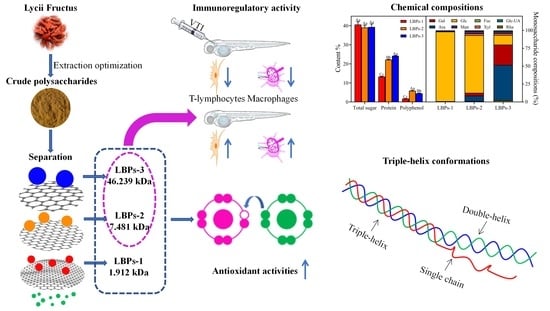
Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Crude Polysaccharide Extraction Process by RSM
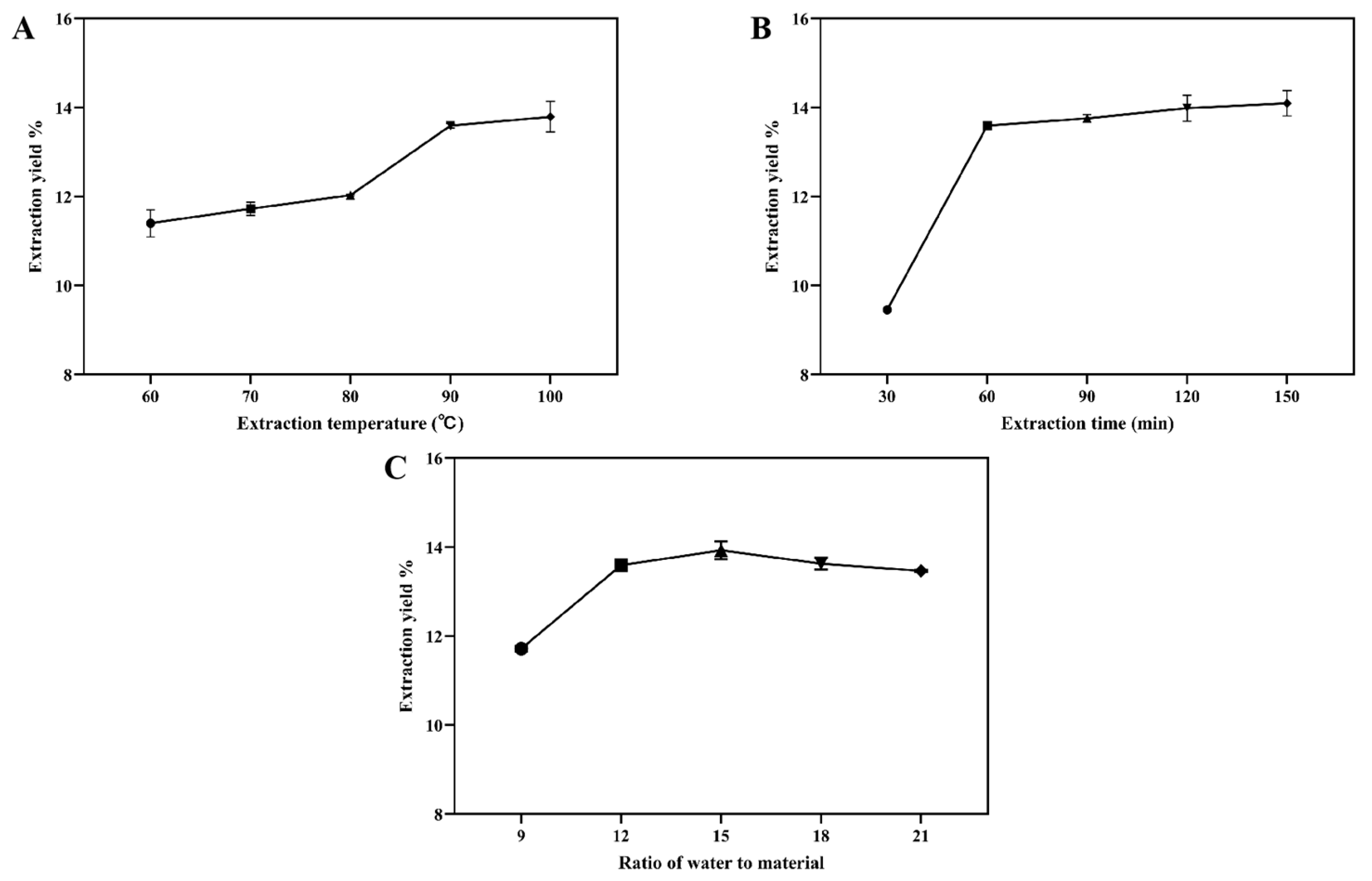
2.1.1. Single-Factor Tests
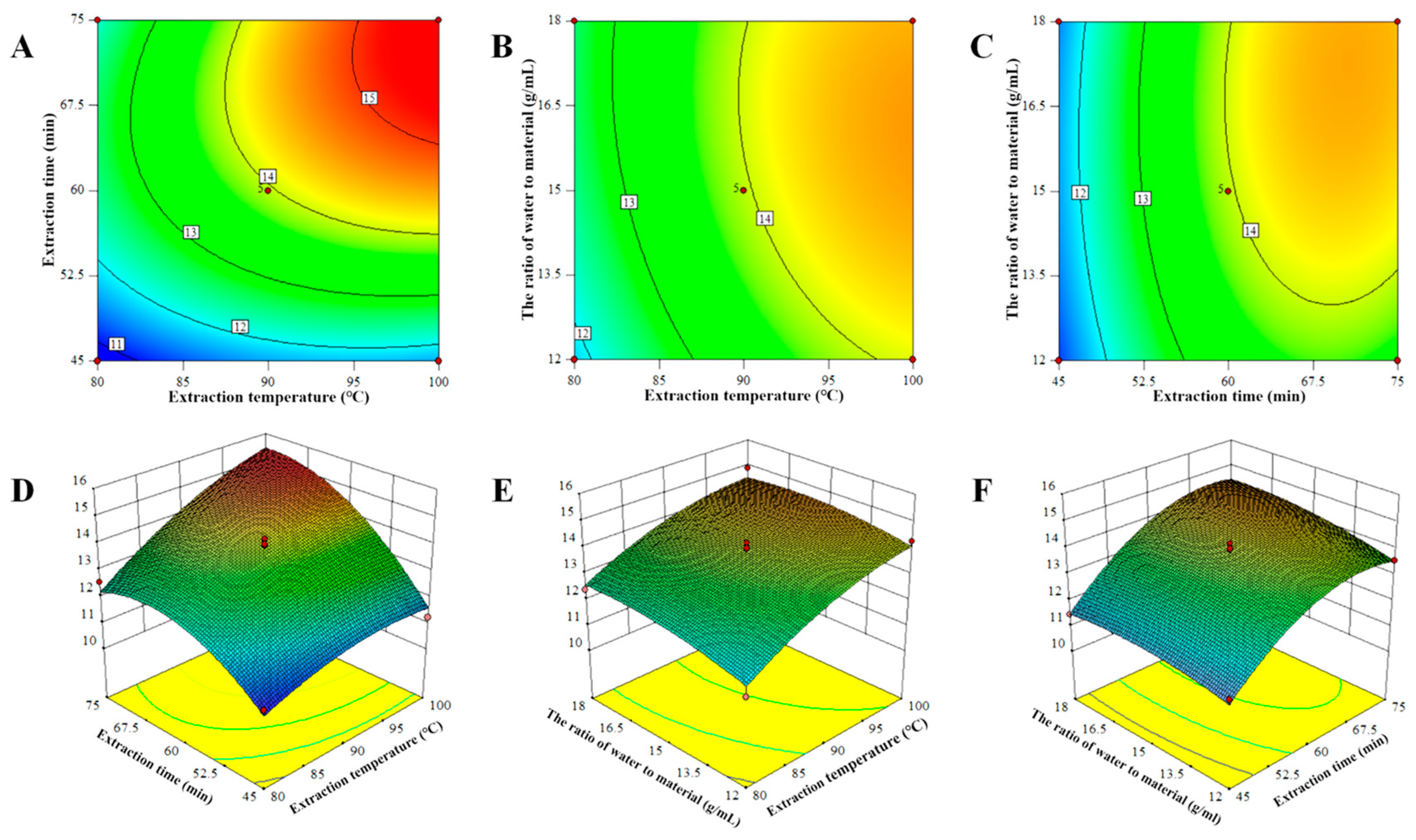
2.1.2. Response Surface Analysis
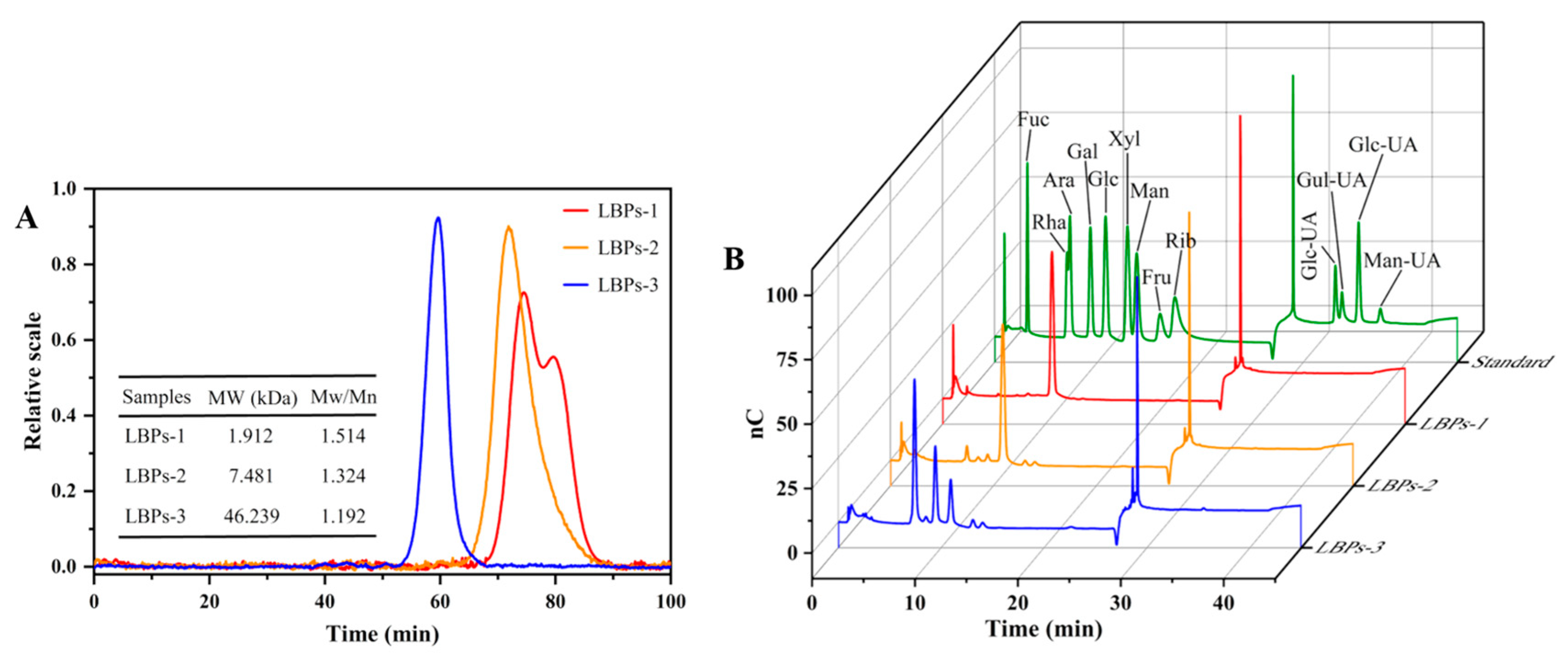
2.2. The Physicochemical Properties of LBPs with Different Molecular Weights
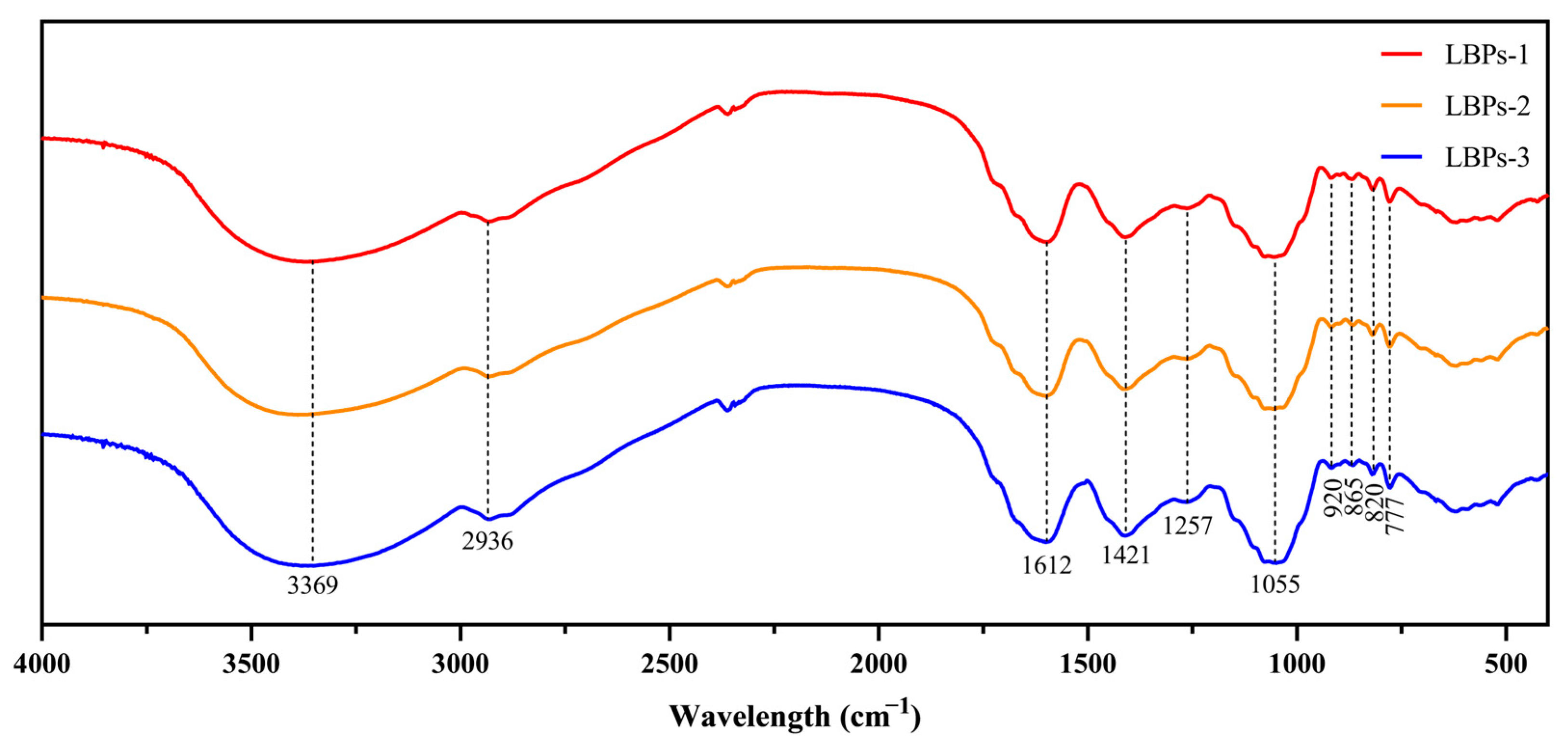
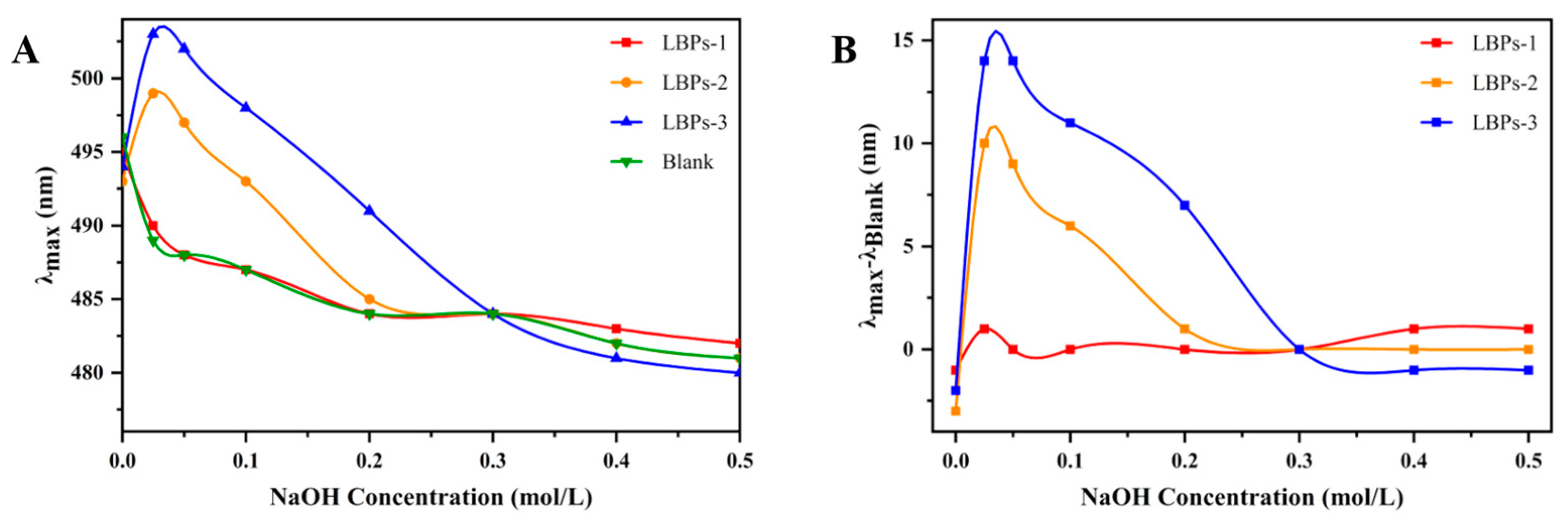
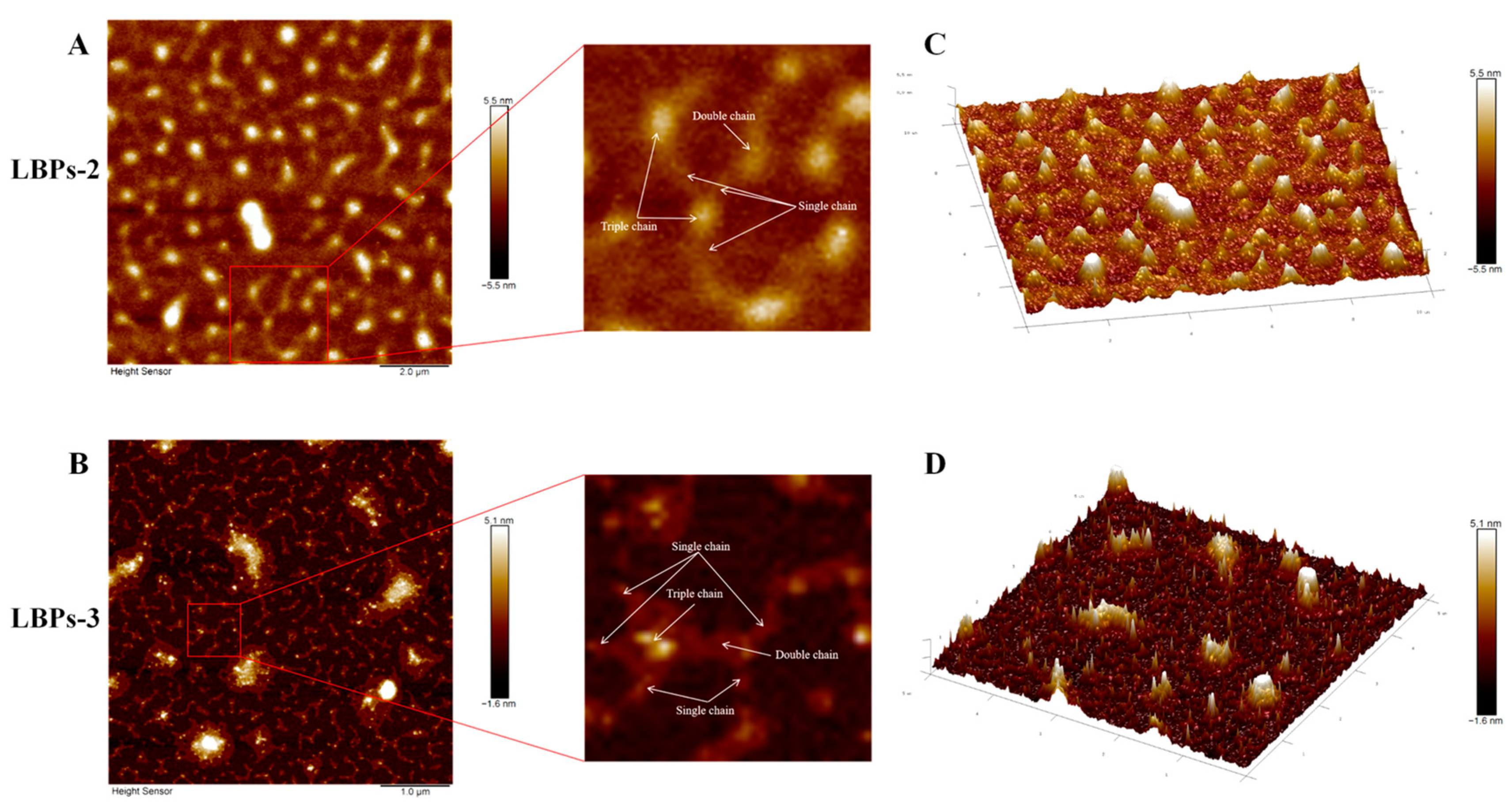
2.3. The Structural Features and Conformation of LBPs with Different Molecular Weights
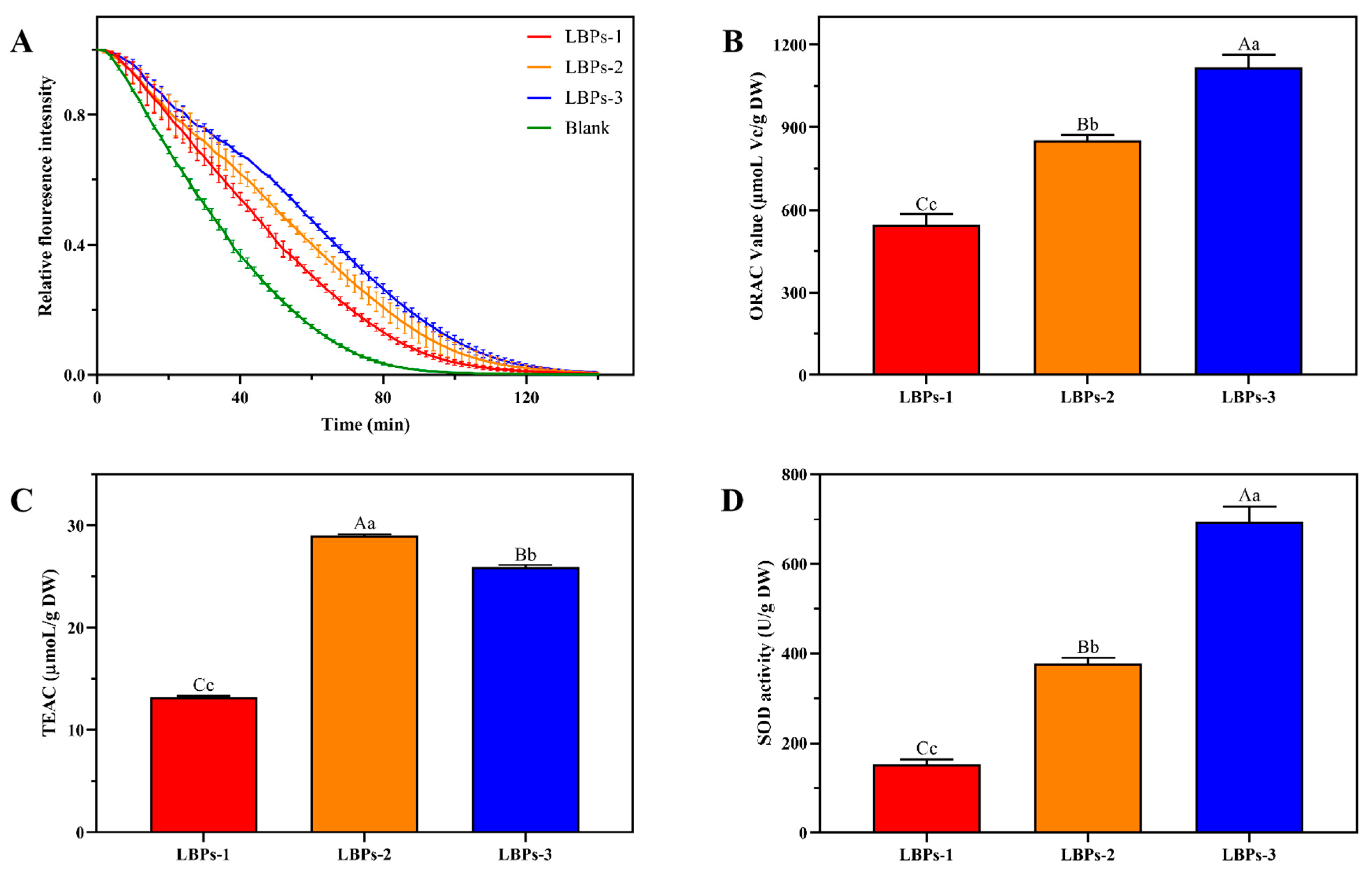
2.4. The Antioxidant Activities of LBPs In Vitro
2.5. In Vivo Immunomodulatory Effect of LBPs-2 and LBPs-3
2.5.1. MTC Assessment
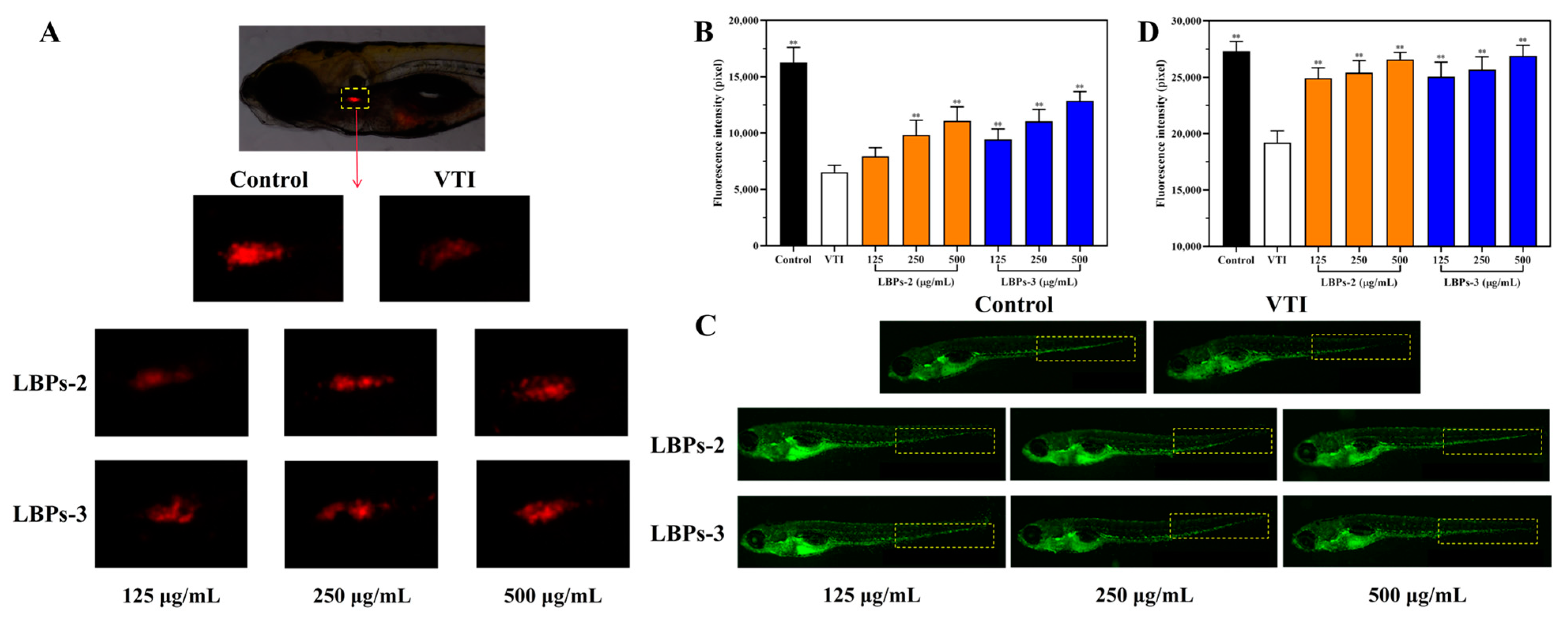
2.5.2. The Effect of LBPs-2 and LBPs-3 on T-Lymphocytes and Macrophages in VTI-Treated Zebrafish
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Crude Polysaccharides
3.3. Separation of Polysaccharides
3.4. Molecular Weight Analysis
3.5. Determination of the Chemical Composition and Monosaccharide Composition
3.6. FT-IR Spectroscopy Analysis
3.7. Congo Red Experiment
3.8. Atomic Force Microscopy (AFM) Analysis
3.9. Nuclear Magnetic Resonance (NMR) Analysis
3.10. Determination of the In Vitro Antioxidant Activities of LBPs
3.10.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.10.2. Scavenging Experiment of LBPs on ABTS+
3.10.3. Superoxide Dismutase (SOD) Activity
3.11. In Vivo Immunoregulatory Activity
3.11.1. Zebrafish Husbandry and Egg Collection
3.11.2. MTC Assessment of LBPs-2 and LBPs-3 Model Zebrafish
3.11.3. Effect of LBPs-2 and LBPs-3 on the T-Lymphocytes and Macrophages in VTI-Treated Zebrafish
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, B.; Zhao, J.Y.; Lv, M.S.; Li, X.Y.; Wang, J.H.; Yue, Z.R.; Shi, J.M.; Zhang, G.C.; Sui, G.C. Comparative study of structural properties and biological activities of polysaccharides extracted from Chroogomphus rutilus by four different approaches. Int. J. Biol. Macromol. 2021, 188, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Liu, D.Y.; Zhu, K.; Zhang, W.T.; Wang, Q. Extraction of polysaccharide from lotus leaf and its anticancer effect. J. King Saud Univ. Sci. 2020, 32, 1702–1706. [Google Scholar]
