, F.-Z.; Yuan, X.-H.; Yu, B.; Liu, H.-M. Synthesis, Structure-Activity Relationship Studies and Biological Characterization of New [1,2,4]triazolo[1,5-a]pyrimidine-Based LSD1/KDM1A Inhibitors. Eur. J. Med. Chem. 2019, 167, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kakkar, R. Nitric Oxide Synthases and Their Inhibitors: A Review. Lett. Drug Des. Discov. 2020, 17, 228–252. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored Plasticity Opens Doors for Selective Inhibitor Design in Nitric Oxide Synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef]
- Zuniga, E.S.; Korkegian, A.; Mullen, S.; Hembre, E.J.; Ornstein, P.L.; Cortez, G.; Biswas, K.; Kumar, N.; Cramer, J.; Masquelin, T.; et al. The Synthesis and Evaluation of Triazolopyrimidines as Anti-Tubercular Agents. Bioorg. Med. Chem. 2017, 25, 3922–3946. [Google Scholar] [CrossRef]
- Bhagat, S.; Sharma, R.; Sawant, D.M.; Sharma, L.; Chakraborti, A.K. LiOH·H2O as a Novel Dual Activation Catalyst for Highly Efficient and Easy Synthesis of 1,3-Diaryl-2-Propenones by Claisen–Schmidt Condensation under Mild Conditions. J. Mol. Catal. A Chem. 2006, 244, 20–24. [Google Scholar] [CrossRef]
- Marcovicz, C.; Camargo, G.d.A.; Scharr, B.; Sens, L.; Levandowski, M.N.; Rozada, T.d.C.; Castellen, P.; Inaba, J.; de Oliveira, R.N.; Miné, J.C.; et al. Schistosomicidal Evaluation of Synthesized Bromo and Nitro Chalcone Derivatives. J. Mol. Struct. 2022, 1258, 132647. [Google Scholar] [CrossRef]
- Ramalho, S.D.; Bernades, A.; Demetrius, G.; Noda-Perez, C.; Vieira, P.C.; dos Santos, C.Y.; da Silva, J.A.; de Moraes, M.O.; Mousinho, K.C. Synthetic Chalcone Derivatives as Inhibitors of Cathepsins K and B, and Their Cytotoxic Evaluation. Chem. Biodivers. 2013, 10, 1999–2006. [Google Scholar] [CrossRef]
- Karaman, İ.; Gezegen, H.; Gürdere, M.B.; Dingil, A.; Ceylan, M. Screening of Biological Activities of a Series of Chalcone Derivatives against Human Pathogenic Microorganisms. Chem. Biodivers. 2010, 7, 400–408. [Google Scholar] [CrossRef]
- Wei, W.; Qunrong, W.; Liqin, D.; Aiqing, Z.; Duoyuan, W. Synthesis of Dinitrochalcones by Using Ultrasonic Irradiation in the Presence of Potassium Carbonate. Ultrason. Sonochem. 2005, 12, 411–414. [Google Scholar] [CrossRef]
- He, X.; Kassab, S.E.; Heinzl, G.; Xue, F. Base-Catalyzed One-Step Synthesis of 5,7-Disubstituted-1,2,4-Triazolo[1,5-a]Pyrimidines. Tetrahedron Lett. 2015, 56, 1034–1037. [Google Scholar] [CrossRef]
- Ovchinnikova, I.G.; Valova, M.S.; Matochkina, E.G.; Kodess, M.I.; Tumashov, A.A.; Slepukhin, P.A.; Fedorova, O.V.; Rusinov, G.L.; Charushin, V.N. Specific Features of Heterocyclization of (E)-3-(2-Ethoxyphenyl)-1-Phenylprop-2-En-1-One with Aminoazoles. Russ. Chem. Bull. 2014, 63, 1552–1576. [Google Scholar] [CrossRef]
- Cho, H.; Shima, K.; Hayashimatsu, M.; Ohnaka, Y.; Mizuno, A.; Takeuchi, Y. Synthesis of Novel Dihydropyrimidines and Tetrahydropyrimidines. J. Org. Chem. 1985, 50, 4227–4230. [Google Scholar] [CrossRef]
