Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity
Abstract
1. Introduction
2. Phytochemistry of CEO
Coriander Seed Oil and Leaf Oil
3. Variation in EO across Varieties/Germplasm/Accessions
4. Variation of Essential Oil at Different Stages
5. Variation of Essential Oil Constituents from Different Geographical Locations
6. Variation in EO Due to Other Factors
7. Extraction and Analysis of Coriander EO
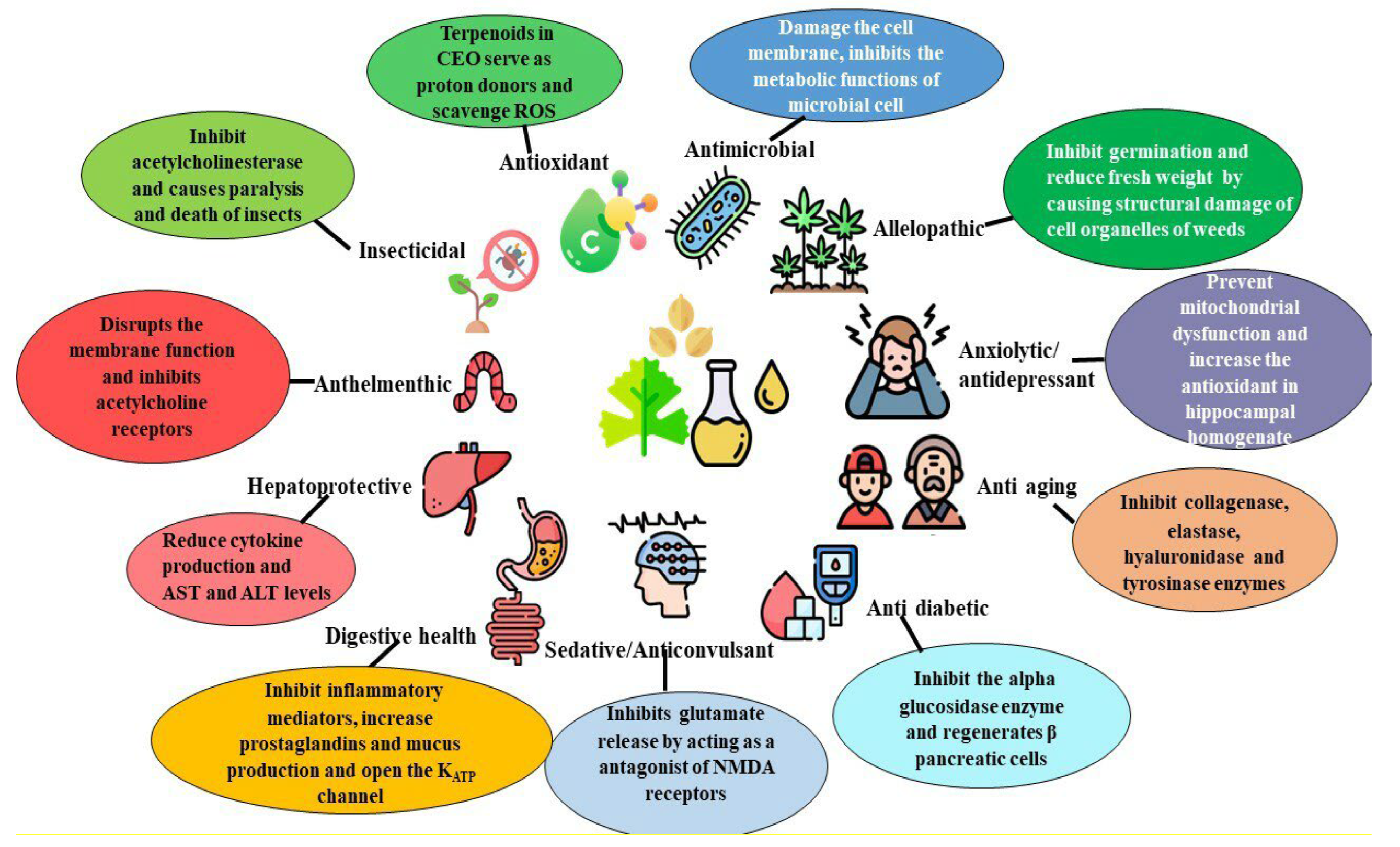
8. Biological Activity of Coriander Essential Oil
8.1. Antioxidant Activity
8.2. Antimicrobial Activity
8.2.1. Antibacterial Activity
8.2.2. Antifungal Activity
8.3. Anthelmintic Activity
8.4. Insecticidal Activity
8.5. Antidiabetic Activity
8.6. Antihyperlipidemic/Hypolipidemic Activity
8.7. Maintenance of Good Digestive Health
8.8. Hepatoprotective Activity
8.9. Anti-Aging Properties
8.10. Sedative/Anticonvulsant Properties
8.11. Anxiolytic-Antidepressant Properties
8.12. Allelopathy
9. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in Zein nanoparticles prepared by liquid–liquid dispersion method. LWT-Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Guenther, E. The essential oils. J. Am. Pharm. Assoc. 1948, 37, 214. [Google Scholar] [CrossRef]
- CheBi. Volatile Oil Component (CHEBI:27311). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:27311 (accessed on 12 August 2022).
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.L.; Phebe, D. Physiological production of essential oil in plants-Ontogeny, secretory structures and seasonal variations: Review. Pertanika J. Sci. Technol. 2016, 2, 1–10. [Google Scholar]
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009, 179, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Fernandes, E.S.; da Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Gkogka, E.; Hazeleger, W.C.; Posthumus, M.A.; Beumer, R.R. The antimicrobial activity of the essential oil of Pistacia lentiscus var. Chia. J. Essent. Oil-Bear. Plants. 2013, 16, 714–729. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.; Hammer, K. The infraspecific taxa of coriander (Coriandrum sativum L.). Genet. Resour. Crop Evol. 2003, 50, 33–63. [Google Scholar] [CrossRef]
- Khan, I.U.; Dubey, W.; Gupta, V. Taxonomical aspect of coriander (Coriandrum sativum L.). Int. J. Curr. Res. Rev. 2014, 6, 9926–9930. [Google Scholar]
- Yeung, E.C.; Bowra, S. Embryo and endosperm development in coriander (Coriandrum sativum). Botany 2011, 89, 263–273. [Google Scholar] [CrossRef]
- Gardenate. Growing Coriander. Available online: https://www.gardenate.com/plant/Coriander?page=1&co=ALL (accessed on 12 October 2022).
- Ware, M. Cilantro (Coriander): Benefits, nutrition, and preparation tips. Available online: https://www.medicalnewstoday.com/articles/277627 (accessed on 16 October 2022).