- Chen, F.; Huang, G.L. Extraction and antioxidant activities of cushaw polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1646–1649. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Yu, X.F.; Ge, Q.; Li, J.; Wang, D.J.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T.H. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, Y.Y.; Ren, G.X. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Huang, H.L. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pang, W.S.; Chen, J.L.; Bai, S.W.; Zheng, Z.Z.; Wu, X.H. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement. Altern. Med. 2013, 267, 1472. [Google Scholar] [CrossRef]
- Li, K.; Cao, Y.; Jiao, S.; Du, G.; Du, Y.; Qin, X. Structural characterization and immune activity screening of polysaccharides with different molecular weights from Astragali Radix. Front. Pharmacol. 2020, 11, 582091. [Google Scholar] [CrossRef]
- Xu, J.Q.; Zhang, J.L.; Sang, Y.M.; Wei, Y.; Chen, X.G.; Wang, Y.X.; Xue, H.K. Polysaccharides from medicine and food homology materials: A review on their extraction, purification, structure, and biological activities. Molecules 2022, 27, 3215. [Google Scholar] [CrossRef]
- Guo, X.F.; Kang, J.; Xu, Z.Y.; Guo, Q.B.; Zhang, L.F.; Ning, H.F.; Cui, W.S. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hu, S.Z.; Nie, S.P.; Yu, Q.; Xie, M.Y. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2015, 2016, 5692852. [Google Scholar]
- Yang, J.; Wei, Y.Q.; Ding, J.B.; Li, Y.L.; Ma, J.L.; Liu, J.L. Research and application of Lycii Fructus in medicinal field. Chin. Herb. Med. 2018, 10, 339–352. [Google Scholar] [CrossRef]
- Wu, D.T.; Guo, H.; Lin, S.; Lam, S.C.; Zhao, L.; Lin, D.R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Techol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Deng, Q.; Zhou, W.X.; Zhang, Y.X. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 2022, 229, 107921. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.Y.; Zhang, N.; Bing, T.; Liu, X.J.; Zhang, Z.P.; Shangguan, D.H. Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.S.; Bu, Y.; Lo, A.C.; Chan, T.C.; So, K.F.; Lai, J.S.; Shih, K.C. A systematic review of potential therapeutic use of Lycium barbarum polysaccharides in disease. BioMed Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.L.; Chen, B.H.; Yi, F.L.; Zou, S.S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 140, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.J.; Chen, T.Q.; Wu, Y.B.; Wu, J.G.; Wu, J.Z. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015, 72, 151–157. [Google Scholar] [CrossRef]
- Feng, Y.N.; Zhang, X.F. Polysaccharide extracted from Huperzia serrata using response surface methodology and its biological activity. Int. J. Biol. Macromol. 2020, 157, 267–275. [Google Scholar] [CrossRef]
