Highly Valuable Fish Oil: Formation Process, Enrichment, Subsequent Utilization, and Storage of Eicosapentaenoic Acid Ethyl Esters
Abstract
1. Introduction
| Health Effect | Reference |
|---|---|
| Reduction in depression or major depressive disorder | [13,14] |
| Potential beneficial effects on atherosclerotic plaques | [15] |
| Neuroprotective effects after stroke | [16] |
| Control inflammation and tissue homeostasis | [17] |
| Cardiovascular benefits | [4] |
| Improve diabetes | [18] |
| Improve dry eye syndrome | [19] |
| Benefits for the prevention and management of skin diseases | [20] |
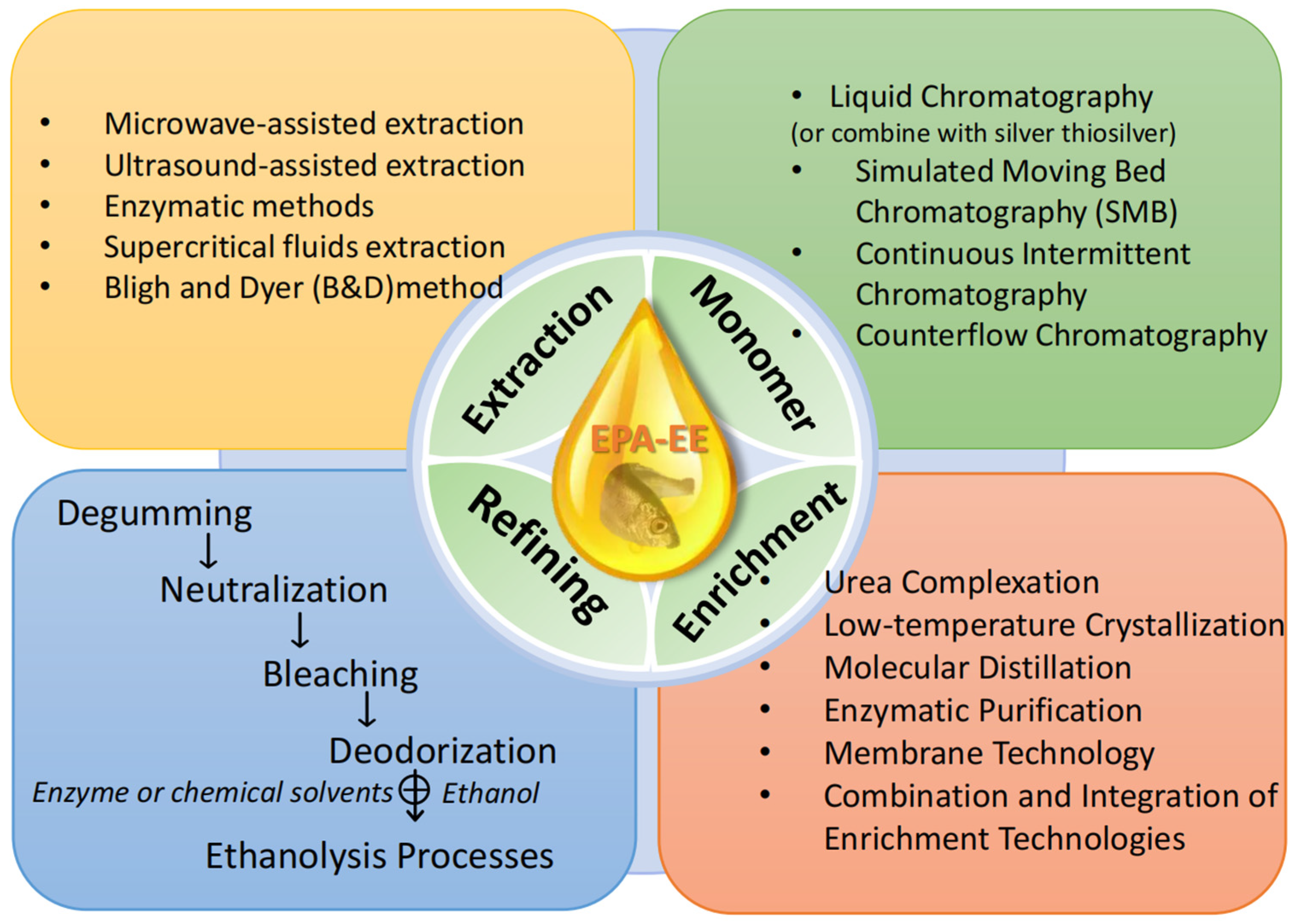
2. EPA-EE Formation Progress
2.1. Fish Oil Extraction
2.1.1. Traditional Fish Oil Extraction
| Year | Location | SFAs | MUFAs | PUFAs | EPA | DHA | Reference |
|---|---|---|---|---|---|---|---|
| 2020 | Pearl River Estuary, China | 38.90 | 31.10 | 20.90 | 5.72 | 7.44 | [22] |
| 2019 | Chile | 40.82 | 39.44 | 18.71 | 1.49 | 2.67 | [31] |
| 2018 | Central Europe | 24.76 | 39.99 | 34.14 | 3.74 | 8.73 | [32] |
| 2018 | Czech Republic | 23.69 | 37.50 | 37.28 | 4.43 | 13.15 | [24] |
| 2014 | Brazil | 25.40 | 31.10 | 31.51 | 7.85 | 19.20 | [21] |
| 2014 | Panama | 39.80 | 31.80 | 28.30 | 6.24 | 15.30 | [33] |
| 2014 | Yangtze Basin, China | 33.05 | 34.47 | 32.81 | 2.53 | 7.49 | [23] |
| 2013 | Egypt | 25.60 | 48.30 | 26.10 | 1.15 | 10.30 | [34] |
| 2012 | Southern Italy | 43.60 | 24.90 | 31.50 | 6.82 | 13.80 | [35] |
| 2012 | India | 37.10 | 23.70 | 32.30 | 4.28 | 3.08 | [36] |
| 2012 | Black Sea | 31.30 | 28.40 | 27.00 | 6.30 | 14.50 | [37] |
2.1.2. Emerging Green Fish Oil Extraction Technology
2.2. Fish Oil Refining
2.3. Production Process of EPA-Rich Ethyl Ester Fish Oil
3. EPA-EE Mixture Enrichment and Its Monomer Preparation
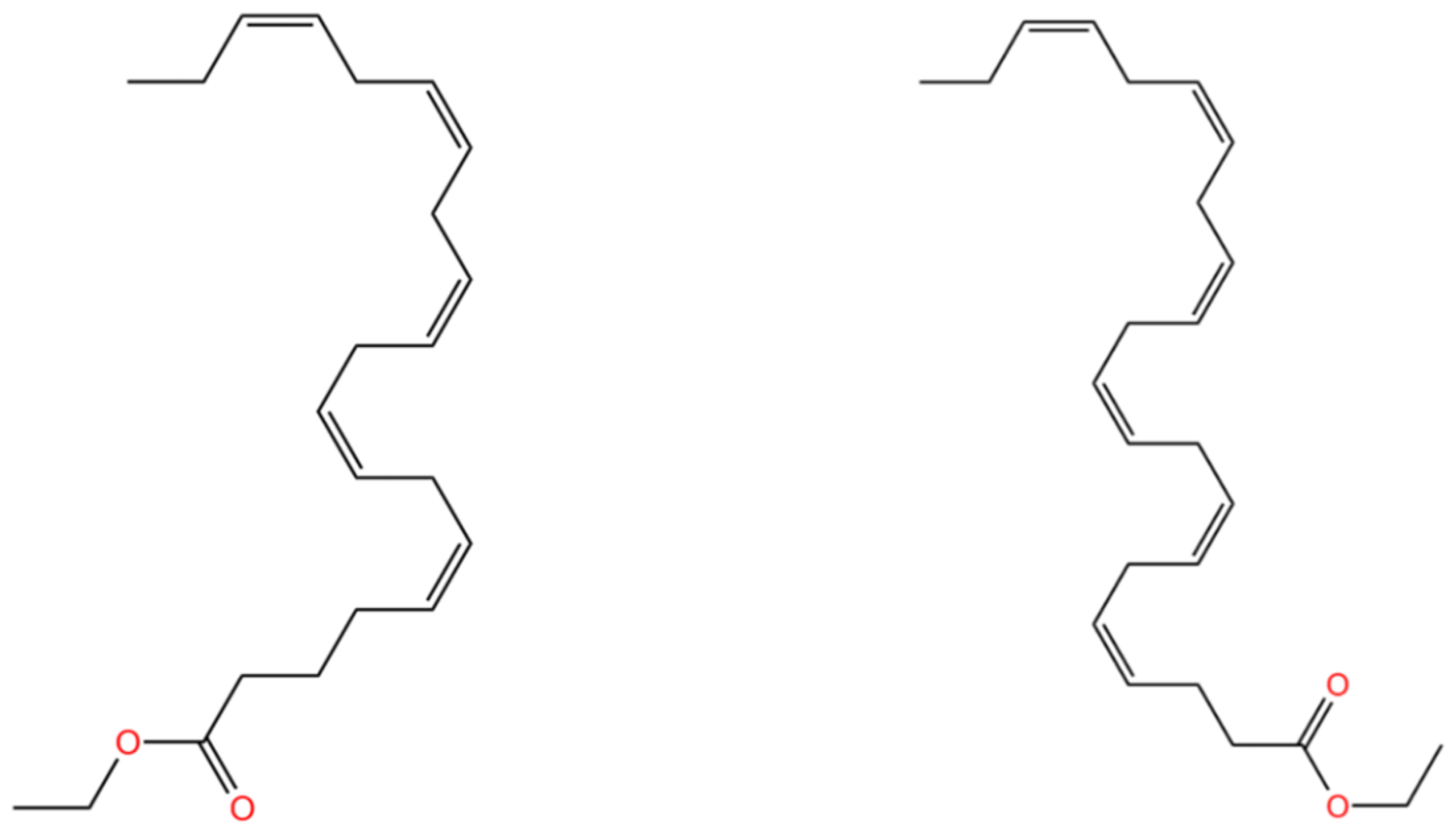
3.1. EPA-EE and DHA-EE Physical and Chemical Properties
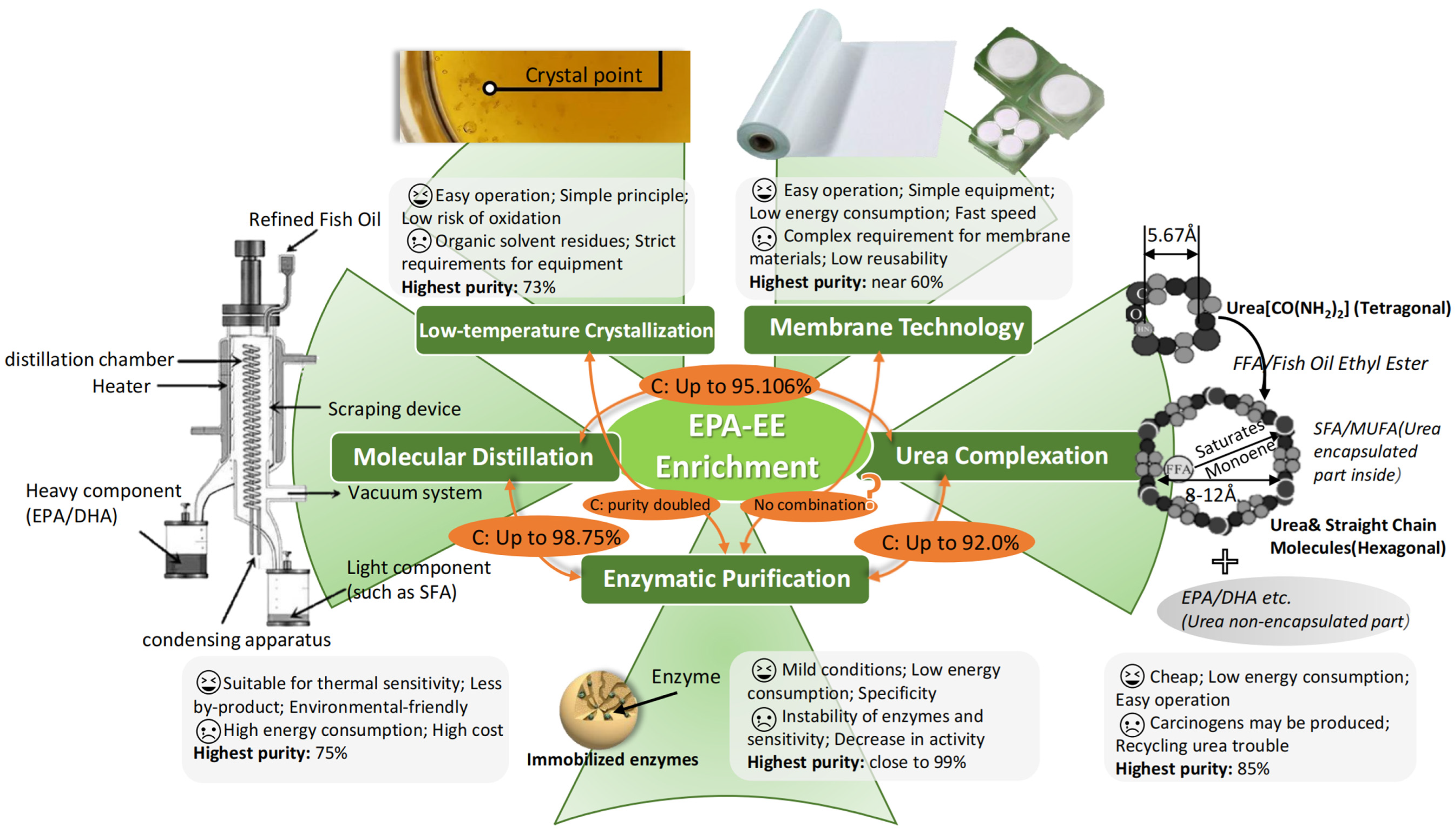
3.2. EPA-EE Mixture Enrichment
3.2.1. Urea Complexation
3.2.2. Low-Temperature Crystallization
3.2.3. Molecular Distillation
3.2.4. Enzymatic Purification
3.2.5. Membrane Technology
3.2.6. Combination and Integration of Enrichment Technologies
3.3. High-Purity EPA-EE Preparation
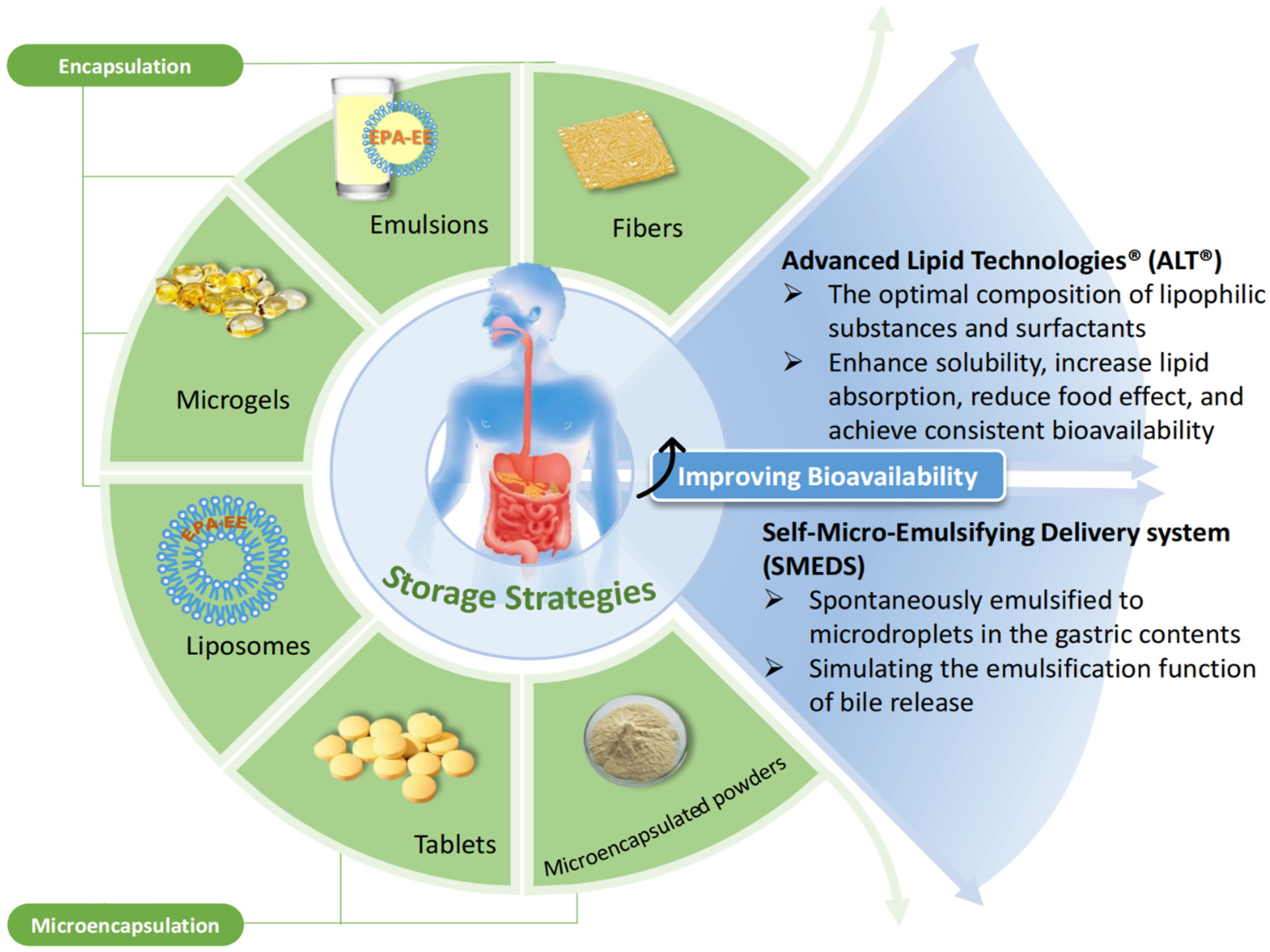
4. Subsequent EPA-EE Use and Its Storage Characteristics
4.1. EPA-EE Bioavailability
4.2. EPA-EE Storage Properties and Oxidative Stability
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Aslani, S.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Implications on the Therapeutic Potential of Statins via Modulation of Autophagy. Oxid. Med. Cell. Longev. 2021, 2021, 9599608. [Google Scholar] [CrossRef] [PubMed]
- U.S Food & Drug Administration Home Page. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-use-drug-reduce-risk-cardiovascular-events-certain-adult-patient-groups (accessed on 24 August 2022).
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Taskinen, M.R.; Bjornson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.W.; Murphy, E.W.; McCarty, H.B.; Snyder, B.D.; Schrank, C.S.; McCann, P.J.; Crimmins, B.S. Variation in the essential fatty acids EPA and DHA in fillets of fish from the Great Lakes region. J. Great Lakes Res. 2017, 43, 150–160. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guenard, F.; Barbier, O.; Vohl, M.C. Effect of different concentrations of omega-3 fatty acids on stimulated THP-1 macrophages. Genes Nutr. 2017, 12, 7. [Google Scholar] [CrossRef]