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M.; Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: Volatile and non-volatile extracts. Int. J. Food Prop. 2012, 15, 736–747. [Google Scholar] [CrossRef]
- Chahal, K.K.; Singh, R.; Kumar, A.; Bhardwaj, U. Chemical composition and biological activity of Coriandrum sativum L.: A review. IOSR J. Pharm. 2016, 6, 17–42. [Google Scholar]
- Sarkic, A.; Stappen, I. Essential Oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Farooq, A.; Muhammad, S.; Abdullah, I.H.; Nazamid, S.; Shahid, I.; Umer, R. Physicochemical composition of hydro-distilled essential oil from coriander (Coriandrum sativum L.) seeds cultivated in Pakistan. J. Med. Arom. Plant Sci. 2011, 5, 3537–3544. [Google Scholar]
- SurgingLife Coriander Essential Oil Its Uses and What It Is. Available online: https://surginglife.com/wellness/essential-oils/guide/coriander/ (accessed on 13 August 2022).
- Wahba, H.E.; Abd Rabbu, H.S.; Ibrahim, M.E. Evaluation of essential oil isolated from dry coriander seeds and recycling of the plant waste under different storage conditions. Bull. Natl. Res. Cent. 2020, 44, 192. [Google Scholar] [CrossRef]
- Wei, J.-N.; Liu, Z.-H.; Zhao, Y.-P.; Zhao, L.-L.; Xue, T.-K.; Lan, Q.-K. Phytochemical and bioactive profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef]
- Morsy, N.F.S. Chemical structure, quality indices and bioactivity of essential oil constituents. In Active Ingredients from Aromatic And Medicinal Plants; IntechOpen: London, UK, 2017. [Google Scholar]
- Burdock, G.A.; Carabin, I.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Faqir, M.A.; Muhammad, I.K.; Tehseen, S.; El-Ghorab, A.; Javed, I.S. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): A Review. Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Begum, J.; Sultana, M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh J. Pharmacol. 2009, 4, 150–153. [Google Scholar] [CrossRef]
- Chung, I.-M.; Ahmad, A.; Kim, S.-J.; Naik, P.M.; Nagella, P. Composition of the essential oil constituents from leaves and stems of korean Coriandrum sativum and their immunotoxicity activity on the Aedes aegypti L. Immunopharmacol. Immunotoxicol. 2012, 34, 152–156. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool–A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Satyal, P.; Setzer, W.N. Chemical compositions of commercial essential oils from Coriandrum sativum fruits and aerial parts. Nat. Prod. Commun. 2020, 15, 421–428. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-Ol: A novel and promising therapeutic agent for human gastrointestinal cancers. PLoS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, H.; Li, W.; Li, X.; Xiang, H.; Zhang, G.; Liu, H.; Wang, Q.; Wang, Y.; Xian, M.; et al. Production of γ-terpinene by metabolically engineered Escherichia coli using glycerol as feedstock. RSC Adv. 2018, 8, 30851–30859. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Hadzopoulou-Cladaras, M. Camphene, a plant derived monoterpene, exerts its hypolipidemic action by affecting srebp-1 and mtp expression. PLoS ONE 2016, 11, e0147117. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-what are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor-a fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon-a review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive activity and redox profile of the monoterpenes (+)-camphene, p-cymene, and geranyl acetate in experimental models. ISRN Toxicol 2013, 2013, 459530. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Sun, H.-L.; Chen, S.-Y.; Zeng, L.; Wang, T.-T. Anti-fungal activity, mechanism studies on α-phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wen, J.; Wang, Z.; Wang, J. Multiple regulation and targeting effects of borneol in the neurovascular unit in neurodegenerative diseases. Basic Clin. Pharmacol. Toxicol. 2022, 130, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-caryophyllene: A sesquiterpene with countless biological properties. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. citronellol, a monoterpene alcohol with promising pharmacological activities-a systematic review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Forbes, W.M.; Gallimore, W.A.; Mansingh, A.; Reese, P.B.; Robinson, R.D. Eryngial (trans-2-Dodecenal), a bioactive compound from eryngium foetidum: Its identification, chemical isolation, characterization and comparison with Ivermectin in vitro. Parasitology 2014, 141, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.M.; Simeoni, L.A.; Silveira, D. Genus Pouteria: Chemistry and biological activity. Rev. Bras. Farmacogn. 2009, 19, 501–509. [Google Scholar] [CrossRef]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Rosselli, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oil from flowers of Eryngium triquetrum (apiaceae) collected wild in Sicily. Nat. Prod. Commun. 2016, 11, 1019–1024. [Google Scholar] [CrossRef]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Mazzanti, G.; Uccella, N.; Castelli, F. Study on the mechanisms of the antibacterial action of some plant alpha, beta-unsaturated aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef]
- Marques, C.N.H.; Morozov, A.; Planzos, P.; Zelaya, H.M. The fatty acid signaling molecule cis-2-decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial-susceptible state. Appl. Environ. Microbiol. 2014, 80, 6976–6991. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Nejad, E.S.; Hadian, J.; Ranjbar, H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Pickett, K.M.; Caldwell, C.D.; Pincock, J.A.; Roberts, J.C.; Mapplebeck, L. Cultivar and sowing date effects on seed yield and oil composition of coriander in Atlantic Canada. Ind. Crops Prod. 2008, 28, 88–94. [Google Scholar] [CrossRef]
- Beyzi, E.; Karaman, K.; Gunes, A.; BuyukkilicBeyzi, S. Change in some biochemical and bioactive properties and essential oil composition of coriander seed (Coriandrum sativum L.) varieties from Turkey. Ind. Crops Prod. 2017, 109, 74–78. [Google Scholar] [CrossRef]
- Abou El-Nasr, T.H.S.; Ibrahim, M.M.; Aboud, K.A.; El-Enany, M.A. Assessment of genetic variability for three coriander (Coriandrum sativum L.) cultivars grown in Egypt, using morphological characters, essential oil composition and ISSR markers. World Appl. Sci. J. 2013, 25, 839–849. [Google Scholar]
- Saxena, S.N.; Rathore, S.S.; Saxena, R.; Barnwal, P.; Sharma, L.K.; Singh, B. Effect of cryogenic grinding on essential oil constituents of coriander (Coriandrum sativum l.) genotypes. J. Essent. Oil-Bear. Plants 2014, 17, 385–392. [Google Scholar] [CrossRef]