- Ye, C.L.; Jiang, C.J. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr. Polym. 2011, 84, 495–502. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, X.; Li, L.; Wu, X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Deng, X.L.; Luo, S.; Luo, X.; Hu, M.H.; Ma, F.L.; Wang, Y.Y.; Zhou, L.; Huang, R.R. Fraction from Lycium barbarum polysaccharides reduces immunotoxicity and enhances antitumor activity of doxorubicin in mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Wang, H.X.; Huang, F.; Wang, L.M. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Yan, Y.X.; Zhao, J.; Li, S.P. Advanced analysis of polysaccharides from Lycii fructus. Chin. J. Pharm. Anal. 2018, 38, 3–12. [Google Scholar]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, H.; Fu, L.C.; Li, J.; Yang, L.N.; Liu, H. Effect of extraction method on the chemical profiles and bioactivities of soybean hull polysaccharides. Food Sci. Nutr. 2021, 9, 5928–5938. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Jin, C.H. Synthesis of chitosan-gallic acid conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef]
- Vittorio, O.; Brandl, M.; Cirillo, G.; Kimpton, K.; Hinde, E.; Gaus, K.; Yee, E.; Kumar, N.; Duong, H.; Fleming, C.; et al. Dextran-catechin: An anticancer chemically-modified natural compound targeting copper that attenuates neuroblastoma growth. Oncotarget 2016, 7, 47479–47493. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wang, Y.M.; Zhang, L.L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef]
- Liu, G.; Ye, J.; Li, W.; Zhang, J.; Wang, Q.; Zhu, X.A.; Miao, J.Y.; Huang, Y.H.; Chen, Y.J.; Cao, Y. Extraction, structural characterization, and immunobiological activity of ABP Ia polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 162, 975–984. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, Y.L.; Ma, X.Q.; Ren, H.W.; Fan, W.G.; Leng, F.F.; Yang, M.J.; Wang, X.L. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Ind. Crop. Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Xu, J.N.; Liu, W.; Liu, C.J.; Chai, Y.; Gao, X.D.; Yao, W.B. Comparative study of the content and structural features of polysaccharides from different Lycium. J. Chin. Inst. Food Sci. Technol. 2015, 15, 233–239. [Google Scholar]