- Hoyos Concha, J.L.; Bonilla, J.R. Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Cienc. Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar] [CrossRef]
- Xie, D.; Chen, Y.; Yu, J.; Yang, Z.; Wang, X.; Wang, X. Progress in enrichment of n-3 polyunsaturated fatty acid: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–17, online ahead of print. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Xie, B.; Zhang, H.M.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.F.; Lu, C.Y.; McLntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhe, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Wani, O.; May, H.T.; Budoff, M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul. Pharmacol. 2017, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Miyamoto, N.; Yamashiro, K.; Tanaka, R.; Hattori, N. Omega-3 Polyunsaturated Fatty Acids and Stroke Burden. Int. J. Mol. Sci. 2019, 20, 5549. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K. New Areas of Interest: Is There a Role for Omega-3 Fatty Acid Supplementation in Patients With Diabetes and Cardiovascular Disease? Curr. Diab. Rep. 2019, 19, 6. [Google Scholar] [CrossRef]
- O’Byrne, C.; O’Keeffe, M. Omega-3 fatty acids in the management of dry eye disease-An updated systematic review and meta-analysis. Acta Ophthalmol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Thomsen, B.J.; Chow, E.Y.; Sapijaszko, M.J. The Potential Uses of Omega-3 Fatty Acids in Dermatology: A Review. J. Cutan. Med. Surg. 2020, 24, 481–494. [Google Scholar] [CrossRef]
- Fernandes, C.E.; Vasconcelos, M.A.d.S.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; Filho, A.B.d.M. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.Q.; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Xie, C.; Li, D.; Xu, J.; Zhang, M.; Zhang, M. Lipid Contents, Fatty Acid Profiles and Nutritional Quality of Nine Wild Caught Freshwater Fish Species of the Yangtze Basin, China. J. Food Nutr. Res. 2014, 2, 388–394. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S.; Zajic, T.; Krejsa, J.; Masilko, J.; Mraz, J. Nutritional value of several commercially important river fish species from the Czech Republic. PeerJ 2018, 6, e5729. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Polat, A.; Uçak, İ.; Ozogul, F. Seasonal fat and fatty acids variations of seven marine fish species from the Mediterranean Sea. Eur. J. Lipid Sci. Technol. 2011, 113, 1491–1498. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Zhang, Y.; Shen, Y.; Su, H.; Jin, J.; Jin, Q.; Wang, X. Characterization of Positional Distribution of Fatty Acids and Triacylglycerol Molecular Compositions of Marine Fish Oils Rich in Omega-3 Polyunsaturated Fatty Acids. Biomed. Res. Int. 2018, 2018, 3529682. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and stabilization of omega-3 oils: A review. Trends Food Sci. Technol. 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Villarreal-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of fatty acid profiles of dried and raw by-products from cultured and wild fishes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600516. [Google Scholar] [CrossRef]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Rincon-Cervera, M.A.; Gonzalez-Barriga, V.; Valenzuela, R.; Lopez-Arana, S.; Romero, J.; Valenzuela, A. Profile and distribution of fatty acids in edible parts of commonly consumed marine fishes in Chile. Food Chem. 2019, 274, 123–129. [Google Scholar] [CrossRef]
