C3-Symmetric Ligands in Drug Design: When the Target Controls the Aesthetics of the Drug
Abstract
1. Introduction
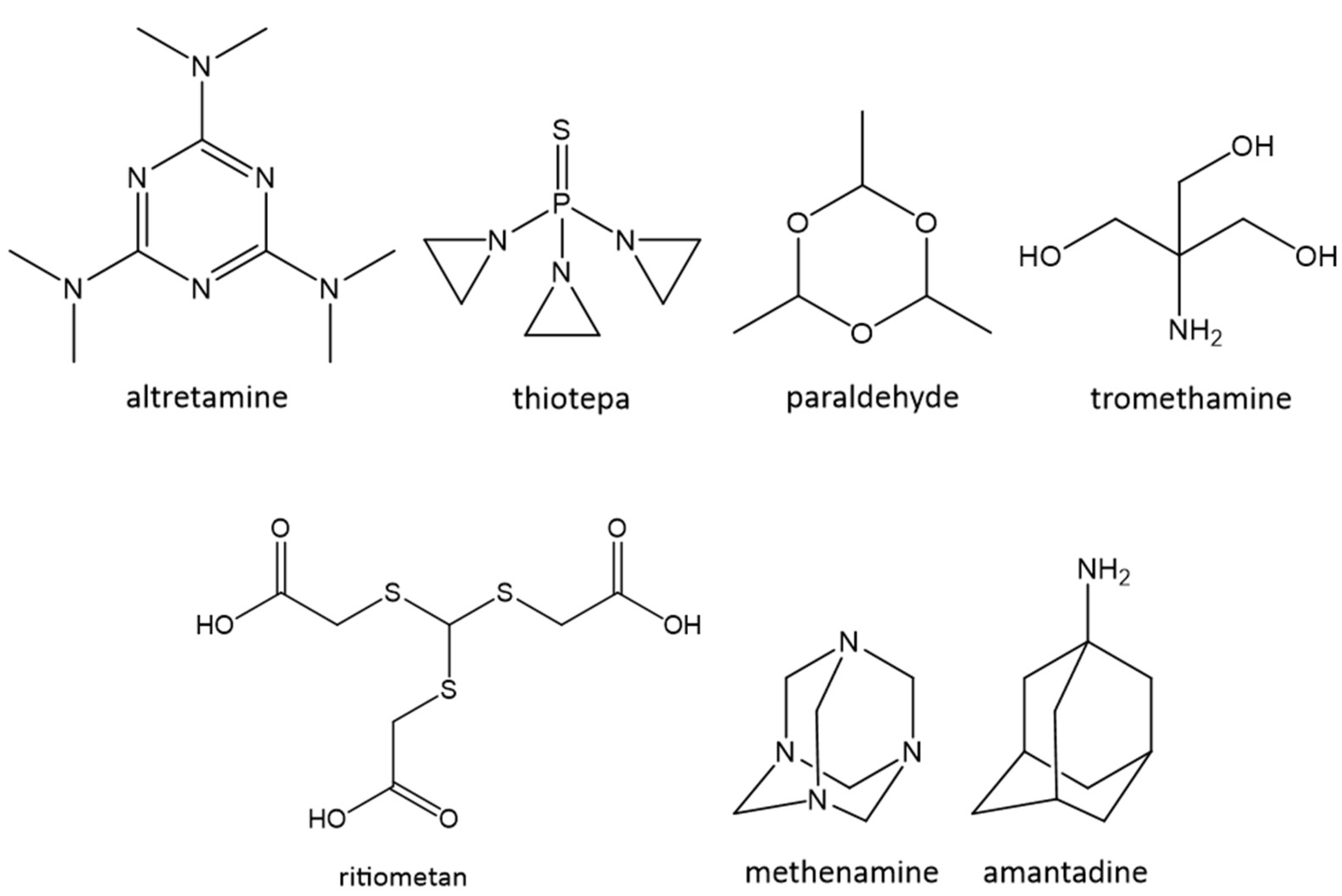
2. Currently Marketed C3-Drugs
3. Recent Works concerning Novel C3-Symmetric Drugs
3.1. Adamantane-Based Dendrons for Improving Ligand–Target Interactions
3.2. Benzene Rings
3.2.1. C3-Opioids as DNA Condensation Agents
3.2.2. Tris Triazole Compounds as G-Quadruplex Stabilising Ligands
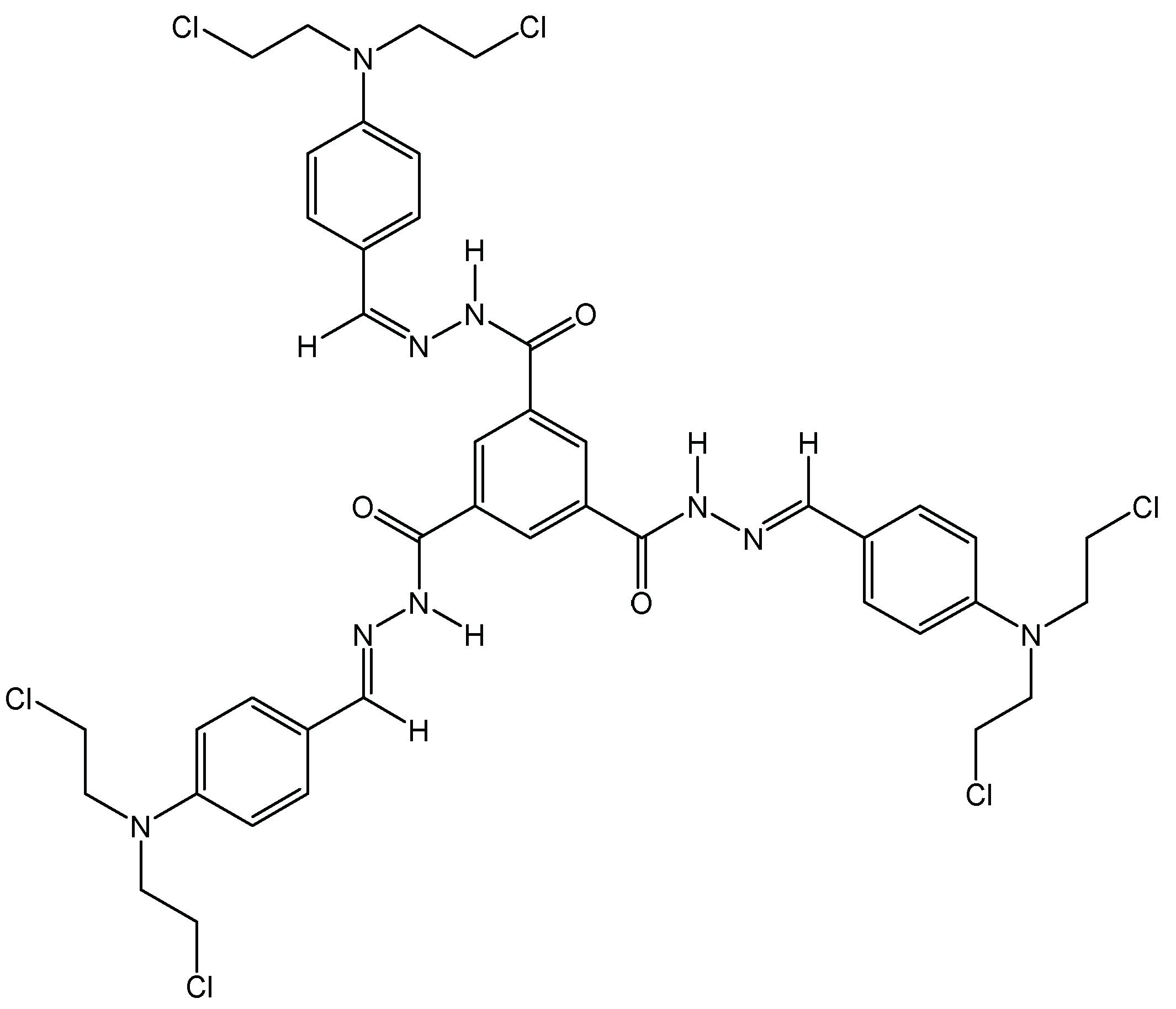
3.2.3. Tripodal Nitrogen Mustards for Aggregation Induced Emission and DNA Alkylation
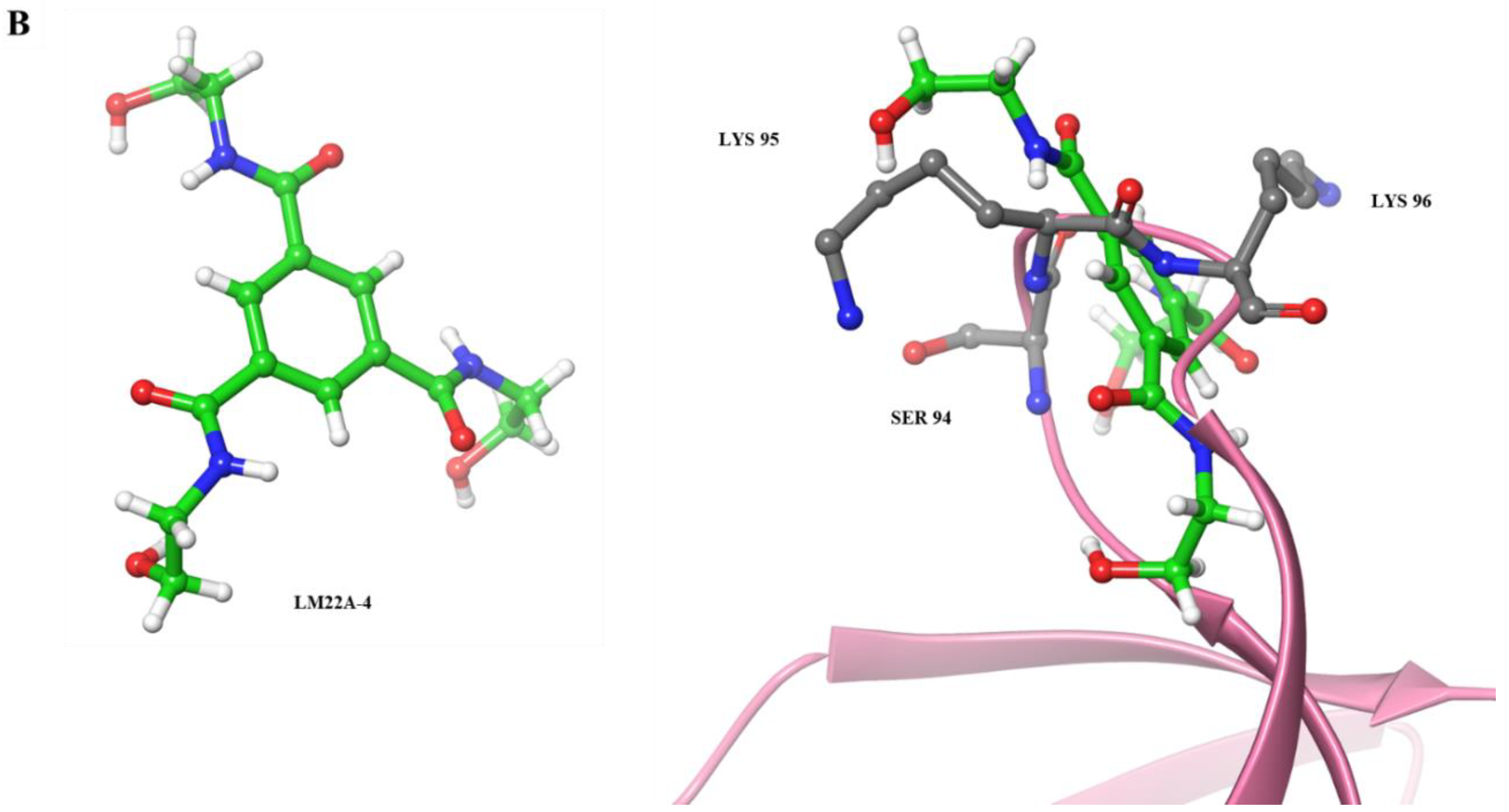
3.2.4. Benzenetricarboxamide as a Neurotrophic Agent against Neurodegenerative Diseases
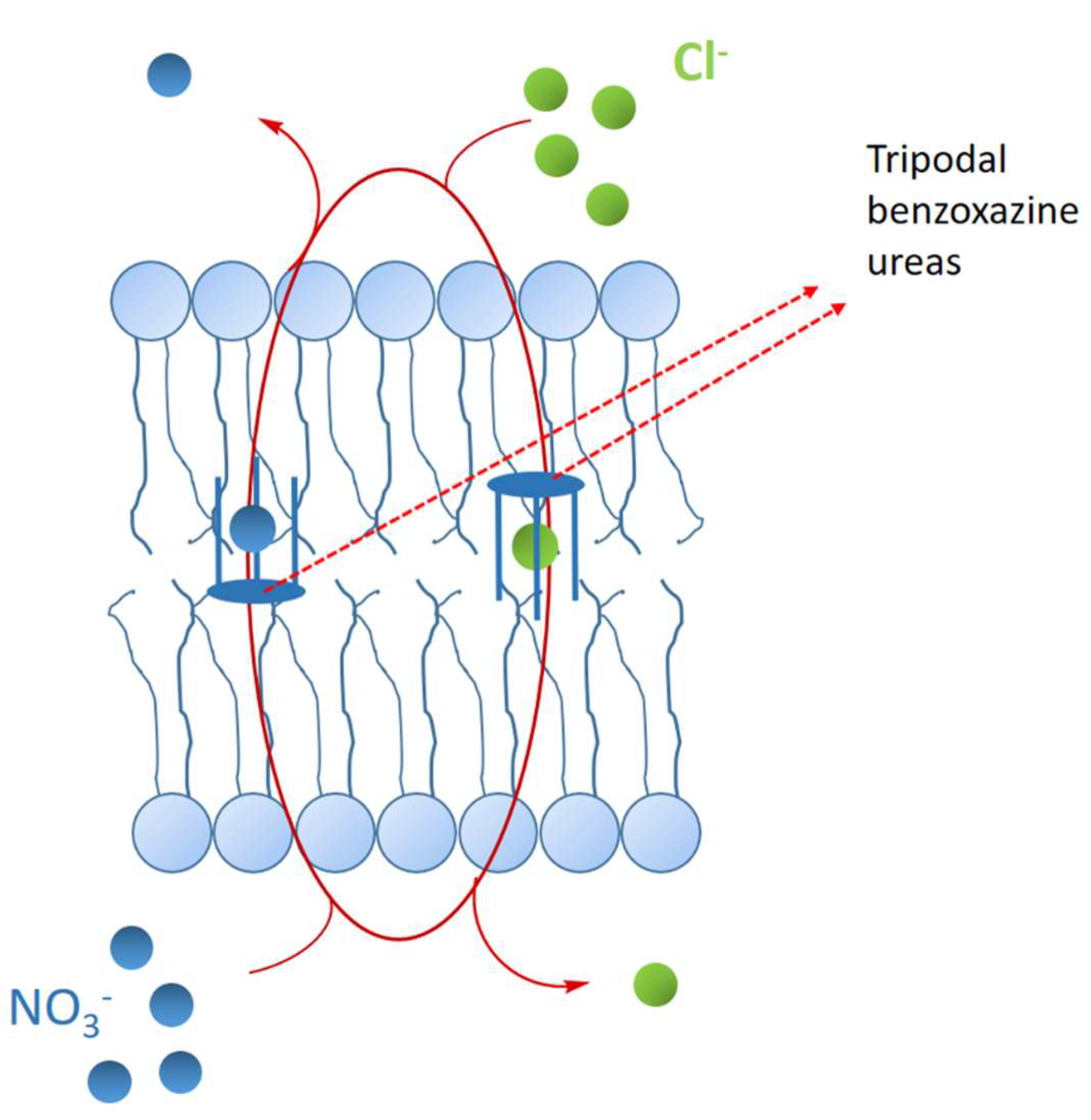
3.2.5. Benzoxazine Ureas as Synthetic Chloride Transmembrane Transporters
3.2.6. Trivalent Hemagglutinin Inhibitors against Flu
3.3. Triazine Derivatives as Tripodal Scaffolds
3.3.1. Trisusbtituted Triazines as Antiviral Triplet Drugs
3.3.2. Star-Shaped Tripodal Triazine-Related β-Lactams with Antioxidant and Antibacterial Properties
3.4. Aurintricarboxylic Acid as a Potent Allosteric Antagonist of P2X Receptors
3.5. triPEGnitromethane Derivatives for AIDS Treatment
3.6. Nitrilotriacetic Acid Derivatives
3.6.1. Nitriloacetic Acid as a Scaffold for a Synthetic Antigen Inducing Neutralizing Antibodies against HIV
3.6.2. Nitrilotriacetic Acid Derivatives as Copper Chelators within Wilson’s Disease Treatment
3.7. C3-Symmetric Cyclic Peptides as CD40L Mimetics
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, S.E.; Castaldi, M.P. Applications of chiral C3-symmetric molecules. Chem. Commun. 2006, 29, 3045–3062. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.E.; Castaldi, M.P. C3 symmetry: Molecular design inspired by nature. Angew. Chem. Int. Ed. 2006, 45, 4718–4720. [Google Scholar] [CrossRef] [PubMed]
- Moberg, C. C3 Symmetry in Asymmetric Catalysis and Chiral Recognition. Angew. Chem. Int. Ed. 1998, 37, 248–268. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-874969-1. [Google Scholar]