- Guan, X.F.; Wang, Q.; Lin, B.; Su, M.L.; Zheng, Q.; Huang, J.Q.; Lai, G.T. Structural characterization of a soluble polysaccharide SSPS1 from soy whey and its immunoregulatory activity in macrophages. Int. J. Biol. Macromol. 2022, 217, 131–141. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, Z.Y.; Pan, L.C.; Sun, H.Q.; Song, Q.Y.; Zhang, Y.M. Structure analysis and anti-fatigue activity of a polysaccharide from Lepidium meyenii Walp. Nat. Prod. Res. 2019, 33, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Huang, G.L. Extraction, structural characterization, and physicochemical properties of polysaccharide from purple sweet potato. Chem. Biol. Drug Des. 2021, 98, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.Y.; Yang, W.J.; Huang, G.L.; Huang, H.L. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Zhang, X.J.; Kong, X.R.; Hao, Y.L.; Zhang, X.L.; Zhu, Z.Y. Chemical structure and inhibition on a-glucosidase of polysaccharide with alkaline-extracted from glycyrrhiza inflata residue. Int. J. Biol. Macromol. 2020, 147, 1125–1135. [Google Scholar] [CrossRef]
- Adamcik, J.; Jung, J.M.; Flakowski, J.; Rios, P.D.L.; Dietler, G.; Mezzenga, R. Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotechnol. 2010, 5, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jiang, M.F.; Wu, K.; Yang, H.; Ni, X.W.; Yan, W.L.; Phillips, G.O.; Jiang, F.T. Investigation on curdlan dissociation by heating in water. Food Hydrocolloids 2017, 70, 57–64. [Google Scholar] [CrossRef]
- Li, X.C.; Han, W.J.; Mai, W.Q.; Wang, L. Antioxidant activity and mechanism of tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar] [CrossRef]
- Yalcin, G.; Sogut, O. Influence of extraction solvent on antioxidant capacity value of oleaster measured by ORAC method. Nat. Prod. Res. 2014, 28, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.J.; Huang, G.L. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Aisikaer, G.; Khan, R.U.; Bu, J.W.; Jiang, Z.H.; Ni, Z.D.; Ying, T.J. Effects of composite chemical pretreatment on maintaining quality in button mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2014, 95, 36–41. [Google Scholar] [CrossRef]
- Wu, H.; Min, T.; Li, X.F.; Li, L.; Lai, F.R.; Tang, Y.Q.; Yang, X.H. Physicochemical properties and antioxidant activities of acidic polysaccharides from wampee seeds. Int. J. Biol. Macromol. 2013, 59, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.C.; Ren, Z.X.; Qiu, W.M.; Chen, J.L.; Jiang, Q.; Chen, B.; Chen, D.F. Antioxidant and cytoprotective effects of Tibetan Tea and its phenolic components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef]
- Tang, W.W.; Xia, J.L.; Chen, Y. Analysis of functional composition, antioxidant activity and their correlation in stem, leaf and flower from Dendrobium officinale. Food Mach. 2021, 37, 45–50. [Google Scholar]