- Linhartová, Z.; Krejsa, J.; Zajíc, T.; Másílko, J.; Sampels, S.; Mráz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Murillo, E.; Rao, K.S.; Durant, A.A. The lipid content and fatty acid composition of four eastern central Pacific native fish species. J. Food Compost. Anal. 2014, 33, 1–5. [Google Scholar] [CrossRef]
- Abouel-Yazeed, A.M. Fatty acids profile of some marine water and freshwater fish. J. World Aquac. Soc. 2013, 8, 283–292. [Google Scholar]
- Prato, E.; Biandolino, F. Total lipid content and fatty acid composition of commercially important fish species from the Mediterranean, Mar Grande Sea. Food Chem. 2012, 131, 1233–1239. [Google Scholar] [CrossRef]
- Dhaneesh, K.V.; Noushad, K.M.; Kumar, T.T.A.; Bacurau, R.F.P. Nutritional Evaluation of Commercially Important Fish Species of Lakshadweep Archipelago, India. PLoS ONE 2012, 7, e45439. [Google Scholar] [CrossRef]
- Kocatepe, D.; Turan, H. Proximate and fatty acid composition of some commercially important fish species from the Sinop region of the Black Sea(Article). Lipids 2012, 47, 635–641. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ozogul, Y.; Ucar, Y.; Takadaş, F.; Durmus, M.; Köşker, A.R.; Polat, A. Comparision of Green and Conventional Extraction Methods on Lipid Yield and Fatty Acid Profiles of Fish Species. Eur. J. Lipid Sci. Technol. 2018, 120, 1800107. [Google Scholar] [CrossRef]
- Afolabi, H.K.; Mudalip, S.K.A.; Alara, O.R. Microwave-assisted extraction and characterization of fatty acid from eel fish (Monopterus albus). Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 465–470. [Google Scholar] [CrossRef]
- De la Fuente, B.; Pinela, J.; Mandim, F.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction. Food Chem. 2022, 386, 132778. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, B.K.K.K.; Moreda-Piñeiro, A.; Fowler, S.W. Ultrasound-Assisted Extraction in Analytical Applications for Fish and Aquatic Living Resources, a Review. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liao, H.Z.; Wang, Y.H.; Wang, H.M.D.; Shieh, C.J.; Tseng, C.Y. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- Aitta, E.; Marsol-Vall, A.; Damerau, A.; Yang, B. Enzyme-Assisted Extraction of Fish Oil from Whole Fish and by-Products of Baltic Herring (Clupea harengus membras). Foods 2021, 10, 1811. [Google Scholar] [CrossRef]
- Sangkharak, K.; Paichid, N.; Yunu, T.; Klomklao, S. Improvement of extraction and concentration method for polyunsaturated fatty acid production from Nile tilapia processing waste. Biomass Convers. Biorefin. 2020, 12, 3995–4007. [Google Scholar] [CrossRef]
- Aarthy, M.; Saravanan, P.; Ayyadurai, N.; Gowthaman, M.K.; Kamini, N.R. A two step process for production of omega 3-polyunsaturated fatty acid concentrates from sardine oil using Cryptococcus sp. MTCC 5455 lipase. J. Mol. Catal. B Enzym. 2016, 125, 25–33. [Google Scholar] [CrossRef]
