Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris pedata
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Adsorbents for Magnetic MOFs
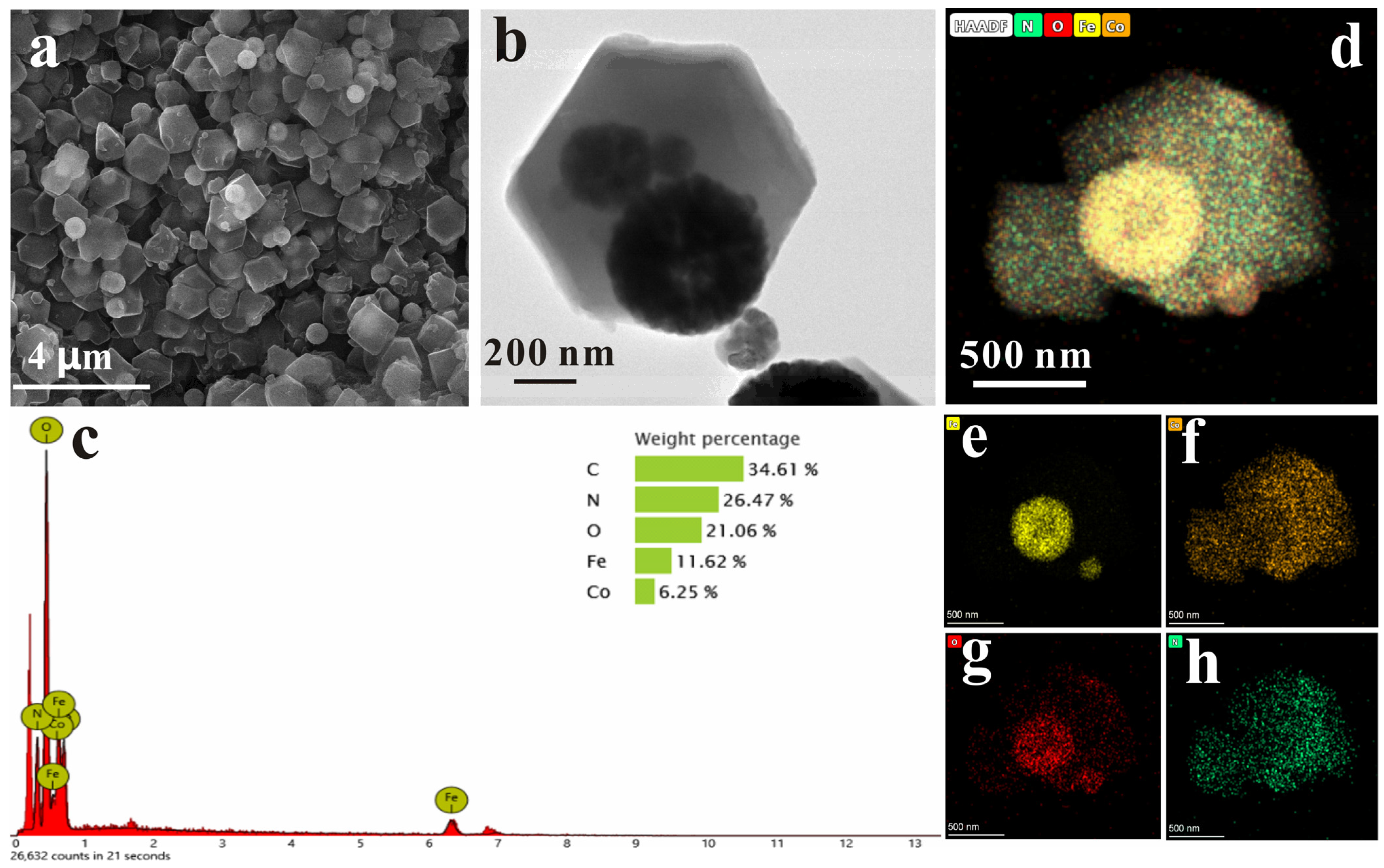
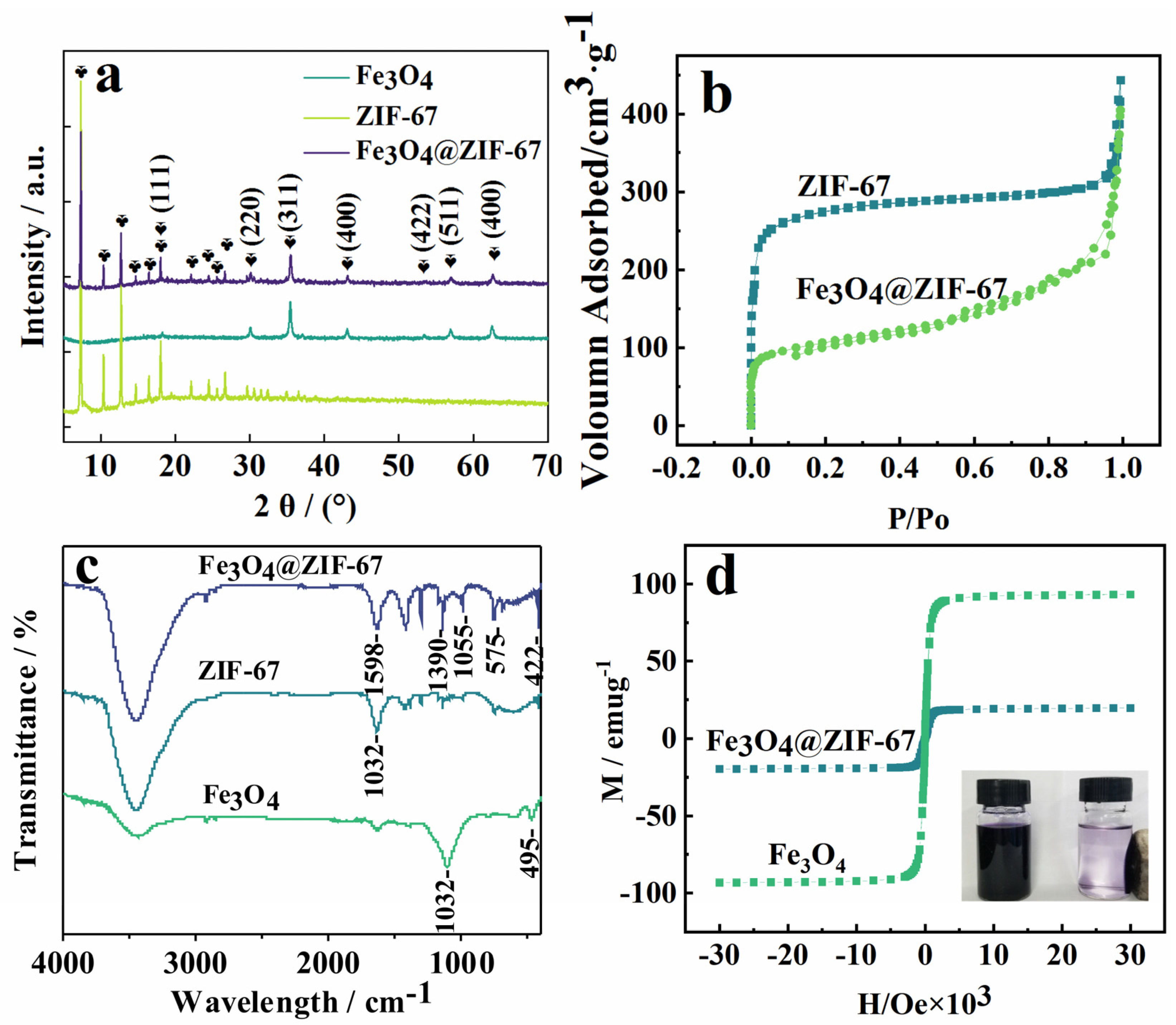
2.2. Characterization of Fe3O4@ZIF−67
2.3. Optimization of MSPE Extraction Conditions
2.3.1. Selection of Desorbing Solvent
2.3.2. Effect of the Amount of Fe3O4@ZIF−67
2.3.3. Optimization of the Desorbing Solvent Volume
2.3.4. Effect of the Proportion of Acetonitrile in the Desorbing Solvent
2.3.5. Effect of Formic Acid Concentration to Acidify Desorbing Solvent
2.3.6. Effect of Salt Ion Concentration in the Sample Solution
2.3.7. Effect of Vortex Time on Adsorption Capacity
2.4. Stability and Reusability of Fe3O4 @ZIF−67
2.5. Validation of Method
2.6. Method Application
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Chromatographic Conditions
3.3. Preparation of Fe3O4 NPs
3.4. Preparation of Fe3O4@ZIF−67
3.5. Sample Preparation
3.6. MSPE Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, H.Y.; Lim, Y.Y.; Tan, S.P. Antioxidative, Tyrosinase Inhibiting and Antibacterial Activities of Leaf Extracts From Medicinal Ferns. Biosci. Biotechnol. Biochem. 2009, 73, 1362–1366. [Google Scholar] [CrossRef]
- Goswami, H.K.; Sen, K.; Mukhopadhyay, R. Pteridophytes: Evolutionary boon as medicinal plants. Plant Genet. Resour. C 2016, 14, 328–355. [Google Scholar] [CrossRef]
- Eugene, C.; Kim, H.W.; Baek, H.; Kim, H.; Jo, G.U.; Park, S.; Oh, C.; Deuk-sil, O.; Kim, J. A study on the antioxidant and anticancer activities of Dicranopteris pedeta aqueous extract. J. Adv. Eng. Technol. 2020, 13, 39–44. [Google Scholar]
- Baharuddin, A.A.; Roosli, R.A.; Zakaria, Z.A.; Tohid, S.F.M. Dicranopteris linearis A potential medicinal plant with anticancer properties. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2021, 20, 28–37. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Kamisan, F.H.; Kek, T.L.; Salleh, M.Z. Hepatoprotective and antioxidant activities of Dicranopteris linearis leaf extract against paracetamol-induced liver intoxication in rats. Pharm. Biol. 2020, 58, 478–489. [Google Scholar] [CrossRef]