- López, P.A.; Widrlechner, M.P.; Simon, P.W.; Rai, S.; Boylston, T.D.; Isbell, T.A.; Bailey, T.B.; Gardner, C.A.; Wilson, L.A. Assessing phenotypic, biochemical, and molecular diversity in coriander (Coriandrum sativum L.) germplasm. Genet. Resour. Crop Evol. 2008, 55, 247–275. [Google Scholar] [CrossRef]
- Saxena, S.N.; Swarup Meena, R.; Vishal, M.K.; John, S.; Kumar Sharma, L.; Mishra, B.K.; Agarwal, D. Variation in essential oil constituents of coriander (Coriandrum sativum L.) germplasm across coriander growing regions in India. J. Essent. Oil Res. 2022, 34, 173–180. [Google Scholar] [CrossRef]
- Sampaio, T.S.; Nogueira, P.C.L. Volatile components of Mangaba fruit (Hancornia speciosa Gomes) at three stages of maturity. Food Chem. 2006, 95, 606–610. [Google Scholar] [CrossRef]
- Visai, C.; Vanoli, M. Volatile compound production during growth and ripening of peaches and nectarines. Sci. Hortic. 1997, 70, 15–24. [Google Scholar] [CrossRef]
- Vendramini, A.L.; Trugo, L.C. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Nguyen, Q.-H.; Talou, T.; Evon, P.; Cerny, M.; Merah, O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020, 326, 127034. [Google Scholar] [CrossRef] [PubMed]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, B. Changes on essential oil composition of coriander (Coriandrum sativum l.) fruits during three stages of maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Telci, I.; Bayram, E.; Avci, B. Changes in yields, essential oil and linalool contents of Coriandrum sativum varieties (var. Vulgare Alef. and var. Microcarpum DC.) harvested at different development stages. Eur. J. Hortic. Sci. 2006, 71, 267–271. [Google Scholar]
- Priyadarshi, S.; Borse, B.B. Effect of the environment on content and composition of essential oil in coriander. Int. J. Sci. Eng. Res. 2014, 5, 57–65. [Google Scholar]
- Shams, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dashtaki, H.N.; Dursun, A.; Yildirim, E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). Indian J. Sci. Technol. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Punetha, D.; Tewari, G.; Pande, C. Compositional variability in inflorescence essential oil of Coriandrum sativum from North India. J. Essent. Oil Res. 2018, 30, 113–119. [Google Scholar] [CrossRef]
- Gil, A.; De La Fuente, E.B.; Lenardis, A.E.; López Pereira, M.; Suárez, S.A.; Bandoni, A.; Van Baren, C.; Di Leo Lira, P.; Ghersa, C.M. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. [Google Scholar] [CrossRef]
- İzgı, M.N.; Telci, İ.; Elmastaş, M. Variation in essential oil composition of coriander (Coriandrum sativum L.) Varieties Cultivated in Two Different Ecologies. J. Essent. Oil Res. 2017, 29, 494–498. [Google Scholar] [CrossRef]
- Msaada, K.; Taarit, M.B.; Hosni, K.; Hammami, M.; Marzouk, B. Regional and maturational effects on essential oils yields and composition of coriander (Coriandrum sativum L.) fruits. Sci. Hortic. 2009, 122, 116–124. [Google Scholar] [CrossRef]
- Gandova, V.; Tasheva, S.; Marinova, K.; Dimov, M.; Dobreva, K.; Prodanovastefanova, V.; Stoyanova, A. Investigation of chemical composition, thermodynamic and thermal properties of coriander (Coriandrum sativum. L) essential oil. Oxid. Commun. 2020, 43, 85. [Google Scholar]
- Ravi, R.; Prakash, M.; Bhat, K.K. Aroma Characterization of coriander (Coriandrum sativum L.) oil samples. Eur. Food Res. Technol. 2007, 225, 367–374. [Google Scholar] [CrossRef]
- Al amrani, K.; Barbouchi, M.; Elidrissi, M.; Amechrouq, A.; Chokrad, M. Chemical composition and physicochemical properties of the essential oil of coriander (Coriandrum sativum L.) grown in Morocco. RHAZES: Green App. Chem. 2019, 4, 35–50. [Google Scholar]
- Huzar, E.; Dzięcioł, M.; Wodnicka, A.; Örün, H.; İçöz, A.; Çiçek, E. Influence of hydrodistillation conditions on yield and composition of coriander (Coriandrum sativum L.) essential oil. Pol. J. Food Nutr. Sci. 2018, 68, 243–250. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Alexa, E.; Popescu, I.; Negrea, M.; Radulov, I.; Obistioiu, D.; Cocan, I. Exploring ecological alternatives for crop protection using Coriandrum sativum essential oil. Molecules 2019, 24, 2040. [Google Scholar] [CrossRef]
- Choi, S.-A.; Lee, H.-S. Insecticidal activities of Russia coriander oils and these constituents against Sitophilus oryzae and Sitophilus zeamais. J. Appl. Biol. Chem. 2018, 61, 239–243. [Google Scholar] [CrossRef]
- Kiralan, M.; Calikoglu, E.; Ipek, A.; Bayrak, A.; Gurbuz, B. Fatty Acid and volatile oil composition of different coriander (Coriandrum sativum) registered varieties cultivated in Turkey. Chem. Nat. Compo. 2009, 45, 100–102. [Google Scholar] [CrossRef]
- Georgieva, R.; Delibaltova, V.; Chavdarov, P. Change in agronomic characteristics and essential oil composition of coriander after application of foliar fertilizers and biostimulators. Ind. Crops Prod. 2022, 181, 114819. [Google Scholar] [CrossRef]
- Khalid, K.A. Effect of macro and micro nutrients on essential oil of coriander fruits. J. Mat. Environ. Sci. 2015, 6, 2060–2065. [Google Scholar]
- Özyazici, G. Influence of organic and inorganic fertilizers on coriander (Coriandrum sativum L.) agronomic traits, essential oil and components under semi-arid climate. Agronomy 2021, 11, 1427. [Google Scholar] [CrossRef]
- Rasouli, F.; Nasiri, Y.; Asadi, M.; Hassanpouraghdam, M.B.; Golestaneh, S.; Pirsarandib, Y. Fertilizer type and humic acid improve the growth responses, nutrient uptake, and essential oil content on Coriandrum sativum L. Sci. Rep. 2022, 12, 7437. [Google Scholar] [CrossRef]
- Msaada, K.; Taârit, M.B.; Hosni, K.; Nidhal, S.; Tammar, S.; Bettaieb, I.; Hammami, M.; Limam, F.; Marzouk, B. Comparison of different extraction methods for the determination of essential oils and related compounds from coriander (Coriandrum sativum L.). Acta Chim. Slov. 2012, 59, 803–813. [Google Scholar]
- Sourmaghi, M.H.S.; Kiaee, G.; Golfakhrabadi, F.; Jamalifar, H.; Khanavi, M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015, 52, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Zeković, Z. Isolation of coriander (Coriandrum sativum L.) essential oil by green extractions versus traditional techniques. J. Supercrit. Fluids 2015, 99, 23–28. [Google Scholar] [CrossRef]