- Semenoglou, I.; Eliasson, L.; Uddstål, R.; Tsironi, T.; Taoukis, P.; Xanthakis, E. Supercritical CO2 extraction of oil from Arctic charr side streams from filleting processing. Innov. Food Sci. Emerg. Technol. 2021, 71, 102712. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of Oil from High-Moisture Tuna Livers by Subcritical Dimethyl Ether: A Comparison with Different Extraction Methods. Eur. J. Lipid Sci. Technol. 2018, 121, 1800087. [Google Scholar] [CrossRef]
- Keley, M.T.; Keihan, A.H.; Nobakht, M.; Rezaei, M.H. Effects of Supercritical CO2 on Quantity and Quality of Extracted Oil from Myctophidae Fish and Comparison It with the Wet Pressing as a Commercial Method. Turk. J. Fish. Aquat. Sci. 2022, 22, TRJFAS21017. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1959, 226, 497–509. [Google Scholar] [CrossRef]
- Costa, D.d.S.V.; Bragagnolo, N. Development and validation of a novel microwave assisted extraction method for fish lipids. Eur. J. Lipid Sci. Technol. 2016, 119, 1600108. [Google Scholar] [CrossRef]
- Zhao, B.; Niu, Y.; Liu, Q.; Liu, D.; Zhang, J. Ultrasound-assisted extraction of rainbow trout oil and its fatty acid composition. China Oils Fats 2019, 44, 20–24. [Google Scholar]
- Wang, X.; Wang, X.; Wei, Z.; Chen, Y.; Wang, P.; Li, Y.; Qiao, L.; Liu, R. Ultrasound-assisted extraction of tuna cooking liquor fish oil process research and quality analysis. Fish Sci. Technol. Inf. 2022, 49, 82–90. [Google Scholar]
- Qing, W.X.Z.L.W. Ultrasound-assisted extraction of Salmon oil and its fatty acid composition. China Oils Fats 2019, 44, 23–26. [Google Scholar]
- Marsol-Vall, A.; Aitta, E.; Guo, Z.; Yang, B. Green technologies for production of oils rich in n-3 polyunsaturated fatty acids from aquatic sources. Crit. Rev. Food Sci. Nutr. 2022, 62, 2942–2962. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Fabiano-Tixier, A.S.; Lino, C.; Avellone, G.; Chemat, F.; Pagliaro, M. Omega-3 Extraction from Anchovy Fillet Leftovers with Limonene: Chemical, Economic, and Technical Aspects. ACS Omega 2019, 4, 15359–15363. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Avellone, G.; Pagliaro, M. A Circular Economy Approach to Fish Oil Extraction. ChemistrySelect 2019, 4, 5106–5109. [Google Scholar] [CrossRef]
- Soldo, B.; Šimat, V.; Vlahović, J.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. High Quality Oil Extracted from Sardine By-Products as an Alternative to Whole Sardines: Production and Refining. Eur. J. Lipid Sci. Technol. 2019, 121, 1800513. [Google Scholar] [CrossRef]
- Lamas, D.L. Effect of enzymatic degumming process on the physicochemical and nutritional properties of fish byproducts oil. Appl. Food Res. 2022, 2, 100170. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Petenuci, M.E.; Fonseca, G.G. Production and characterization of crude and refined oils obtained from the co-products of Nile tilapia and hybrid sorubim processing. Food Chem. 2014, 157, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Joseph, D. Production and characterization of refined oils obtained from Indian oil sardine (Sardinella longiceps). J. Agric. Food Chem. 2015, 63, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of edible oils: A critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Charanyaa, S.; Belur, P.D.; Regupathi, I. A New Strategy to Refine Crude Indian Sardine Oil. J. Oleo Sci. 2017, 66, 425–434. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Ning, Z.; Wang, Y.; Yang, B.; Ma, Y.; Yang, X. A process for the synthesis of PUFA-enriched triglycerides from high-acid crude fish oil. J. Food Eng. 2012, 109, 366–371. [Google Scholar] [CrossRef]