- Chen, X.Y.; Tang, R.; Liu, T.T.; Dai, W.; Liu, Q.; Gong, G.P.; Song, S.; Hu, M.H.; Huang, L.J.; Wang, Z.F. Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int. J. Biol. Macromol. 2019, 131, 744–751. [Google Scholar] [CrossRef]
- Zhang, J.J.; Meng, G.Y.; Zhai, G.Y.; Yang, Y.H.; Zhao, H.J.; Jia, L. Extraction, characterization and antioxidant activity of polysaccharides of spent mushroom compost of Ganoderma lucidum. Int. J. Biol. Macromol. 2016, 82, 432–439. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, S.Y.; Kim, E.A.; Lee, J.H.; Kim, Y.S.; Yu, S.K.; Chae, J.B.; Choe, I.H.; Cho, J.H.; Jeon, Y.J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.; Ho, C.T.; Li, B.; Xie, Y.Z.; Chen, S.D.; Hu, H.P.; Chen, Z.Z.; Wu, Q.P. Purification, physicochemical properties, and antioxidant activities of two low-molecular-weight polysaccharides from Ganoderma leucocontextum fruiting bodies. Antioxidants 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, Q.; Zhang, Z.; Wang, J. In vitro antioxidant activity of polysaccharides extracted from Bryopsis plumose. Carbohydr. Polym. 2010, 80, 1057–1061. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.Y.; Li, H.R.; Ren, Y.M.; Xiao, Y.H.; Song, Q.Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocolloids 2020, 100, 105412. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Feng, S.L.; Liu, J.; Zhou, L.J.; Yuan, M.; Ding, C.B. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.B.; Gao, X.D. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Wang, J.L.; Niu, S.F.; Zhao, B.T.; Luo, T.; Liu, D.; Zhang, J. Catalytic synthesis of sulfated polysaccharides. II: Comparativestudies of solution conformation and antioxidant activities. Carbohydr. Polym. 2014, 107, 221–231. [Google Scholar] [CrossRef]
- Falcão, M.A.P.; De Souza, L.S.; Dolabella, S.S.; Guimarães, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environ. Sci. Pollut. Res. 2018, 25, 35015–35026. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Camil, C.B.; Iris, S. The immune system and aging: A review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Li, H.; Yue, X.L.; Xie, J.Y.; Zhang, Y.Y.; Di, H.Y.; Chen, D.F. Macrophage immunomodulatory activity of the polysaccharides from the roots of Bupleurum smithii var. Parvifolium. J. Ethnopharmacol. 2010, 130, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.P.; Han, R.; Li, H.S.; Song, J.; Yan, H.; Li, G.; Liu, A.; Cao, X.; Guo, J.; Zhai, S.; et al. Agronomic traits and molecular marker identification of wheat-Aegilops caudata addition lines. Front. Plant Sci. 2017, 8, 1743. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Du, X.H.; Li, L.H.; Liu, Y.D.; Zhang, L.; Pan, X.; Li, Y.; Li, H.; Lu, H. Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ. J. Plant Physiol. 2017, 64, 224–234. [Google Scholar] [CrossRef]