- Song, E.-J.; Ko, M.-J. Extraction of monoterpenes from coriander (Coriandrum sativum L.) Seeds Using Subcritical Water Extraction (SWE) Technique. J. Supercrit. Fluids 2022, 188, 105668. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Essential oil composition of the coriander (Coriandrum sativum L.) herb depending on the development stage. Acta Agrobot. 2013, 66, 53–60. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Mortazavi, S.A.; Yazdi, F.T.; Mohammadi, M. Microwave-assisted hydrodistillation extraction of essential oil from coriander seeds and evaluation of their composition, antioxidant and antimicrobial activity. Heliyon 2020, 6, e04893. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Zekovic, Z.; Adamovic, D.; Cetkovic, G.; Radojkovic, M.; Vidovic, S. Essential oil and extract of coriander (Coriandrum sativum L.). Acta Period. Technol. 2011, 281–288. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Sulaiman, R.Z. Detection of some active compounds in the leaves and stems of local coriander plant-Coriandrum sativum L. Tikrit J. Pure Sci. 2018, 23, 6–15. [Google Scholar]
- Darughe, F.; Barzegar, M.; Sahari, M.A. Antioxidant and antifungal activity of coriander (Coriandrum sativum L.) essential oil in cake. Food Chem. Toxicol. 2012, 19, 1253–1260. [Google Scholar]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Norashikin, S.; Rozita, O.; Wan, A.H.M.Y.; Rossuriati, D.H. Subcritical water extraction of essential oil from coriander (Coriandrum sativum L.) Seeds. Malays. J. Anal. Sci. 2008, 12, 22–24. [Google Scholar]
- Mhemdi, H.; Rodier, E.; Kechaou, N.; Fages, J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J. Food Eng. 2011, 105, 609–616. [Google Scholar] [CrossRef]
- Illés, V.; Daood, H.G.; Perneczki, S.; Szokonya, L.; Then, M. Extraction of coriander seed oil by CO2 and propane at super- and subcritical conditions. J. Supercrit. Fluids 2000, 17, 177–186. [Google Scholar] [CrossRef]
- Anitescu, G.; Doneanu, C.; Radulescu, V. Isolation of coriander oil: Comparison between steam distillation and supercritical CO2 extraction. Flavour Fragr. J. 1997, 12, 173–176. [Google Scholar] [CrossRef]
- Shrirame, B.S.; Geed, S.R.; Raj, A.; Prasad, S.; Rai, M.K.; Singh, A.K.; Singh, R.S.; Rai, B.N. Optimization of supercritical extraction of coriander (coriandrum sativum l.) seed and characterization of essential ingredients. J. Essent. Oil-Bear. Plants 2018, 21, 330–344. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.B.; de Almeida Melo, E.; Filho, J.M. antioxidant compounds from coriander (Coriandrum sativum L.) etheric extract. J. Food Compost. Anal. 2005, 18, 193–199. [Google Scholar] [CrossRef]
- Önder, A. Coriander and its phytoconstituents for the beneficial effects. In Potential of Essential Oils; El-Shemy, H.A., Ed.; IntechOpen: Rijeka, Croatia, 2018; ISBN 9781789237801. [Google Scholar]
- Baccouri, B.; Rajhi, I. Potential antioxidant activity of terpenes. In Terpenes and Terpenoids-Recent Advances; Perveen, S., Al-Taweel, A.M., Eds.; Intechopen: London, UK, 2021; ISBN 9781838819163. [Google Scholar]
- Dua, A.; Agrawal, S.; Kaur, A.; Mahajan, R. Antioxidant profile of Coriandrum sativum methanolic extract. Int. Res. J. Pharm. 2014, 5, 220–224. [Google Scholar] [CrossRef]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Raghuramulu, N.; Madhavan Nair, K.; Kalyanasundaram, S. A Manual of Laboratory Techniques; Jami-Osmania: Hyderabad, India, 1983. [Google Scholar]
- Dua, A.; Mittal, A.; Gupta, S.; Mahajan, R. Bioreactive compounds and antioxidant properties of methanolic extract of fennel (Foeniculum vulgare Miller). Int. Res. J. Pharm. 2013, 4, 241–245. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Mehdizadeh, L.; Moghaddam, M. Essential oils: Biological activity and therapeutic potential. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 167–179. ISBN 9780128146255. [Google Scholar]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Keskin, D.; Toroglu, S. Studies on antimicrobial activities of solvent extracts of different spices. J. Environ. Biol. 2011, 32, 251–256. [Google Scholar]
- Duarte, A.; Ferreira, S.; Silva, F.; Domingues, F.C. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 2012, 19, 236–238. [Google Scholar] [CrossRef]
- Zare-Shehneh, M.; Askarfarashah, M.; Ebrahimi, L.; Kor, N.M.; Zare-Zardini, H.; Soltaninejad, H.; Hashemian, Z.; Jabinian, F. Biological activities of a new antimicrobial peptide from Coriandrum sativum. Int. J. Biosci. 2014, 4, 89–99. [Google Scholar]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Innocent, B.X. Studies on the immunostimulant activity of Coriandrum sativum and resistance to Aeromonas hydrophila in Catla catla. J. Appl. Pharm. Sci. 2011, 1, 132–135. [Google Scholar]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- de Freires, I.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; de Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef]
- Lalitha, V.; Kiran, B.; Raveesha, K.A. Antifungal and antibacterial potentiality of six essential oils extracted from plant source. Int. J. Eng. Sci. Technol. 2011, 3, 3029–3038. [Google Scholar]
- Soares, B.V.; Morais, S.M.; dos Santos Fontenelle, R.O.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.C.; Brito, E.S.; Neto, M.A.S.; Brito, E.H.S.; Cavalcante, C.S.P.; et al. Antifungal activity, toxicity and chemical composition of the essential oil of Coriandrum sativum L. Fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.E.; Cave, G.W.V.; Morrison, A.A.; Bartley, D.J.; Elsheikha, H.M. Nematocidal effects of a coriander essential oil and five pure principles on the infective larvae of major ovine gastrointestinal nematodes in vitro. Pathogens 2020, 9, 740. [Google Scholar] [CrossRef]
- Eguale, T.; Tilahun, G.; Debella, A.; Feleke, A.; Makonnen, E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacol. 2007, 110, 428–433. [Google Scholar] [CrossRef]
- Zoubiri, S.; Baaliouamer, A. Essential oil composition of Coriandrum sativum seed cultivated in Algeria as food grains protectant. Food Chem. 2010, 122, 1226–1228. [Google Scholar] [CrossRef]
- Ngamo, T.; Ngatanko, I.; Ngassou, M.; Mapongmestem, P.; Hance, T. Insecticidal efficiency of essential oils of 5 aromatic plants tested both alone and in combination towards Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Adv. Pharm. Technol. Res. 2007, 2, 75–80. [Google Scholar]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Hansen, L.S.; Jensen, K.M.V. Effect of temperature on parasitism and host-Feeding of Trichogramma turkestanica (Hymenoptera: Trichogrammatidae) on Ephestia kuehniella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2002, 95, 50–56. [Google Scholar] [CrossRef]