- Zahrina, I.; Nasikin, M.; Krisanti, E.; Mulia, K. Deacidification of palm oil using betaine monohydrate-based natural deep eutectic solvents. Food Chem. 2018, 240, 490–495. [Google Scholar] [CrossRef]
- Sander, A.; Petračić, A.; Zokić, I.; Vrsaljko, D. Scaling up extractive deacidification of waste cooking oil. J. Environ. Manag. 2022, 316, 115222. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.L.; Monte, M.L.; Pohndorf, R.S.; Crexi, V.T.; Pinto, L.A.A. Bleaching with blends of bleaching earth and activated carbon reduces color and oxidation products of carp oil. Eur. J. Lipid Sci. Technol. 2015, 117, 829–836. [Google Scholar] [CrossRef]
- Wang, X.; Wen, Y.; Bi, S.; Li, Z.; Xue, Y.; Xue, C.; Jiang, X. Bleaching with the Mixed Adsorbents of Activated Earth and Activated Alumina to Reduce Color and Oxidation Products of Anchovy Oil. J. Ocean Univ. China 2021, 20, 1167–1174. [Google Scholar] [CrossRef]
- Igansi, A.V.; Engelmann, J.; Lütke, S.F.; Porto, F.B.; Pinto, L.A.A.; Cadaval, T.R.S. Isotherms, kinetics, and thermodynamic studies for adsorption of pigments and oxidation products in oil bleaching from catfish waste. Chem. Eng. Commun. 2019, 206, 1399–1413. [Google Scholar] [CrossRef]
- Ortiz, X.; Carabellido, L.; Marti, M.; Marti, R.; Tomas, X.; Diaz-Ferrero, J. Elimination of persistent organic pollutants from fish oil with solid adsorbents. Chemosphere 2011, 82, 1301–1307. [Google Scholar] [CrossRef]
- De Oliveira, D.A.; Minozzo, M.G.; Licodiedoff, S.; Waszczynskyj, N. Physicochemical and sensory characterization of refined and deodorized tuna (Thunnus albacares) by-product oil obtained by enzymatic hydrolysis. Food Chem. 2016, 207, 187–194. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, S.; Zhang, J.; Liu, S.; Ding, Y.; Liu, J. Deodorisation of fish oil by nanofiltration membrane process: Focus on volatile flavour compounds and fatty acids composition. Int. J. Food Sci. Technol. 2018, 53, 692–699. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Peng, X.; Yu, X.; Dai, Z.; Shen, Q. Effect of deodorization method on the chemical and nutritional properties of fish oil during refining. LWT 2018, 96, 560–567. [Google Scholar] [CrossRef]
- Simat, V.; Vlahovic, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalic Mekinic, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. [Google Scholar] [CrossRef]
- Song, G.; Dai, Z.; Shen, Q.; Peng, X.; Zhang, M. Analysis of the Changes in Volatile Compound and Fatty Acid Profiles of Fish Oil in Chemical Refining Process. Eur. J. Lipid Sci. Technol. 2017, 120, 1700219. [Google Scholar] [CrossRef]
- Fiori, L.; Volpe, M.; Lucian, M.; Anesi, A.; Manfrini, M.; Guella, G. From Fish Waste to Omega-3 Concentrates in a Biorefinery Concept. Waste Biomass Valorization 2017, 8, 2609–2620. [Google Scholar] [CrossRef]