- Drugbank. Available online: https://go.drugbank.com (accessed on 3 January 2023).
- Harding, C.; Mossop, H.; Homer, T.; Chadwick, T.; King, W.; Carnell, S.; Lecouturier, J.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: Multicentre, open label, randomised, non-inferiority trial. BMJ 2022, 376, e068229. [Google Scholar] [CrossRef] [PubMed]
- Thomaston, J.L.; Polizzi, N.F.; Konstantinidi, A.; Wang, J.; Kolocouris, A.; Degrado, W.F. Inhibitors of the M2 Proton Channel Engage and Disrupt Transmembrane Networks of Hydrogen-Bonded Waters. J. Am. Chem. Soc. 2018, 140, 15219–15226. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Lamanna, G.; Grillaud, M.; Macri, C.; Chaloin, O.; Muller, S.; Bianco, A. Adamantane-based dendrons for trimerization of the therapeutic P140 peptide. Biomaterials 2014, 35, 7553–7561. [Google Scholar] [CrossRef]
- Malina, J.; Farrell, N.P.; Brabec, V. Substitution-Inert Trinuclear Platinum Complexes Efficiently Condense/Aggregate Nucleic Acids and Inhibit Enzymatic Activity. Angew. Chem. Int. Ed. 2014, 53, 12812–12816. [Google Scholar] [CrossRef]
- Vijayanathan, V.; Thomas, T.; Shirahata, A.; Thomas, T.J. DNA condensation by polyamines: A laser light scattering study of structural effects. Biochemistry 2001, 40, 13644–13651. [Google Scholar] [CrossRef]
- Kankia, B.I.; Buckin, V.; Bloomfield, V.A. Hexamminecobalt(III)-induced condensation of calf thymus DNA: Circular dichroism and hydration measurements. Nucleic Acids Res. 2001, 29, 2795–2801. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Hirte, H.W.; Bacchetti, S.; Harley, C.B. Telomerase activity in human ovarian carcinoma. Proc. Natl. Acad. Sci. USA 1994, 12, 2900–2904. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.E.; Ritson, D.J.; Zhang, F.; Lombardo, C.M.; Haider, S.; Oldham, N.; Neidle, S. A click chemistry approach to C3 symmetric, G-quadruplex stabilising ligands. Org. Biomol. Chem. 2010, 8, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.F.; Chen, Y.Y.; Zhang, Y.M.; Fan, Y.Q.; Zhou, Q.; Yang, H.L.; Zhang, Q.P.; Yao, H.; Wei, T.B.; Lin, Q. A novel bis-component AIE smart gel with high selectivity and sensitivity to detect CN−, Fe3+ and H2PO4−. Soft Matter 2019, 15, 6348–6352. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhang, Q.P.; Zhang, Y.M.; Gong, G.F.; Chen, Y.Y.; Yao, H.; Wei, T.B.; Lin, Q. A novel strong AIE bi-component hydrogel as a multi-functional supramolecular fluorescent material. Dyes Pigm. 2019, 171, 107745. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Huang, Q.; Zhang, Y.M.; Wang, J.; Guan, X.W.; Chen, Y.Y.; Yao, H.; Wei, T.B.; Lin, Q. Forming a water-soluble supramolecular polymer and an AIEE hydrogel: Two novel approaches for highly sensitive detection and efficient adsorption of aldehydes. Polym. Chem. 2019, 10, 6489–6494. [Google Scholar] [CrossRef]
- Massa, S.M.; Yang, T.; Xie, Y.; Shi, J.; Bilgen, M.; Joyce, J.N.; Nehama, D.; Rajadas, J.; Longo, F.M. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Investig. 2010, 120, 1774–1785. [Google Scholar] [CrossRef]
- Henry, R.A.; Hughes, S.M.; Connor, B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur. J. Neurosci. 2007, 25, 3513–3525. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Ribeiro, F.F.; Rodrigues, R.S.; Sebastião, A.M. Brain-Derived Neurotrophic Factor (BDNF) Role in Cannabinoid-Mediated Neurogenesis. Front. Cell. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo-Santos, A.; Vaz, S.H.; Parreira, S.; Rapaz-Lérias, S.; Caetano, A.P.; Buée-Scherrer, V.; Castrén, E.; Valente, C.A.; Blum, D.; Sebastião, A.M.; et al. Dysregulation of TrkB Receptors and BDNF Function by Amyloid- β Peptide is Mediated by Calpain. Cereb. Cortex 2015, 25, 3107–3121. [Google Scholar] [CrossRef] [PubMed]
- Howells, D.W.; Porritt, M.J.; Wong, J.Y.F.; Batchelor, P.E.; Kalnins, R. Reduced BDNF mRNA Expression in the Parkinson’s Disease Substantia Nigra. Exp. Neurol. 2000, 166, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; Ku, A.; Bretas, T.L.; Cardoso, F. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010, 257, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cattaneo, E. Huntington’s Disease. In Neurotrophic Factors; Springer: Heidelberg, Germany, 2014; pp. 357–409. [Google Scholar] [CrossRef]