- Yang, W.; Ye, M.; Qiao, X.; Wang, Q.; Bo, T.; Guo, D. Collision-induced Dissociation of 40 Flavonoid Aglycones and Differentiation of the Common Flavonoid Subtypes Using Electrospray Ionization Ion-trap Tandem Mass Spectrometry and Quadrupole Time-of-Flight Mass Spectrometry. Eur. J. Mass Spectrom. 2012, 18, 493–503. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Yue, D.; Chen, H. Solid-phase extraction methods for nucleic acid separation. A review. J. Sep. Sci. 2022, 45, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Kotova, A.A.; Thiebaut, D.; Vial, J.; Tissot, A.; Serre, C. Metal-organic frameworks as stationary phases for chromatography and solid phase extraction: A review. Coord. Chem. Rev. 2022, 455, 214364. [Google Scholar] [CrossRef]
- Muguruza, A.R.; De Luis, R.F.; Iglesias, N.; Bazan, B.; Urtiaga, M.K.; Larrea, E.S.; Fidalgo-Marijuan, A.; Barandika, A.G. Encapsulation of beta-alanine model amino-acid inzirconium (IV) metal (IV) metal organic frameworks: Defect engineering to improve host guest interactions. J. Inorg. Biochem. 2020, 205, 110977. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ma, P.; Piao, H.; Qin, Z.; Tao, S.; Sun, Y.; Wang, X.; Song, D. Solid-phase microextraction of triazine herbicides via cellulose paper coated with a metal-organic framework of type MIL-101(Cr), and their quantitation by HPLC-MS. Microchim. Acta 2019, 186, 742. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A. On the use of metal-organic frameworks for the extraction of organic compounds from environmental samples. Environ. Sci. Pollut. Res. 2021, 28, 59015–59039. [Google Scholar] [CrossRef]
- Si, T.; Lu, X.; Zhang, H.; Wang, S.; Liang, X.; Guo, Y. Metal-organic framework-based core-shell composites for chromatographic stationary phases. TrAC Trends Anal. Chem. 2022, 149, 116545. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Gao, G.; Zhao, P.; Lu, N.; Lun, X.; Hou, X. Magnetic Solid Phase Extraction of Non-steroidal Anti-inflammatory Drugs From Water Samples Using a Metal Organic Framework of Type Fe3O4/MIL-101(Cr), and Their Quantitation By UPLC-MS/MS. Microchim. Acta 2017, 184, 2981–2990. [Google Scholar] [CrossRef]