- Ayvaz, A.; Albayrak, S.; Karaborklu, S. Gamma radiation sensitivity of the eggs, larvae and pupae of Indian meal moth Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2008, 64, 505–512. [Google Scholar] [CrossRef]
- Sighamony, S.; Anees, I.; Chandrakala, T.; Osmani, Z. Efficacy of certain indigenous plant products as grain protectants against Sitophilus oryzae (L.) and Rhyzopertha dominica (F.). J. Stored Prod. Res. 1986, 22, 21–23. [Google Scholar] [CrossRef]
- SritiEljazi, J.; Bachrouch, O.; Salem, N.; Msaada, K.; Aouini, J.; Hammami, M.; Boushih, E.; Abderraba, M.; Limam, F.; Mediouni Ben Jemaa, J. Chemical composition and insecticidal activity of essential oil from coriander fruit against Tribolium castaenum, Sitophilus oryzae, and Lasioderma serricorne. Int. J. Food Prop. 2017, 20, S2833–S2845. [Google Scholar] [CrossRef]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Stored Prod. Res. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasan, M.M.; Xiong, W.; Zhang, S.C.; Lei, C.L. Fumigant and repellent activities of essential oil from Coriandrum sativum (L.) (Apiaceae) against red flour beetle Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Pest Sci. 2009, 82, 171–177. [Google Scholar] [CrossRef]
- Khani, A.; Rahdari, T. Chemical composition and insecticidal activity of essential oil from Coriandrum sativum seeds against Tribolium confusum and Callosobruchus maculatus. ISRN Pharm. 2012, 2012, 263517. [Google Scholar] [CrossRef]
- Aligita, W.; Susilawati, E.; Septiani, H.; Atsil, R. Antidiabetic activity of Coriander (Coriandrum sativum L.) leaves’ ethanolic extract. Int. J. Pharm. Biol. Arch. 2018, 8, 59–63. [Google Scholar]
- Lipinski, B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Complicat. 2001, 15, 203–210. [Google Scholar] [CrossRef]
- Dakhlaoui, S.; Wannes, W.A.; Sari, H.; Hmida, M.B.; Frouja, O.; Limam, H.; Tammar, S.; Bachkouel, S.; Jemaa, M.B.; Jallouli, S.; et al. Combined effect of essential oils from Lavender (Lavandula officinalis L.) aerial parts and coriander (Coriandrum sativum L.) seeds on antioxidant, anti-diabetic, anti-cancer and anti-inflammatory activities. J. Essent. Oil-Bear. Plants. 2022, 25, 188–199. [Google Scholar] [CrossRef]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Ali, N.; Mostafa, I.; Hasan, R.A.; Sobeh, M. Coriander oil reverses dexamethasone-induced insulin resistance in rats. Antioxidants 2022, 11, 441. [Google Scholar] [CrossRef]
- El-Soud, N.H.A.; El-Lithy, N.A.; El-Saeed, G.S.M.; Wahby, M.S.; Khalil, M.Y.; El-Kassem, L.T.A.; Morsy, F.; Shaffie, N. Efficacy of Coriandrum sativum L. essential oil as antidiabetic. J. Appl. Sci. Res. 2012, 8, 3646–3655. [Google Scholar]
- Deepa, B.; Venkatraman Anuradha, C. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- More, T.A.; Kulkarni, B.R.; Nalawade, M.L.; Arvindekar, A.U. Antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: A combinatorial therapy approach. Int. J. Pharm. Pharm. Sci. 2014, 6, 159–163. [Google Scholar]
- Garikiparithi, M. 10 Best Essential Oils for High Cholesterol Reduction. Available online: https://www.belmarrahealth.com/10-best-essential-oils-high-cholesterol-reduction/ (accessed on 16 August 2022).
- Lal, A.A.S.; Kumar, T.; Murthy, P.B.; Pillai, K.S. Hypolipidemic effect of Coriandrum sativum L. in triton-induced hyperlipidemic rats. Indian J. Exp. Biol. 2004, 42, 909–912. [Google Scholar]
- Ramadan, M.F.; Amer, M.M.A.; Awad, A.E.-S. Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed a diet containing cholesterol. Eur. Food Res. Technol. 2008, 227, 1173–1182. [Google Scholar] [CrossRef]
- Vimala, G.; Gricilda Shoba, F. A review on antiulcer activity of few Indian medicinal plants. Int. J. Microbiol. 2014, 2014, 519590. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Andrade, L.N.; de Sousa, E.B.V.; de Sousa, D.P. Anti-ulcer activity of essential oil constituents. Molecules 2014, 19, 5717–5747. [Google Scholar] [CrossRef]
- Heidari, B.; Sajjadi, S.E.; Minaiyan, M. Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid- induced acute colitis in rats. Avicenna J. Phytomed. 2016, 6, 205–214. [Google Scholar] [PubMed]
- Jia, X.-Y.; Zhang, Q.-A.; Zhang, Z.-Q.; Wang, Y.; Yuan, J.-F.; Wang, H.-Y.; Zhao, D. Hepatoprotective effects of almond oil against carbon tetrachloride induced liver injury in rats. Food Chem. 2011, 125, 673–678. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Dhibi, S.; Dhifi, W.; Mnif, W.; Ben Nasr, H.; Hfaiedh, N. Chemical composition and Hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCL4-induced acute hepatotoxicity in rats. RSC Adv. 2019, 9, 3777–3787. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; de Souza Silva-Comar, F.M.; Silva, E.L.; da Rocha, E.M.T.; Comar, J.F.; Silva-Filho, S.E.; Zagotto, M.; Uchida, N.S.; Bersani-Amado, C.A.; Cuman, R.K.N. Lavender (Lavandula officinalis) essential oil prevents acetaminophen-induced hepatotoxicity by decreasing oxidative stress and inflammatory response. Res. Soc. Dev. 2021, 10, e43410313461. [Google Scholar] [CrossRef]
- Özbek, H.; Kırmızı, N.İ.; Cengiz, N.; Erdoğan, E. Hepatoprotective effects of Coriandrum sativum essential oil against acute hepatotoxicity induced by carbon tetrachloride on rats. ACTA Pharm. Sci. 2016, 54, 35. [Google Scholar] [CrossRef]
- Altınok-Yipel, F.; Ozan Tekeli, İ.; Özsoy, Ş.Y.; Güvenç, M.; Kaya, A.; Yipel, M. Hepatoprotective activity of linalool in rats against liver injury induced by carbon tetrachloride. Int. J. Vitam. Nutr. Res. 2020, 90, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mazani, M.; Rezagholizadeh, L.; Shamsi, S.; Mahdavifard, S.; Ojarudi, M.; Salimnejad, R.; Salimi, A. protection of CCl4-induced hepatic and renal damage by linalool. Drug Chem. Toxicol. 2022, 45, 963–971. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Sadaka, C.; Beyrouthy, M.E.; Hfaiedh, M.; Dhifi, W.; Brini, F.; Saad, R.B.; Mnif, W. Immunomodulatory effect of linalool (Lin) against CCl4 -induced hepatotoxicity and oxidative damage in rats. Biotechnol. Appl. Biochem. 2022. [Google Scholar] [CrossRef]
- Scattergood, G. Apiaceous Opportunity: Coriander Oil Displays Anti-Ageing Skin Care Nenefits—New Research. Available online: https://www.cosmeticsdesign-asia.com/Article/2022/05/04/corinader-oil-has-the-potential-to-be-effective-anti-ageing-ingredient (accessed on 16 August 2022).