- Simmons, D.A.; Belichenko, N.P.; Yang, T.; Condon, C.; Monbureau, M.; Shamloo, M.; Jing, D.; Massa, S.M.; Longo, F.M. A Small Molecule TrkB Ligand Reduces Motor Impairment and Neuropathology in R6/2 and BACHD Mouse Models of Huntington’s Disease. J. Neurosci. 2013, 33, 18712–18727. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.Y. Low-molecular mimetics of nerve growth factor and brain-derived neurotrophic factor: Design and pharmacological properties. Med. Res. Rev. 2020, 41, 2746–2774. [Google Scholar] [CrossRef]
- Yu, G.; Wang, W. Protective effects of LM22A-4 on injured spinal cord nerves. Int. J. Clin. Exp. Pathol. 2015, 8, 6526–6532. [Google Scholar]
- Ali Shariati, M.; Kumar, V.; Yang, T.; Chakraborty, C.; Barres, B.A.; Longo, F.M.; Liao, Y.J. A Small Molecule TrkB Neurotrophin Receptor Partial Agonist as Possible Treatment for Experimental Nonarteritic Anterior Ischemic Optic Neuropathy. Curr. Eye Res. 2018, 43, 1489–1499. [Google Scholar] [CrossRef]
- Schmid, D.A.; Yang, T.; Ogier, M.; Adams, I.; Mirakhur, Y.; Wang, Q.; Massa, S.M.; Longo, F.M.; Katz, D.M. A TrkB Small Molecule Partial Agonist Rescues TrkB Phosphorylation Deficits and Improves Respiratory Function in a Mouse Model of Rett Syndrome. J. Neurosci. 2012, 32, 1803–1810. [Google Scholar] [CrossRef]
- Boltaev, U.; Meyer, Y.; Tolibzoda, F.; Jacques, T.; Gassaway, M.; Xu, Q.; Wagner, F.; Zhang, Y.L.; Palmer, M.; Holson, E.; et al. Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists. Sci. Signal. 2017, 10, eaal1670. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Wood, R.J.; Prawdiuk, A.R.; Furness, S.G.B.; Chen, A.I.; Murray, S.S. TrkB Agonist LM22A-4 Increases Oligodendroglial Populations During Myelin Repair in the Corpus Callosum. Front. Mol. Neurosci. 