- Plotka-wasylka, J.; Szczepańska, N.; De la Guardia, M.; Namiesnik, J. Miniaturized Solid-phase Extraction Techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.; Lin, J. Recent Advances in Graphene-based Magnetic Composites for Magnetic Solid-phase Extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Suleiman, J.; Hu, B.; Peng, H.; Huang, C. Separation/preconcentration of Trace Amounts of Cr, Cu and Pb in Environmental Samples By Magnetic Solid-phase Extraction with Bismuthiol-ii-immobilized Magnetic Nanoparticles and Their Determination By ICP-OES. Talanta 2009, 77, 1579–1583. [Google Scholar] [CrossRef]
- Li, X.; Zhu, G.; Luo, Y.; Yuan, B.; Feng, Y. Synthesis and Applications of Functionalized Magnetic Materials in Sample Preparation. TrAC Trends Anal. Chem. 2013, 45, 233–247. [Google Scholar] [CrossRef]
- Boontongto, T.; Burakham, R. Simple magnetization of Fe3O4/MIL-53(Al)-NH2 for a rapid vortex-assisted dispersive magnetic solid-phase extraction of phenol residues in water samples. J. Sep. Sci. 2020, 43, 3083–3092. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Yang, H.; Ji, X.; Zhang, H.; Li, Y.; Zhou, M.; Wang, J.; Qian, M. A magnetic solid phase extraction based on UiO-67@GO@Fe3O4 coupled with UPLC-MS/MS for the determination of nitroimidazoles and benzimidazoles in honey. Food Chem. 2022, 373, 131512. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Y.; Zhou, Y.; Chen, J.; Wei, X.; Ni, R.; Liu, Z.; Xu, F. A composite consisting of a deep eutectic solvent and dispersed magnetic metal-organic framework (type UiO-66-NH2) for solid-phase extraction of RNA. Microchim. Acta 2020, 187, 58. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, A.; Wu, F.; Luo, X.; Zhang, J. In-situ growth of boronic acid- decorated metal-organic framework on Fe3O4 nanospheres for specific enrichment of cis-diol containing nucleosides. Anal. Chim. Acta 2022, 1206, 339772. [Google Scholar] [CrossRef]