- Wang, T.; Dai, M.Z.; Li, Y.C.; Peng, Y.; Li, C.Q. Study of the immunity-enhancing effect and mechanism of Chinese herbalcompound extract. Acta Lab. Anim. Sci. Sin. 2022, 30, 198–207. [Google Scholar]
- Gong, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.L.; Zou, Z.H.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.J.; Wang, Z.F. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Peng, X.M.; Tian, G.Y. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 2001, 331, 95–99. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, Y.; Sun, Y.J.; Mou, Q.; Wang, B.; Zhang, Y.; Huang, L.J. Structural characterization of LbGp1 from the fruits of Lycium barbarum L. Food Chem. 2014, 159, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Tian, S.; Shu, X.Y.; Du, H.T.; Li, N.; Wang, J.R. Extraction, characterization and immunological activity of polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef]
- Ding, J.B. Preparation Technology of Gouqi Yishen Capsule. CN Patent CN1457873A, 23 May 2003. [Google Scholar]
- Long, X.Y.; Yan, Q.; Cai, L.J.; Li, G.Y.; Luo, X.G. Box-Behnken design-based optimization for deproteinization of crude polysaccharides in Lycium barbarum berry residue using the Sevag method. Heliyon 2020, 6, e03888. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, X.L.; Wang, F.; Zhang, Q.; Zhang, Z.S. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Huang, Q.L.; Wong, K.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.X.; Hu, Y.D.; Yang, Y.Q.; Yuan, J.; Wu, Y.F.; Li, S.J.; Lin, J.Z.; He, L.; Hou, S.Z.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, J.; Ah-Hen, K.S.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total phenolics, anthocyanin profile and antioxidant activity of maqui, Aristotelia chilensis (Mol.) Stuntz, berries extract in freeze-dried polysaccharides microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Huang, R.M.; Wen, P.; Song, Y.; He, B.L.; Tan, J.L.; Hao, H.L.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, R.F.; Dong, L.H.; Guo, J.X.; Deng, Y.Y.; Yi, Y.; Zhang, M.W. Antioxidant and antiproliferative activities of polysaccharide fractions from litchi pulp. Food Funct. 2015, 6, 2589–2606. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.; Zahid, H.; Ansari, R.; Lee, R.C.Y.; Partap, A.; Gerlai, R. Breeding zebrafish: A review of different methods and a discussion on standardization. Zebrafish 2017, 14, 561–573. [Google Scholar] [CrossRef]