- El Rady, E.A. Three-Component Uncatalyzed Eco-Friendly Reactions for One-Pot Synthesis of 4,7-Dihydro[1,2,4]Triazolo[1,5-a]Pyrimidine Derivatives: Three-Component One-Pot Synthesis of [1,2,4]Triazolo[1,5-a]Pyrimidine. J. Heterocycl. Chem. 2014, 51, 869–875. [Google Scholar] [CrossRef]
- Tsuda, Y.; Mishina, T.; Obata, M.; Araki, K.; Inui, J.; Nakamura, T. A Polyazaheterocyclic Compound. European Patent EP0217142A2, 8 April 1987. [Google Scholar]
- Wang, B.S.; Huang, X.; Chen, L.Z.; Liu, M.M.; Shi, J.B. Design and Synthesis of Novel Pyrazolo[4,3-d]pyrimidines as Potential Therapeutic Agents for Acute Lung Injury. J. Enzym. Inhib. Med. Chem. 2019, 34, 1121–1130. [Google Scholar] [CrossRef]
- Kim, B.R.; Cho, Y.C.; Cho, S. Anti-Inflammatory Effects of a Novel Compound, MPQP, Through the Inhibition of IRAK1 Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages. BMB Rep. 2018, 51, 308–313. [Google Scholar] [CrossRef]
- Becker, J.; Grasso, R.J. Suppression of Phagocytosis by Dexamethasone in Macrophage Cultures: Inability of Arachidonic Acid, Indomethacin, and Nordihydroguaiaretic Acid to Reverse the Inhibitory Response Mediated by a Steroid-Inducible Factor. Int. J. Immunopharmacol. 1985, 7, 839–847. [Google Scholar] [CrossRef]
- Grasso, R.J.; West, L.A.; Guay, R.C.; Klein, T.W. Inhibition of Yeast Phagocytosis by Dexamethasone in Macrophage Cultures: Reversibility of the Effect and Enhanced Suppression in Cultures of Stimulated Macrophages. J. Immunopharmacol. 1982, 4, 265–278. [Google Scholar] [CrossRef]
- Simons, R.K.; Junger, W.G.; Loomis, W.H.; Hoyt, D.B. Acute Lung Injury in Endotoxemic Rats Is Associated with Sustained Circulating IL-6 Levels and in-Trapulmonary CINC Activity and Neutrophil Recruitment—Role of Circulating TNF-α and IL-β? Int. Congr. Ser. 1996, 6, 39–45. [Google Scholar]
- Faffe, D.S.; Seidl, V.R.; Chagas, P.S.; Gonçalves de Moraes, V.L.; Capelozzi, V.L.; Rocco, P.R.; Zin, W.A. Respiratory Effects of Lipopolysaccharide-Induced Inflammatory Lung Injury in Mice. Eur. Respir. J. 2000, 15, 85–91. [Google Scholar] [CrossRef]
- Spasov, A.; Kosolapov, V.; Babkov, D.; Klochkov, V.; Sokolova, E.; Miroshnikov, M.; Borisov, A.; Velikorodnaya, Y.; Smirnov, A.; Savateev, K.; et al. Discovery of Nitro-Azolo[1,5-a]Pyrimidines with Anti-Inflammatory and Protective Activity against LPS-Induced Acute Lung Injury. Pharmaceuticals 2022, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.; Ozerov, A.; Kosolapov, V.; Gurova, N.; Kucheryavenko, A.; Naumenko, L.; Babkov, D.; Sirotenko, V.; Taran, A.; Borisov, A.; et al. Guanidine Derivatives of Quinazoline-2,4(1H,3H)-Dione as NHE-1 Inhibitors and Anti-Inflammatory Agents. Life 2022, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Sert, d.N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- D’Alessio, F.R. Mouse Models of Acute Lung Injury and ARDS. Methods Mol. Biol. 2018, 1809, 341–350. [Google Scholar] [CrossRef]