- Salem, M.A.; Manaa, E.G.; Osama, N.; Aborehab, N.M.; Ragab, M.F.; Haggag, Y.A.; Ibrahim, M.T.; Hamdan, D.I. Coriander (Coriandrum sativum L.) essential oil and oil-loaded nano-formulations as an anti-aging potentiality via TGFβ/SMAD pathway. Sci. Rep. 2022, 12, 6578. [Google Scholar] [CrossRef]
- Bahr, T.A.; Rodriguez, D.; Beaumont, C.; Allred, K. The effects of various essential oils on epilepsy and acute seizure: A systematic review. Evid. Based. Complement. Alternat. Med. 2019, 2019, 6216745. [Google Scholar] [CrossRef] [PubMed]
- Gastón, M.S.; Cid, M.P.; Vázquez, A.M.; Decarlini, M.F.; Demmel, G.I.; Rossi, L.I.; Aimar, M.L.; Salvatierra, N.A. Sedative effect of central administration of Coriandrum sativum essential oil and its major component linalool in neonatal chicks. Pharm. Biol. 2016, 54, 1954–1961. [Google Scholar] [CrossRef]
- Emam, G.M.; Heydari, H.G. Effect of extract and essential oil of Coriandrum sativum seed against Pentylenetetrazole induced seizure. Pharm. Sci. 2008, 14, 1–10. [Google Scholar]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-Methyl D-Aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef] [PubMed]
- NIMH Anxiety Disorders. Available online: https://www.nimh.nih.gov/health/topics/anxiety-disorders (accessed on 18 August 2022).
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Trifan, A.; Hancianu, M. Inhalation of coriander volatile oil increased anxiolytic-antidepressant-like behaviors and decreased oxidative status in beta-amyloid (1-42) Rat model of Alzheimer’s disease. Physiol. Behav. 2014, 131, 68–74. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Murphy, K.M.; Dawson, J.C.; Jones, S.S. Relationship among phenotypic growth traits, yield and weed suppression in spring wheat landraces and modern cultivars. Field Crops Res. 2008, 105, 107–115. [Google Scholar] [CrossRef]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed Control Research and Safener Technology: The Path to Modern Agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical characterization and allelopathic potential of volatile oil of Eucalyptus tereticornis against Amaranthus viridis. J. Plant Interact. 2011, 6, 297–302. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Azirak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 2010, 58, 457–465. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Mousavizadeh, S.J.; Mohammadi, H.; Rokhzadi, A.; Majidi, M.; Amini, S. Allelopathic effect of some essential oils on seed germination of Lathyrus annuus and Vicia villosa. J. Biodivers. 2013, 3, 67–73. [Google Scholar]
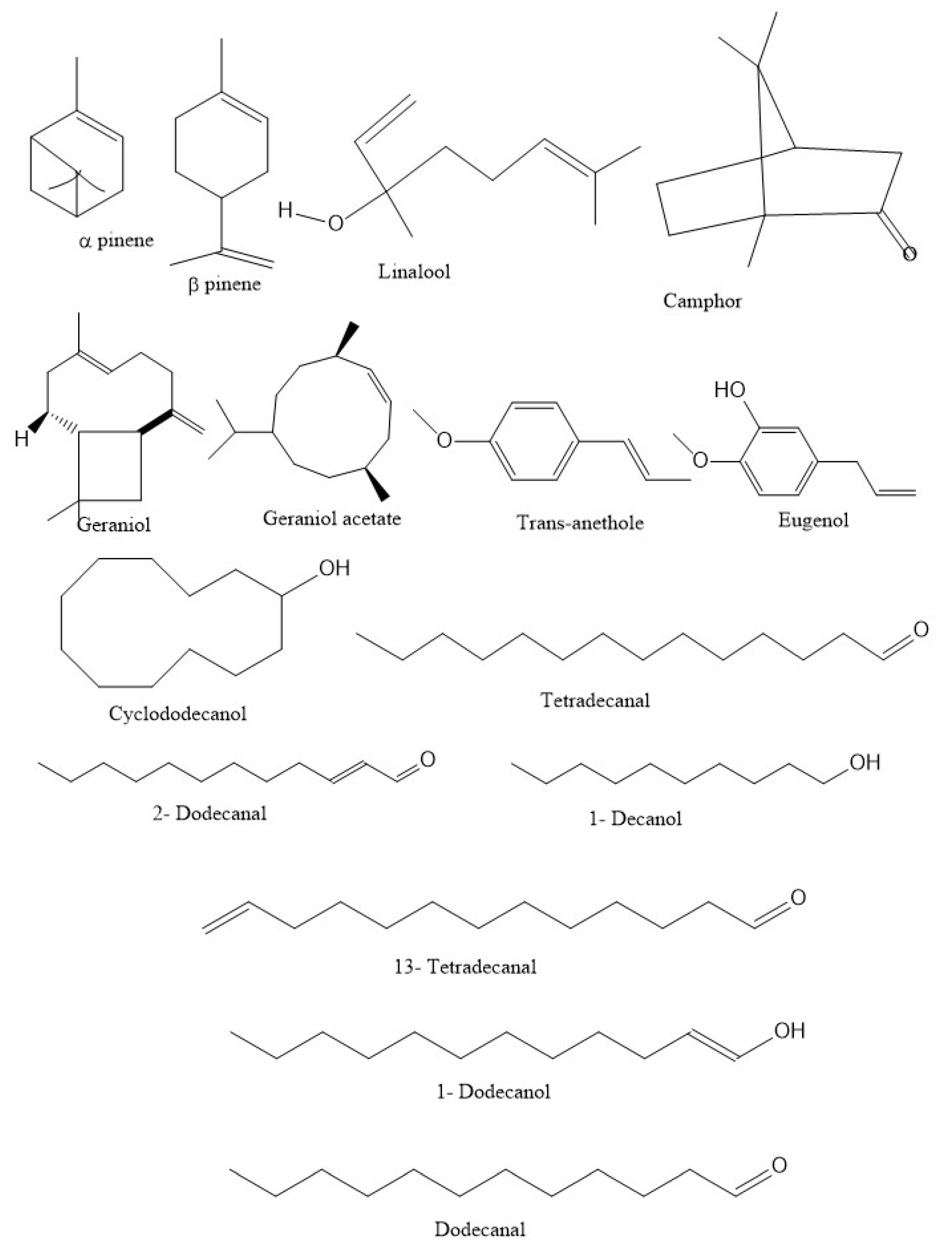
| Chemical Group | Constituents |
|---|---|
| Alcohols | Linalool (60–80%), geraniol (1.2–4.6%), terpinen-4-ol (3%), α-terpineol (0.5%) |
| Hydrocarbons | Limonene (0.5–4.0%), g-terpinene (1–8%), p-cymene (3.5%), α-pinene (0.2–8.5%), camphene (1.4%), myrcene (0.2–2.0%) |
| Ketones | Camphor (0.9–4.9%) |
| Esters | Geranyl acetate (0.1–4.7%), linalyl acetate (0–2.7%) |
| Constituent | Concentration (%) | Function | References | |
|---|---|---|---|---|
| Seed | Leaf | |||
| Linalool | 40.9−79.9 | 0–13 | Antimicrobial, anti-inflammatory, anticancer, antioxidant properties | [30,31] |
| Geraniol | 0.5–3.0 | - | Insecticidal and repellent properties | [32] |
| Terpinen-4-ol | 0.43–1 | 0.2 | Promotes anti-inflammatory cytokine production, enhances the effect of several chemotherapeutic and biological agents | [33] |
| α-terpineol | 0.5–1.5 | - | Anticancer, anticonvulsant, antiulcer, antihypertensive | [34] |
| γ-terpinene | 0.3–11.2 | 0.9–3.1 | Potential biofuel alternative | [35] |
| p-cymene | 0.5–1.5 | - | Natural antioxidant, antimicrobial activity | [36] |
| Limonene | 2.0–5.0 | - | Anticancer activity | [37] |
| α-pinene | 0.2–10.9 | 1.9–2.5 | Antimicrobial, apoptotic, antimetastatic, and antibiotic | [38] |