- Shi, S.; Li, H.Q.; Wang, L.; Li, N.; Tan, S.J.; Liu, H.; Peng, G.Y.; Li, Y.N.; Zhang, C.; Feng, H.T. Protective effects of three types of dairy products on immune vulnerability in vinorelbine-treated zebrafish. Food Sci. 2022, 43, 113–120. [Google Scholar]

| Number | Factor | Actual Value (%) | Predicted Value (%) | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 1 | 90 (0) | 45 (−1) | 12 (0) | 11.35 | 11.18 |
| 2 | 80 (−1) | 60 (0) | 12 (0) | 11.41 | 11.81 |
| 3 | 90 (0) | 60 (0) | 15 (0) | 13.97 | 13.95 |
| 4 | 90 (0) | 75 (1) | 18 (1) | 14.26 | 14.44 |
| 5 | 100 (1) | 60 (0) | 12 (−1) | 14.26 | 14.07 |
| 6 | 90 (0) | 60 (0) | 15 (0) | 13.61 | 13.95 |
| 7 | 90 (0) | 60 (0) | 15 (0) | 13.98 | 13.95 |
| 8 | 90 (0) | 60 (0) | 15 (0) | 14.20 | 13.95 |
| 9 | 100 (1) | 75 (1) | 15 (0) | 15.24 | 15.44 |
| 10 | 90 (0) | 60 (0) | 15 (0) | 14.00 | 13.95 |
| 11 | 90 (0) | 45 (−1) | 18 (1) | 11.45 | 11.48 |
| 12 | 80 (−1) | 75 (1) | 15 (0) | 12.57 | 12.20 |
| 13 | 90 (0) | 75 (1) | 12 (−1) | 13.56 | 13.54 |
| 14 | 80 (−1) | 45 (−1) | 15 (0) | 10.91 | 10.70 |
| 15 | 80 (−1) | 60 (0) | 18 (1) | 12.40 | 12.59 |
| 16 | 100 (1) | 60 (0) | 18 (1) | 14.88 | 14.49 |
| 17 | 100 (1) | 45 (−1) | 15 (0) | 11.24 | 11.62 |
| Source | Sum of Squares | Degrees of Freedom | Mean Squares | F | p |
|---|---|---|---|---|---|
| Model | 31.12 | 9 | 3.46 | 24.28 | 0.0002 |
| A | 8.70 | 1 | 8.70 | 61.12 | 0.0001 |
| B | 14.25 | 1 | 14.25 | 100.08 | <0.0001 |
| C | 0.73 | 1 | 0.73 | 5.09 | 0.0586 |
| AB | 1.37 | 1 | 1.37 | 9.60 | 0.0173 |
| AC | 0.035 | 1 | 0.035 | 0.25 | 0.6354 |
| BC | 0.087 | 1 | 0.087 | 0.61 | 0.4593 |
| A2 | 0.81 | 1 | 0.81 | 5.71 | 0.0482 |
| B2 | 4.39 | 1 | 4.39 | 30.83 | 0.0009 |
| C2 | 0.31 | 1 | 0.31 | 2.20 | 0.1815 |
| Residual | 1.00 | 7 | 0.14 | ||
| Lack of fit | 0.81 | 3 | 0.27 | 5.94 | 0.0590 |
| Pure error | 0.18 | 4 | 0.046 | ||
| Cor. total | 32.12 | 16 |
| Index | LBPs-1 | LBPs-2 | LBPs-3 |
|---|---|---|---|
| Total sugar (%) | 40.62 ± 1.48 Aa | 39.04 ± 1.26 Aa | 39.34 ± 1.55 Aa |
| Protein (%) | 13.31 ± 0.14 Cc | 22.23 ± 0.14 Bb | 24.19 ± 0.17 Aa |
| Total polyphenol (%) | 1.77 ± 0.05 Cc | 5.89 ± 0.23 Aa | 4.51 ± 0.15 Bb |
| Monosaccharide composition (mol%) | |||
| Fucose | - | 0.38 | 0.50 |
| Rhamnose | 0.10 | 0.82 | 2.03 |
| Arabinose | 0.58 | 7.58 | 49.27 |
| Galactose | 0.31 | 3.93 | 28.35 |
| Glucose | 97.39 | 80.56 | 13.38 |
| Xylose | 0.25 | 2.49 | 2.42 |
| Mannose | 0.72 | 3.64 | 2.73 |
| Glucuronic Acid | 0.64 | 0.60 | 1.32 |
| Glycosyl Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| →4)-β-Galp-(1→ | H | 4.50 | 3.28 | 3.54 | 3.93 | 3.23 | 3.09/3.23 |