- Cassiano, N.M.; Lima, V.V.; Oliveira, R.V.; De Pietro, A.C.; Cass, Q.B. Development of Restricted-access Media Supports and Their Application to the Direct Analysis of Biological Fluid Samples Via High-performance Liquid Chromatography. Anal. Bioanal. Chem. 2006, 385, 1580. [Google Scholar] [CrossRef]
- Bagheri, N.; Lawati, H.A.; Hassanzadeh, J. Simultaneous Determination of Total Phenolic Acids and Total Flavonoids in Tea and Honey Samples Using an Integrated Lab on a Chip Device. Food Chem. 2021, 342, 128338. [Google Scholar] [CrossRef]
- Thornton, A.W.; Jelfs, K.E.; Konstas, K.; Doherty, C.M.; Hill, A.J.; Cheetham, A.K.; Bennett, T.D. Porosity in Metal–organic Framework Glasses. Chem. Commun. 2016, 52, 3750–3753. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.S.; Jegal, J.; Jhung, S.H. Adsorptive Removal of Methyl Orange From Aqueous Solution with Metal-organic Frameworks, porous Chromium-benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef]
- Si, Y.; Wang, W.; El-Sayed, E.M.; Yuan, D. Use of Breakthrough Experiment to Evaluate the Performance of Hydrogen Isotope Separation for Metal-organic Frameworks M-MOF-74 (M=Co, Ni, Mg, Zn). Sci. China Chem. 2020, 63, 881–889. [Google Scholar] [CrossRef]
- Gao, Q.; Lin, C.; Luo, D.; Suo, L.; Chen, J.; Feng, Y. Magnetic Solid-phase Extraction Using Magnetic Hypercrosslinked Polymer for Rapid Determination of Illegal Drugs in Urine. J. Sep. Sci. 2011, 34, 3083–3091. [Google Scholar] [CrossRef]
- Dzah, C.S. Influence of Fruit Maturity on Antioxidant Potential and Chilling Injury Resistance of Peach Fruit (Prunus Persica) During Cold Storage. Afr. J. Food Agric. Nutr. Dev. 2014, 14, 9578–9591. [Google Scholar] [CrossRef]
- Li, T.; Lu, M.; Gao, Y.; Huang, X.; Liu, G.; Xu, D. Double Layer MOFs M-ZIF-8@ZIF−67: The Adsorption Capacity and Removal Mechanism of Fipronil and Its Metabolites From Environmental Water and Cucumber Samples. J. Adv. Res. 2020, 24, 159–166. [Google Scholar] [CrossRef]
- Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2 (R1); (ICH: Geneva); Somatek Inc.: San Diego, CA, USA, 2005; Available online: https://www.ich.org/page/quality-guidelines (accessed on 17 January 2020).