2019, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.C.; Radziejewski, C.; Spraggon, G.; Greenwald, J.; Kostura, M.R.; Burtnick, L.D.; Stuart, D.I.; Choe, S.; Jones, E.Y. The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site. Protein Sci. 1999, 8, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Saha, D.; Mukherjee, A.; Talukdar, P. One-pot synthesis and transmembrane chloride transport properties of C3-symmetric benzoxazine urea. Org. Lett. 2016, 18, 5864–5867. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Du, W.; Somovilla, V.J.; Yu, G.; Haksar, D.; De Vries, E.; Boons, G.J.; De Vries, R.P.; De Haan, C.A.M.; Pieters, R.J. Enhanced Inhibition of Influenza A Virus Adhesion by Di-and Trivalent Hemagglutinin Inhibitors. J. Med. Chem. 2019, 62, 6398–6404. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, M.; Jirmann, R.; Hoelscher, K.; Wienke, M.; Niemeyer, F.C.; Rehders, D.; Meyer, B. A nanomolar multivalent ligand as entry inhibitor of the hemagglutinin of avian influenza. J. Am. Chem. Soc. 2014, 136, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Yang, F.; Su, Y.; Li, W.; Zhang, J.; Xu, H.; Huang, B.; Sun, M.; Mu, Y.; Zhang, Y.; et al. Design and Synthesis of Oleanolic Acid Trimers to Enhance Inhibition of Influenza Virus Entry. ACS Med. Chem. Lett. 2021, 12, 1759–1765. [Google Scholar] [CrossRef]
- Sonawane, R.P.; Sikervar, V.; Sasma, S. Comprehensive Heterocyclic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 181–283. [Google Scholar] [CrossRef]
- Mibu, N.; Yokomizo, K.; Aki, H.; Ota, N.; Fujii, H.; Yuzuriha, A.; Saneyoshi, S.; Tanaka, A.; Koga, A.; Zhou, J.; et al. Synthesis and antiviral evaluation of some C3-symmetrical trialkoxy-substituted 1,3,5-triazines and their molecular geometry. Chem. Pharm. Bull. 2015, 63, 935–944. [Google Scholar] [CrossRef]
- Mibu, N.; Yokomizo, K.; Sano, M.; Kawaguchi, Y.; Morimoto, K.; Shimomura, S.; Sato, R.; Hiraga, N.; Matsunaga, A.; Zhou, J.R.; et al. Preparation and antiviral activity of some new C3-and CS symmetrical tri-substituted triazine derivatives having benzylamine substituents. Chem. Pharm. Bull. 2018, 66, 830–838. [Google Scholar] [CrossRef]