- Wang, F.; Zuo, Z.; Chen, K.; Fang, J.; Cui, H.; Shu, G.; Zhou, Y.; Chen, Z.; Huang, C.; Liu, W. Histopathological Changes Caused by Inflammation and Oxidative Stress in Diet-Induced-Obese Mouse Following Experimental Lung Injury. Sci. Rep. 2018, 8, 14250. [Google Scholar] [CrossRef] [PubMed]





 |  |  | 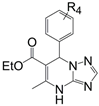 | |||
|---|---|---|---|---|---|---|
| 6 | 7 | 10 | 11 | |||
| Compound | R3 | R4 | R5 | NO IC50 (µM) | IL-6 IC50 (µM) | MTT CC50 (µM) |
| 6a | CH3 | H | 2-NH2 | n.a. * | n.a. | >>100 |
| 6b | CH3 | H | 4-NH2 | 21.12 | 2.18 | 65.6 |
| 6c | CH3 | 4-F | 4-NH2 | 0.37 | 34.9 | >>100 |
| 6d | CH3 | 3-NH2 | 4-NH2 | n.a. | n.a. | >>100 |
| 6e | CH3 | 4-NH2 | 4-NH2 | 16.24 | 18.45 | >>100 |
| 6f | NH2 | 4-NH2 | 4-NH2 | 42.94 | n.a. | 96.11 |
| 6g | SCH3 | 4-NH2 | 4-NH2 | 0.12 | >100 | >>100 |
| 7a | – | 4-F | 4-NH2 | 0.37 | 14.9 | >>100 |
| 7b | – | 3-NH2 | 4-NH2 | 45.32 | 31.44 | >>100 |
| 7c | – | 4-NH2 | 4-NH2 | 15.2 | 2.2 | 47.07 |
| 10a | – | 3-NH2 | – | 322.7 | n.a. | >>100 |
| 10b | – | 4-NH2 | – | 71.82 | n.a. | 177.5 |
| 11a | – | 3-NH2 | – | n.a. | n.a. | >>100 |
| 11b | – | 4-NH2 | – | n.a. | n.a. | >>100 |
| Dexamethasone | – | – | – | 23.38 | 2.5 | 97.39 |
| Group | Mean Injury Score | Interquartile Range |
|---|---|---|
| Vehicle | 0.25 | 0–0.25 |
| LPS | 2.83 | 3–3 |
| LPS+Dexamethasone | 1.33 | 1–1.75 |
| LPS+6e | 1.83 | 1–2.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spasov, A.; Ovchinnikova, I.; Fedorova, O.; Titova, Y.; Babkov, D.; Kosolapov, V.; Borisov, A.; Sokolova, E.; Klochkov, V.; Skripka, M.; et al. Amino Derivatives of Diaryl Pyrimidines and Azolopyrimidines as Protective Agents against LPS-Induced Acute Lung Injury. Molecules 2023, 28, 741. https://doi.org/10.3390/molecules28020741
Spasov A, Ovchinnikova I, Fedorova O, Titova Y, Babkov D, Kosolapov V, Borisov A, Sokolova E, Klochkov V, Skripka M, et al. Amino Derivatives of Diaryl Pyrimidines and Azolopyrimidines as Protective Agents against LPS-Induced Acute Lung Injury. Molecules. 2023; 28(2):741. https://doi.org/10.3390/molecules28020741
Chicago/Turabian StyleSpasov, Alexander, Irina Ovchinnikova, Olga Fedorova, Yulia Titova, Denis Babkov, Vadim Kosolapov, Alexander Borisov, Elena Sokolova, Vladlen Klochkov, Maria Skripka, and et al. 2023. "Amino Derivatives of Diaryl Pyrimidines and Azolopyrimidines as Protective Agents against LPS-Induced Acute Lung Injury" Molecules 28, no. 2: 741. https://doi.org/10.3390/molecules28020741
APA StyleSpasov, A., Ovchinnikova, I., Fedorova, O., Titova, Y., Babkov, D., Kosolapov, V., Borisov, A., Sokolova, E., Klochkov, V., Skripka, M., Velikorodnaya, Y., Smirnov, A., Rusinov, G., & Charushin, V. (2023). Amino Derivatives of Diaryl Pyrimidines and Azolopyrimidines as Protective Agents against LPS-Induced Acute Lung Injury. Molecules, 28(2), 741. https://doi.org/10.3390/molecules28020741