- Rambla-Alegre, M.; Esteve-Romero, J.; Carda-Broch, S. Is it really necessary to validate an analytical method or not? That is the question. J. Chromatogr. A 2012, 1232, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Pereira, J.; Wouter, V.G.; Giró, C.; Câmara, J.S. A fast method using a new hydrophilic–lipophilic balanced sorbent in combination with ultra-high performance liquid chromatography for quantification of significant bioactive metabolites in wines. Talanta 2011, 86, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of phenolic compounds in residual brewing yeast using matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, A.; Prosen, H.; Jelikić-Stankov, M.; Durdević, P. Evaluation of matrix effect in determination of some bioflavonoidsin food samples by LC–MS/MS method. Talanta 2012, 99, 780–790. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic singlecrystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, B.; Wu, X.; Wang, Y.; Zhu, J.; Liu, X.; Zhao, D. Drug Delivery System and in Vitro Release of Luteolin Based on Magnetic Nanocomposite (Fe3O4@ZIF−67). Micro Nano Lett. 2020, 15, 425–429. [Google Scholar] [CrossRef]
- Jarrah, A.; Farhadi, S. Encapsulation of K6p2w18O62 into Magnetic Nanoporous Fe3O4/MIL-101 (Fe) for Highly Enhanced Removal of Organic Dyes. J. Solid. State Chem. 2020, 285. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, X.; Ruan, L.W.; Liu, B.; Wang, Y.M.; Zhu, J.F.; Qiu, L.G. Post-combustion Co2 Capture with the Hkust-1 and MIL-101(Cr) Metal–organic Frameworks: Adsorption, Separation and Regeneration Investigations. Micropor. Mesopor. Mat. 2013, 179, 191–197. [Google Scholar] [CrossRef]
- Rostamnia, S.; Xin, H. Basic Isoreticular Metal–organic Framework (IRMOF-3) Porous Nanomaterial as a Suitable and Green Catalyst for Selective Unsymmetrical Hantzsch Coupling Reaction. App. Organomet. Chem. 2014, 28, 359–363. [Google Scholar] [CrossRef]




| Compound | Linearity Equation | Determination Coefficient (R2) | Linearity Range (μg∙mL−1) | Intra-Day RSD (%, n = 3) | Inter-Day RSD (%, n = 3) | LOD (ng∙mL−1) | LOQ (ng∙mL−1) |
|---|---|---|---|---|---|---|---|
| Rutin | y = 7.7124x − 18.831 | 0.9922 | 4.38–70.00 | 3.6 | 4.9 | 41.4 | 132.4 |
| Quercitrin | y = 10.994x + 118.94 | 0.9913 | 2.34–37.50 | 2.2 | 3.8 | 56.2 | 147.2 |
| Kaempferol-3-O-α-L-rhamnoside | y = 4.8385x + 18.599 | 0.9901 | 1.25–20 | 3.0 | 5.8 | 40.2 | 131.2 |
| Quercetin | y = 12.554x + 5.8314 | 0.9945 | 1.09–17.50 | 2.8 | 4.1 | 39.5 | 130.5 |
| Kaempferol | y = 18.289x + 68.642 | 0.9921 | 2.03–32.50 | 4.0 | 4.1 | 42.8 | 133.8 |