- Bashiri, M.; Jarrahpour, A.; Rastegari, B.; Iraji, A.; Irajie, C.; Amirghofran, Z.; Malek-Hosseini, S.; Motamedifar, M.; Haddadi, M.; Zomorodian, K.; et al. Synthesis and evaluation of biological activities of tripodal imines and β-lactams attached to the 1,3,5-triazine nucleus. Monatsh. Chem. 2020, 151, 821–835. [Google Scholar] [CrossRef]
- Obrecht, A.S.; Urban, N.; Schaefer, M.; Röse, A.; Kless, A.; Meents, J.E.; Lampert, A.; Abdelrahman, A.; Müller, C.E.; Schmalzing, G.; et al. Identification of aurintricarboxylic acid as a potent allosteric antagonist of P2X1 and P2X3 receptors. Neuropharmacology 2019, 158, 107749. [Google Scholar] [CrossRef]
- Lu, M.; Blacklow, S.C.; Kim, P.S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 1995, 2, 1075–1082. [Google Scholar] [CrossRef]
- Hashimoto, C.; Nomura, W.; Ohya, A.; Urano, E.; Miyauchi, K.; Narumi, T.; Aikawa, H.; Komano, J.A.; Yamamoto, N.; Tamamura, H. Evaluation of a synthetic C34 trimer of HIV-1 gp41 as AIDS vaccines. Bioorg. Med. Chem. 2012, 20, 3287–3291. [Google Scholar] [CrossRef] [PubMed]
- Nomura, W.; Hashimoto, C.; Ohya, A.; Miyauchi, K.; Urano, E.; Tanaka, T.; Narumi, T.; Nakahara, T.; Komano, J.A.; Yamamoto, N.; et al. A Synthetic C34 Trimer of HIV-1 gp41 Shows Significant Increase in Inhibition Potency. ChemMedChem 2012, 7, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Nomura, W.; Ohba, K.; Ohya, A.; Tanaka, T.; Hashimoto, C.; Narumi, T.; Murakami, T.; Yamamoto, N.; Tamamura, H. Remodeling of dynamic structures of HIV-1 envelope proteins leads to synthetic antigen molecules inducing neutralizing antibodies. Bioconjug. Chem. 2010, 21, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Nomura, W.; Mizuguchi, T.; Tamamura, H. Multimerized HIV-gp41-derived peptides as fusion inhibitors and vaccines. Biopolymers 2016, 106, 622–628. [Google Scholar] [CrossRef]
- Orphanet. Available online: https://www.orpha.net/consor/cgi-bin/index.php?lng=FR (accessed on 3 January 2023).