| C | 102.92 | 71.23 | 74.02 | 78.17 | 74.96 | 63.77 | |
| α-Glcp-(1→ | H | 4.97 | 3.47 | 3.60 | 3.23 | 3.35 | 3.36 |
| C | 103.13 | 69.36 | 71.12 | 69.77 | 73.00 | 62.84 | |
| →3)-α-Glcp-(1→ | H | 5.08 | 3.79 | 3.90 | 3.54 | 3.45 | 3.19 |
| C | 101.41 | 76.09 | 81.79 | 71.06 | 69.34 | 53.83 | |
| β-Glcp-(1→ | H | 4.50 | 3.23 | 3.47 | 3.54 | 3.28 | 3.19 |
| C | 102.49 | 74.02 | 69.36 | 70.63 | 72.35 | 53.83 | |
| →3,4)-β-Arap-(1→ | H | 4.50 | 3.33 | 3.79 | 3.91 | 3.16 | |
| C | 95.81 | 71.54 | 76.09 | 81.79 | 53.83 | ||
| →3)-α-Arap-(1→ | H | 5.12 | 3.73 | 3.85 | 3.54 | 3.30 | |
| C | 98.18 | 72.57 | 74.08 | 67.40 | 71.28 | ||
| →4)-α-D-GlcpA-(1→ | H | 5.08 | 4.06 | 3.71 | 4.20 | 4.06 | - |
| C | 107.44 | 73.92 | 64.91 | 82.25 | 73.93 | 172.86 | |
| Glycosyl Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| →3,4)-α-Arap-(1→ | H | 5.09 | 3.92 | 4.06 | 3.98 | 3.71 | |
| C | 109.16/109.37 | 76.44 | 79.64 | 80.32 | 59.16 | ||
| →3,4)-α-Galp-(1→ | H | 5.01 | 3.83 | 4.09 | 3.96 | 3.73 | 3.35 |
| C | 107.44 | 76.66 | 83.76 | 80.75 | 76.50 | 71.92 | |
| →3)-α-Galp-(1→ | H | 4.92 | 3.73 | 4.06 | 3.59 | 3.71 | 3.31 |
| C | 107.22 | 74.94 | 81.18 | 77.30 | 74.94 | 70.85 | |
| →4)-β-Arap-(1→ | H | 4.61 | 3.71 | 3.89 | 4.07 | 3.64/3.75 | |
| C | 103.56 | 75.31 | 77.09 | 79.64 | 60.95 | ||
| β-Arap-(1→ | H | 4.68 | 3.62 | 3.69 | 3.58 | 3.49 | |
| C | 103.13 | 71.08 | 72.35 | 74.51 | 61.32 | ||
| →3,4)-α-Galp-(1→ | H | 5.06 | 3.41 | 3.91 | 4.01 | 3.32 | 3.34 |
| C | 102.49 | 75.63 | 81.18 | 83.98 | 74.33 | 68.15 | |
| →3)-α-GlcpA-(1→ | H | 5.19 | 3.72 | 4.62 | 3.61 | 4.49 | - |
| C | 103.13 | 79.03 | 80.10 | 77.52 | 82.04 | 171.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Chen, L.; Xiao, Z.; Li, Y.; Ma, J.; Ding, J.; Yang, J. Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides. Molecules 2023, 28, 701. https://doi.org/10.3390/molecules28020701
Zeng W, Chen L, Xiao Z, Li Y, Ma J, Ding J, Yang J. Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides. Molecules. 2023; 28(2):701. https://doi.org/10.3390/molecules28020701
Chicago/Turabian StyleZeng, Wenjun, Lulu Chen, Zhihui Xiao, Yanping Li, Jianlong Ma, Jianbao Ding, and Jin Yang. 2023. "Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides" Molecules 28, no. 2: 701. https://doi.org/10.3390/molecules28020701
APA StyleZeng, W., Chen, L., Xiao, Z., Li, Y., Ma, J., Ding, J., & Yang, J. (2023). Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides. Molecules, 28(2), 701. https://doi.org/10.3390/molecules28020701