- Melgosa, R.; Sanz, M.T.; Beltrán, S. Supercritical CO2 processing of omega-3 polyunsaturated fatty acids—Towards a biorefinery for fish waste valorization. J. Supercrit. Fluids 2021, 169, 105121. [Google Scholar] [CrossRef]
- Cao, P.; Zheng, L.; Sun, W.; Zhao, L. Brønsted acidic ionic liquid-catalyzed and ultrasound-promoted transesterification of fish oil with ethanol. Can. J. Chem. Eng. 2022, 100, 3717–3726. [Google Scholar] [CrossRef]
- Castejon, N.; Senorans, F.J. Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: A review. N. Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef]
- Chandel, H.; Wang, B.; Verma, M.L. Chapter 28—Microbial lipases and their applications in the food industry. In Value-Addition in Food Products and Process. Through Enzyme Technology; Kuddus, M., Aguilar, C.N., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 381–394. [Google Scholar]
- Kamal, M.Z.; Barrow, C.J.; Rao, N.M. A computational search for lipases that can preferentially hydrolyze long-chain omega-3 fatty acids from fish oil triacylglycerols. Food Chem. 2015, 173, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Kodali, S.; Chen, B.; Guo, Z. The near-ideal catalytic property of Candida antarctica lipase A to highly concentrate n-3 polyunsaturated fatty acids in monoacylglycerols via one-step ethanolysis of triacylglycerols. Bioresour. Technol. 2016, 219, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, X.; Ma, G.; Dai, L.; Du, W.; Liu, D. Enzymatic ethanolysis of fish oil for selective concentration of polyunsaturated fatty acids (PUFAs) with flexible production of corresponding glycerides and ethyl esters. J. Chem. Technol. Biotechnol. 2018, 93, 2399–2405. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Lee, C.-L.; Kuo, W.-C.; Hsieh, S.-L.; Shieh, C.-J. Synthesis of DHA/EPA Ethyl Esters via Lipase-Catalyzed Acidolysis Using Novozym® 435: A Kinetic Study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Borges, J.P.; Quilles Junior, J.C.; Moreno-Perez, S.; Fernandez-Lorente, G.; Boscolo, M.; Gomes, E.; da Silva, R.; Bocchini, D.A.; Guisan, J.M. Ethyl esters production catalyzed by immobilized lipases is influenced by n-hexane and ter-amyl alcohol as organic solvents. Bioprocess Biosyst. Eng. 2020, 43, 2107–2115. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Dezvarei, S.; Yousefi, M.; Ashjari, M. Selective enrichment of polyunsaturated fatty acids by hydrolysis of fish oil using immobilized and stabilized Rhizomucor miehei lipase preparations. Food Bioprod. Process. 2015, 94, 414–421. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Ninow, J.L.; de Oliveira, D.; Pessela, B.C. Stabilization of lipase from Thermomyces lanuginosus by crosslinking in PEGylated polyurethane particles by polymerization: Application on fish oil ethanolysis. Biochem. Eng. J. 2016, 112, 54–60. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Qing, D. Separation Process for Purification of EPA-EE and DHA-EE; Zhejiang University: Hangzhou, China, 2018. [Google Scholar]