| Compound | Sample Content (μg∙mL−1) ± SD | Added (μg∙mL−1) | Detected (μg∙mL−1) ± SD | Recovery (%) | RSD (%) | Average Recovery (%) |
|---|---|---|---|---|---|---|
| Rutin | 18.9 ± 0.33 | 17.5 | 32.3 ± 0.84 | 88.8 | ||
| 8.7 | 26.1 ± 0.80 | 94.5 | 2.2 | 92.2 | ||
| 4.3 | 21.7 ± 0.50 | 93.3 | ||||
| Quercitrin | 19.6 ± 0.26 | 12.5 | 33.5 ± 0.71 | 104.7 | ||
| 6.2 | 25.8 ± 0.68 | 99.8 | 1.4 | 100.7 | ||
| 3.1 | 22.2 ± 1.31 | 96.2 | ||||
| Kaempferol-3-O-α-L-rhamnoside | 3.1 ± 0.07 | 5.0 | 8.1 ± 0.18 | 99.6 | ||
| 2.5 | 5.4 ± 0.15 | 96.8 | 1.9 | 95.6 | ||
| 1.2 | 3.9 ± 0.10 | 90.0 | ||||
| Quercetin | 0 | 5.0 | 4.8 ± 0.13 | 95.7 | ||
| 2.5 | 2.3 ± 0.13 | 92.5 | 1.8 | 92.6 | ||
| 1.3 | 1.1 ± 0.08 | 89.7 | ||||
| Kaempferol | 0 | 10.4 | 10.0 ± 0.16 | 96.5 | ||
| 5.2 | 5.1 ± 0.24 | 98.8 | 0.2 | 96.3 | ||
| 2.6 | 2.4 ± 0.22 | 93.5 |
| Flavonoids | Adsorbent | Analytical Technique | Matrix | LOD (ng∙mL−1) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| Rutin, quercetin, and kaempferol | OasisTM HLB | SPE-UHPLC-PDA | Wine extract | 10.0–59.0 | 63.0–114.0 | [32] |
| Rutin, quercetin, and kaempferol | TiO2 NPs and diatomaceous earth | Multi-step matrix solid-phase dispersion (MSPD)- capillary LC and LC-MS/MS | Residual brewing yeast | 7.0–11.0 | — | [33] |
| Rutin, quercetin, and kaempferol | — | Ultrasound-assisted extraction (UAE)-HPLC-UV | Flos Sophorae Immaturus | 2000.0–4000.0 | 97.7–99.3 | [34] |
| Rutin, quercetin, and kaempferol | Supelco LC-18 | SPE-LC-MS/MS | Red onion, orange peel, and honey | 39.0–76.0 | 86.0–114.0 | [35] |
| Rutin, quercitrin, kaempferol-3-O-α-L-rhamnoside, quercetin, and kaempferol | Fe3O4@ZIF−67 | MSPE-HPLC-UV | Dicranopteris pedata | 39.5–56.2 | 92.2–100.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Zhu, J.; Zhuo, S.; Chen, J.; Huang, W.; Cheng, H.; Li, L.; Tang, T.; Feng, J. Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris pedata. Molecules 2023, 28, 702. https://doi.org/10.3390/molecules28020702
Feng Z, Zhu J, Zhuo S, Chen J, Huang W, Cheng H, Li L, Tang T, Feng J. Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris pedata. Molecules. 2023; 28(2):702. https://doi.org/10.3390/molecules28020702
Chicago/Turabian StyleFeng, Zhiyang, Jiaqing Zhu, Shen Zhuo, Jun Chen, Wenyi Huang, Hao Cheng, Lijun Li, Tai Tang, and Jun Feng. 2023. "Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris pedata" Molecules 28, no. 2: 702. https://doi.org/10.3390/molecules28020702
APA StyleFeng, Z., Zhu, J., Zhuo, S., Chen, J., Huang, W., Cheng, H., Li, L., Tang, T., & Feng, J. (2023). Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris pedata. Molecules, 28(2), 702. https://doi.org/10.3390/molecules28020702