- Jullien, A.S.; Gateau, C.; Lebrun, C.; Kieffer, I.; Testemale, D.; Delangle, P. D-penicillamine tripodal derivatives as efficient copper(I) chelators. Inorg. Chem. 2014, 53, 5229–5239. [Google Scholar] [CrossRef]
- van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef]
- Bianco, A.; Fournel, S.; Wieckowski, S.; Hoebeke, J.; Guichard, G. Solid-phase synthesis of CD40L mimetics. Org. Biomol. Chem. 2006, 4, 1461–1463. [Google Scholar] [CrossRef]
- Yang, L.; Tian, D.; Han, J.B.; Fan, W.; Zhang, Y.; Li, Y.; Sun, W.; Wei, Y.; Tian, X.; Yu, D.D.; et al. A recombinant receptor-binding domain in trimeric form generates protective immunity against SARS-CoV-2 infection in nonhuman primates. Innovation 2021, 2, 100140. [Google Scholar] [CrossRef]
- He, C.; Yang, J.; Hong, W.; Chen, Z.; Peng, D.; Lei, H.; Alu, A.; He, X.; Bi, Z.; Jiang, X.; et al. A self-assembled trimeric protein vaccine induces protective immunity against Omicron variant. Nat. Commun. 2022, 13, 5459. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, J.; Chen, Y. An interpretable machine learning method for homo-trimeric protein interface residue-residue interaction prediction. Biophys. Chem. 2021, 278, 106666. [Google Scholar] [CrossRef] [PubMed]






| C3-Tripodal Core | Target | Ligand Structure and Therapeutic Use | Examples |
|---|---|---|---|
| 3-1-Adamantanes | HSPA8/ HSC70 | Lupus | 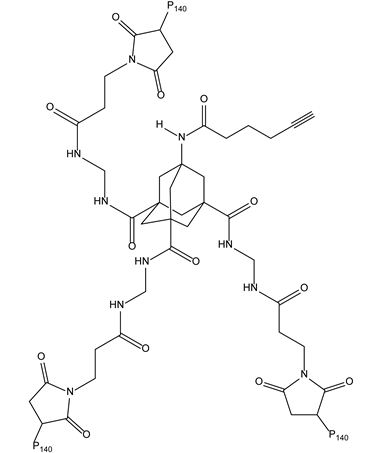 |
| 3-2-Benzene rings | DNA | 3-2-1-C3-opioids as DNA condensation agents |  |
| DNA | 3-2-2-Tris triazole compounds as G quadruplex stabilizing ligands |  | |
| 3-2-Benzene rings (continued) | DNA | 3-2-3-Tripodal nitrogen mustards for aggregation induced emission and DNA alkylation | 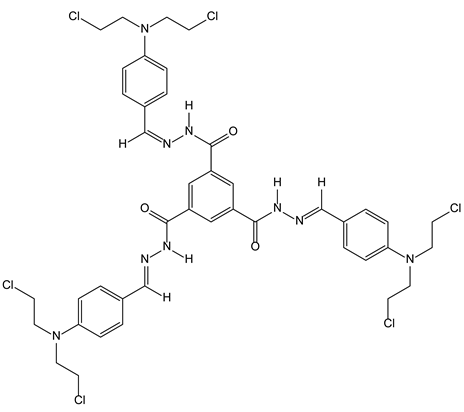 |
| TRKB R | 3-2-4-Benzene tricarboxamide as neurotrophic agent against neurodegenerative diseases | 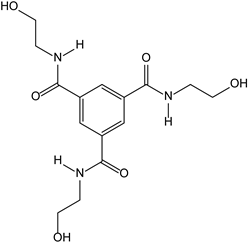 | |
| 3-2-Benzene rings (continued) | Neuronal mem-branes | 3-2-5-Benzoxazine ureas as synthetic chloride transmembrane transporters | 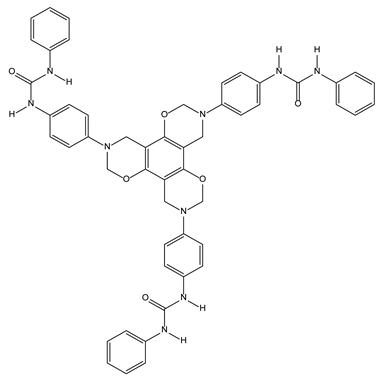 |