| Camphene | 1.3–2 | - | Antileishmanial, hepatoprotective, antiviral and anticancer activity by inducing apoptosis in cancer cells | [39] |
| Myrcene | 0.5–1.5 | - | Anxiolytic, antioxidant, anti-ageing, anti-inflammatory, analgesic properties | [40] |
| Camphor | 0.9–4.9 | - | Insecticidal, antimicrobial, antiviral, anticoccidial, antinociceptive | [41] |
| Geranyl acetate | 0.2–5.4 | - | Antinociceptive activity | [17,42] |
| Linalyl acetate | 0–2.7 | - | Flavoring agent, antimicrobial and anti-inflammatory activity | [43] |
| Eucalyptol | 0.1–1% | 0.5–2 | Anti-inflammatory and antioxidant mainly via the regulation on NF-κB and Nrf2 pathway | [44] |
| β-phellandrene | 0–1.5 | - | Antimicrobial activity | [45] |
| Borneol | 4.5 | - | Acesodyne, sedation, anti-inflammation, antibiosis effect | [46] |
| β-caryophyllene | 3.26 | - | Antibacterial, antioxidant, gastroprotective, anxiolytic, anti-inflammatory | [47] |
| Citronellol | 0.15–0.25 | 8.1–10 | Anti-inflammatory, analgesic and anticonvulsant effects | [48] |
| Caryophyllene oxide | 3.12 | - | Cytotoxic activity, analgesic activity | [49] |
| Thymol | 2.4–3 | - | Antioxidant and antimicrobial properties | [50] |
| Decanal | - | 5.1–8.8 | Antioxidant and antimicrobial properties | [17] |
| (E)-2-Dodecenal | - | 12–14 | Anthelmintic activity | [51] |
| Tridecanal | - | 0.3–0.4 | Antibacterial, antifungal and antioxidant activities | [52] |
| 1-Decanol | - | 5–10% | Bactericidal activity and membrane-damaging activity | [53] |
| (E)-2-Tetradecenal | - | 4.1–8.2 | Antimicrobial, and antioxidant activity | [54] |
| (E)-2-Decenal | - | 20–35 | Antimicrobial, and antioxidant activities | [55] |
| 2-decenoic acid | - | 30.8 | Antimicrobial activity | [56] |
| Capric acid | - | 12.7 | Antibacterial and anti-inflammatory activity | [57] |
| Location | Varieties | Essential Oil Content (%) | Linalool Content (%) | References |
|---|---|---|---|---|
| Turkey | Arslan | 0.30 | 89.46 | [60] |
| Gürbüz | 0.33 | 89.44 | ||
| Erbaa | 0.38 | 91.77 | ||
| Gamze | 0.35 | 89.77 | ||
| Pakistan | Native | 0.15 | 69.64 | [20] |
| Egypt | Russian | 0.19 | 69.50 | [61] |
| Balady | 0.2 | 62.53 | ||
| Selected | 0.09 | 65.48 | ||
| India | Acr1 | 0.25 | 78.22 | [62] |
| Sindhu | 0.32 | 81.3 | ||
| Swathi | 0.34 | 73.2 | ||
| Sadhna | 0.29 | 79.2 | ||
| Sudha | 0.32 | 85.3 |
| Percentage of Important Phytochemicals in Coriander Seed Oil from Different Geographical Locations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Constituent | Bangladesh [28] | Bulgaria [77] | India [78] | Iran [58,71] | Morocco [79] | Pakistan [20] | Poland [80] | Romania [81] | Russia [82] | Turkey [60,83] |
| α-Thujene | - | - | - | - | 0.10 | 0.02 | - | - | - | - |
| α-Pinene | - | 7.14 | 2.81 | 3.3 | 7.69 | 1.63 | 5.03 | 1.62 | 6.44 | 3.06 |
| α-Terpineol | - | 0.66 | - | - | 0.54 | - | 0.63 | - | 0.81 | - |
| β-Pinene | 1.8 | 1.15 | 0.48 | 0.4 | 0.93 | 0.23 | 0.48 | 0.71 | 0.34 | 0.25 |
| β-caryophyllene | 0.3 | 0.17 | - | - | 0.14 | 0.07 | - | 0.44 | - | - |
| p-Cymene | 1.3 | 2.67 | 0.42 | 2 | 0.03 | 1.12 | 0.54 | 8.000 | 7.44 | 0.39 |
| γ-Terpinene | 14.4 | 7.35 | 0.15 | 9.3 | 11.59 | 4.17 | 3.80 | 5.236 | - | 0.20 |
| Borneol | 0.3 | 0.44 | 0.14 | - | 0.42 | 0.18 | - | - | - | - |
| Camphor | - | 8.30 | - | 0.2 | 6.98 | 0.38 | 3.90 | 6.01 | 7.94 | 3.56 |
| Camphene | - | 2.37 | - | 1.07 | 0.64 | 1.09 | - | |||
| Citronella | 1.3 | - | - | 0.3 | - | 0.65 | - | - | - | - |
| Decanal | 0.1 | - | 0.20 | 0.4 | 0.13 | 0.14 | - | - | - | 0.54 |
| Geraniol | 1.9 | - | 24.51 | 1.9 | 2.79 | - | 1.07 | 0.11 | 2.37 | |
| Geranyl acetate | 17.6 | 5.05 | 4.0 | - | 4.99 | 2.13 | 1.423 | 3.19 | 2.77 | |
| Limonene | 0.4 | 5.19 | 0.3 | 3.24 | 0.26 | 2.58 | 9.628 | 3.29 | 0.27 | |
| Linalool | 37.7 | 50.16 | 57.52 | 70.1 | 48.41 | 69.60 | 78.45 | 45.387 | 59.92 | 75.26 |