- Rossi, P.C.; Pramparo Mdel, C.; Gaich, M.C.; Grosso, N.R.; Nepote, V. Optimization of molecular distillation to concentrate ethyl esters of eicosapentaenoic (20:5 omega-3) and docosahexaenoic acids (22:6 omega-3) using simplified phenomenological modeling. J. Sci. Food Agric. 2011, 91, 1452–1458. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Rodriguez, A.; Contreras, E.; Ortiz-Viedma, J.; Munoz, M.; Trigo, M.; Aubourg, S.P.; Espinosa, A. Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea(-)Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology. Molecules 2019, 24, 1642. [Google Scholar] [CrossRef] [PubMed]
- Pando, M.E.; Rodríguez, A.; Galdames, A.; Berríos, M.M.; Rivera, M.; Romero, N.; Valenzuela, M.A.; Ortiz, J.; Aubourg, S.P. Maximization of the docosahexaenoic and eicosapentaenoic acids content in concentrates obtained from a by-product of rainbow trout (Oncorhynchus mykiss) processing. Eur. Food Res. Technol. 2017, 244, 937–948. [Google Scholar] [CrossRef]
- Gu, H.-B.; Ma, X.-Y.; Wu, J.-B.; Zhang, Q.; Yuan, W.-B.; Chen, Y.-P. Concentration of α-Linoleic Acid of Perilla Oil by Gradient Cooling Urea Inclusion. Agric. Sci. China 2009, 8, 685–690. [Google Scholar] [CrossRef]
- Zheng, Z.; Dai, Z.; Shen, Q. Enrichment of polyunsaturated fatty acids from seal oil through urea adduction and the fatty acids change rules during the process. J. Food Process. Preserv. 2018, 42, e13593. [Google Scholar] [CrossRef]
- González-Fernández, M.J.; Fabrikov, D.; Lyashenko, S.; Ferrón-Carrillo, F.; Guil-Guerrero, J.L. Highly concentrated very long-chain PUFA obtainment by Urea complexation methodology. Environ. Technol. Innov. 2020, 18, 100736. [Google Scholar] [CrossRef]
- Sivakumar, R.; Sachin, S.; Priyadarshini, R.; Ghosh, S. Sustainable production of eicosapentaenoic acid-rich oil from microalgae: Towards an algal biorefinery. J. Appl. Microbiol. 2022, 132, 4170–4185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Chen, G.; Zhang, J.; Wang, C.; Liu, B. Extraction and purification of eicosapentaenoic acid and docosahexaenoic acid from microalgae: A critical review. Algal Res. 2019, 43, 101619. [Google Scholar] [CrossRef]
- Vazquez, L.; Prados, I.M.; Reglero, G.; Torres, C.F. Identification and quantification of ethyl carbamate occurring in urea complexation processes commonly utilized for polyunsaturated fatty acid concentration. Food Chem. 2017, 229, 28–34. [Google Scholar] [CrossRef]
- Vazquez, L.; Sanchez-Moyano, M.; de la Iglesia, L.; Reglero, G.; Torres, C.F. A new urea adducts method for PUFA concentration using green food grade solvents and avoiding ethyl carbamate formation. Food Chem. 2022, 392, 133197. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef]
- Vázquez, L.; Akoh, C.C. Concentration of Stearidonic Acid in Free Fatty Acid and Fatty Acid Ethyl Ester Forms from Modified Soybean Oil by Winterization. J. Am. Oil Chem. Soc. 2011, 88, 1775–1785. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, C.Y.; Chen, J.W.; Wang, H.D.; Shieh, C.J. Concentration of Docosahexaenoic and Eicosapentaenoic Acid from Cobia Liver Oil by Acetone Fractionation of Fatty Acid Salts. Appl. Biochem. Biotechnol. 2020, 192, 517–529. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Xie, D.; Li, Y.; Wang, X. One-Step Concentration of Highly Unsaturated Fatty Acids from Tuna Oil by Low-Temperature Crystallization. J. Am. Oil Chem. Soc. 2017, 94, 475–483. [Google Scholar] [CrossRef]