| Influenza virus HA | 3-2-6-Trivalent hemagglutinin inhibitors against flu | 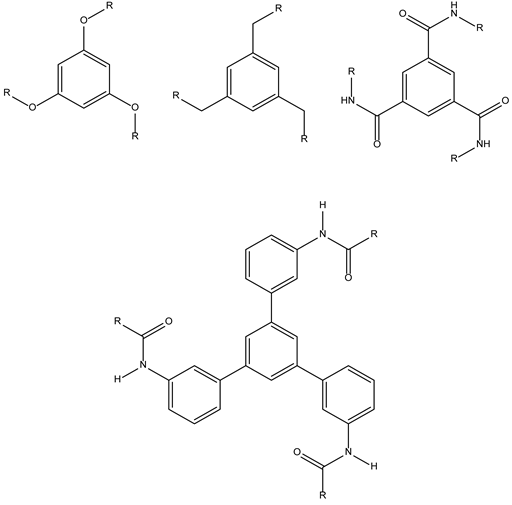 | |
| 3-3-Triazines | Virus Carbohydrates | 3-3-1-Anti-HSV-1 | 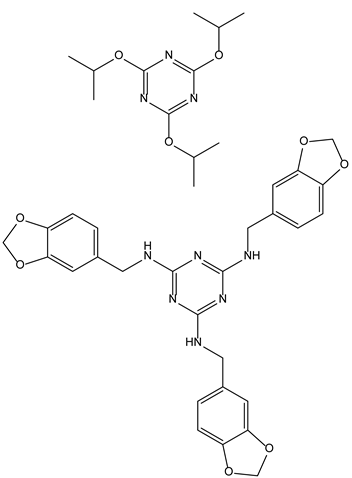 |
| Unkno-wn (pheno-typic test) | 3-3-2-leukemia | 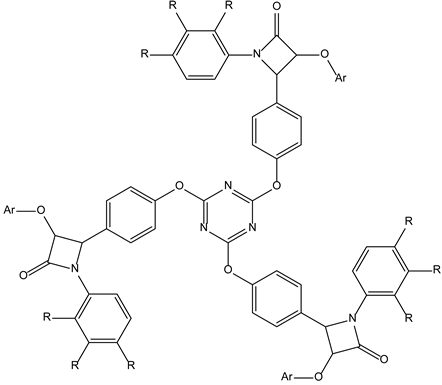 | |
| 3-4-Aurintricarboxylic acid | P2X R | Chronic pain relief | 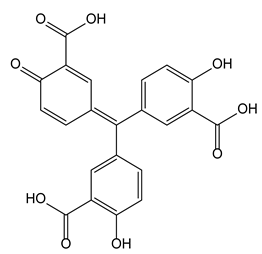 |
| 3-5-triPEGnitrométhane derivatives | Synthetic protein | AntiHIV antibody induction for fusion inhibition | 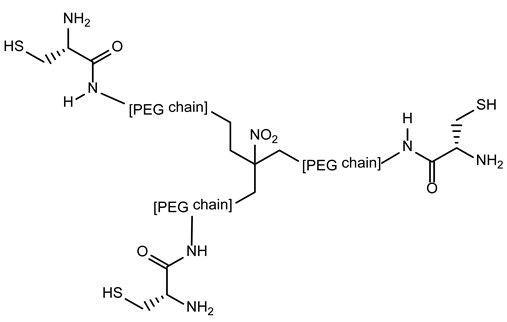 |
| 3-6-Nitrilotriacetic acid derivatives | Synthetic protein Cu(I) | 3-6-1-AntiHIV antibody induction for fusion inhibition | 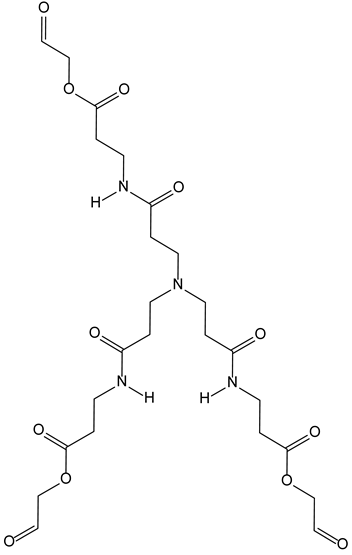 |
| 3-6-Nitrilotriacetic acid derivatives | 3-6-2-Wilson’s disease | 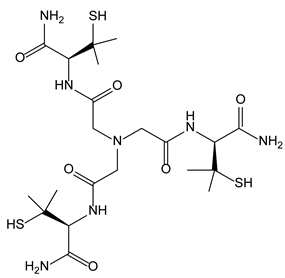 | |
| 3-7-Cyclic peptides | TNFα R | CD40 ligand mimetics with anticancer activity | 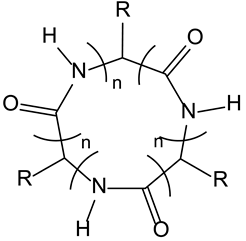 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonijevic, M.; Rochais, C.; Dallemagne, P. C3-Symmetric Ligands in Drug Design: When the Target Controls the Aesthetics of the Drug. Molecules 2023, 28, 679. https://doi.org/10.3390/molecules28020679
Antonijevic M, Rochais C, Dallemagne P. C3-Symmetric Ligands in Drug Design: When the Target Controls the Aesthetics of the Drug. Molecules. 2023; 28(2):679. https://doi.org/10.3390/molecules28020679
Chicago/Turabian StyleAntonijevic, Mirjana, Christophe Rochais, and Patrick Dallemagne. 2023. "C3-Symmetric Ligands in Drug Design: When the Target Controls the Aesthetics of the Drug" Molecules 28, no. 2: 679. https://doi.org/10.3390/molecules28020679
APA StyleAntonijevic, M., Rochais, C., & Dallemagne, P. (2023). C3-Symmetric Ligands in Drug Design: When the Target Controls the Aesthetics of the Drug. Molecules, 28(2), 679. https://doi.org/10.3390/molecules28020679