| Myrcene | 0.6 | 1.78 | 0.37 | 0.2 | 1.16 | 0.18 | 0.47 | 1.504 | 0.20 | 0.42 |
| Neryl acetate | - | - | 6.9 | 6.47 | - | - | - | - | - | |
| Sabinene | 0.2 | 0.73 | 0.2 | 0.58 | 0.12 | - | - | - | 0.10 | |
| Thymol | - | - | 0.2 | 0.06 | 0.41 | - | 0.376 | - | - | |
| Undecanal | 0.1 | - | 0.13 | - | 0.06 | 0.41 | - | - | - | - |
| Extraction Method | Extraction Condition | Quantification Method | Quantification Method (Instrument Used, Model, Conditions) | Quantity of Oil Obtained in % | References |
|---|---|---|---|---|---|
| Hydrodistillation | 80 min | GC-MS | Turbomass system,199 °C for 35 min, 1 μL | 0.18–1.4 | [59] |
| 180 min | GC-MS | ITMS Varian 4000 GC-MS/MS 250 °C for 10 min, 1 μL | 0.29 | [93] | |
| 180 min | GC-MS | Shimadzu GC-9A, Varian 3400, 250 °C | 0.31 | [22] | |
| Microwave-assisted hydrodistillation | 60 min with 500 W power | - | - | 0.32 | [94] |
| 240 min | GC-MS, FID | Shimadzu 15A 260 °C, 1 mL/min | 0.1 | [89] | |
| Rapid solid–liquid dynamic extraction | 8 bar, 6 h | solid-phase microextraction/gas chromatography coupled to mass spectrometry | Clarus 580 GC apparatus coupled to a Clarus SQ 8 S GC/MS, 250 °C | 0.73 | [95] |
| Soxhlet extraction | 40 °C | GC-MS and GC-FID | Agilent GC890N 150 °C, 5 μL | 14.45 | [91] |
| Methylene chloride | GC-MS | Agilent Technologies series 6890N/5975B 280 °C, 20 min, 1 μL | 5.10 | [96] | |
| Petroleum ether, 45 min | GC-MS | Shimadzu, GCMS-QP2010 Ultra, 260 °C | 8.82 | [97] | |
| Steam distillation | 80 min | GC-MS, FID | Agilent Technologies 6890, 340 °C, | [98] | |
| Subcritical water extraction | 125 °C, 0.5 mm, and 2 mL/min | GC-FID and GC-MS | Phillips model PU-4500 50 to 240 C at 3 C/min, 0.5 μL | 14.1 | [99] |
| 10 °C min−1 to 200 °C, 15 min | GC-MS | Agilent gas chromatography model 6890N 200 °C, 15 min | 0.6–0.8 | [100] | |
| extraction | 10 MPa, 35 °C, CO2 - 419.9 kg/m3 | GC-FID and GC-MS | capillary type HP 5890 series II, equipped with a DB-5MS column 280 °C for 53 min, 0.5 μL | 0.84 | [101] |
| 300 bar, 35 °C, | GC | Shimadzu Model RF-353, 50–200 °C, 5 μL | 20 | [102] | |
| 50 °C and 150 bar, 180 min | GC-MS | Fisons Instruments MD 800, 250 °C, 20 min, 0.4 μL | 0.61 | [103] | |
| 350 bar, 35 °C, CO2 -14 g/min | GC-MS | Shimadzu QP2010 Ultra, 280 °C, 30 min | 4.55 | [104] |
| Metabolite | Amount in mg/g Dry Weight of Sample | Method of Analysis | References |
|---|---|---|---|
| Caffeic Acid | 0.08 | HPLC method | [111] |
| Ellagic Acid | 0.162 | HPLC method | [111] |
| Gallic Acid | 0.173 | HPLC method | [111] |
| Kaempferol | 0.233 | HPLC method | [111] |
| Oxidized Ascorbate | 0.15 | Spectrophotometric method | [112] |
| Reduced Ascorbate | 0.136 | Spectrophotometric method | [112] |
| Riboflavin | 0.0046 | Spectrophotometric method | [113] |
| Tocopherol | 0.181 | Spectrophotometric method | [113] |
| Total Ascorbate | 0.287 | Spectrophotometric method | [112] |
| Total Polyphenol | 18.7 | Folin-Ciocalteau method | [114] |
| Quercetin | 0.608 | HPLC method | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity. Molecules 2023, 28, 696. https://doi.org/10.3390/molecules28020696
Al-Khayri JM, Banadka A, Nandhini M, Nagella P, Al-Mssallem MQ, Alessa FM. Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity. Molecules. 2023; 28(2):696. https://doi.org/10.3390/molecules28020696
Chicago/Turabian StyleAl-Khayri, Jameel M, Akshatha Banadka, Murali Nandhini, Praveen Nagella, Muneera Q. Al-Mssallem, and Fatima M. Alessa. 2023. "Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity" Molecules 28, no. 2: 696. https://doi.org/10.3390/molecules28020696
APA StyleAl-Khayri, J. M., Banadka, A., Nandhini, M., Nagella, P., Al-Mssallem, M. Q., & Alessa, F. M. (2023). Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity. Molecules, 28(2), 696. https://doi.org/10.3390/molecules28020696