- Bodkowski, R.; Czyz, K.; Sokola-Wysoczanska, E.; Janczak, M.; Cholewinska, P.; Wyrostek, A. The Effect of Low-Temperature Crystallization of Fish Oil on the Chemical Composition, Fatty Acid Profile, and Functional Properties of Cow’s Milk. Animals 2020, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, R.; De León, G.; Munio, M.; Guadix, A.; Guadix, E. Mass transfer modeling of sardine oil polyunsaturated fatty acid (PUFA) concentration by low temperature crystallization. J. Food Eng. 2016, 183, 16–23. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, L.; Huang, L.; Zhang, C.; Xie, P.; Deng, Y.; Tang, K. Mass Transfer Modeling of α-Eleostearic Acid from Tung Oil Concentration by Low-Temperature Crystallization. ChemistrySelect 2020, 5, 4715–4721. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Tekin, A. Computer simulation and experimental molecular distillation of olive pomace oil deodorizer distillate—A comparative study. Lwt 2018, 96, 636–641. [Google Scholar] [CrossRef]
- Mahrous, E.A.; Farag, M.A. Trends and Applications of Molecular Distillation in Pharmaceutical and Food Industries. Sep. Purif. Rev. 2021, 51, 300–317. [Google Scholar] [CrossRef]
- Rossi, P.; Gayol, M.F.; Renaudo, C.; Pramparo, M.C.; Nepote, V.; Grosso, N.R. The use of artificial neural network modeling to represent the process of concentration by molecular distillation of omega-3 from squid oil. Grasas Aceites 2014, 65, e052. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef]
- Gao, K.; Chu, W.; Sun, J.; Mao, X. Identification of an alkaline lipase capable of better enrichment of EPA than DHA due to fatty acids selectivity and regioselectivity. Food Chem. 2020, 330, 127225. [Google Scholar] [CrossRef]
- Moreno-Perez, S.; Turati, D.F.; Borges, J.P.; Luna, P.; Senorans, F.J.; Guisan, J.M.; Fernandez-Lorente, G. Critical Role of Different Immobilized Biocatalysts of a Given Lipase in the Selective Ethanolysis of Sardine Oil. J. Agric. Food Chem. 2017, 65, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Oviedo, J.; Yara-Varón, E.; Torres, M.; Canela-Garayoa, R.; Balcells, M. Sustainable Synthesis of Omega-3 Fatty Acid Ethyl Esters from Monkfish Liver Oil. Catalysts 2021, 11, 100. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Tsai, M.-L.; Wang, H.-M.D.; Liu, Y.-C.; Hsieh, C.; Tsai, Y.-H.; Dong, C.-D.; Huang, C.-Y.; Shieh, C.-J. Continuous Production of DHA and EPA Ethyl Esters via Lipase-Catalyzed Transesterification in an Ultrasonic Packed-Bed Bioreactor. Catalysts 2022, 12, 404. [Google Scholar] [CrossRef]
- Hao, X.; Suo, H.; Zhang, G.; Xu, P.; Gao, X.; Du, S. Ultrasound-assisted enzymatic preparation of fatty acid ethyl ester in deep eutectic solvent. Renew. Energy 2021, 164, 937–947. [Google Scholar] [CrossRef]
- Liu, Y.; Dave, D. Recent progress on immobilization technology in enzymatic conversion of marine by-products to concentrated omega-3 fatty acids. Green Chem. 2022, 24, 1049–1066. [Google Scholar] [CrossRef]
- Lv, E.; Ding, S.; Lu, J.; Yi, W.; Ding, J. Separation and purification of fatty acids by membrane technology: A critical review. Int. J. Chem. React. Eng. 2020, 18, 20190224. [Google Scholar] [CrossRef]
- Ghasemian, S.; Sahari, M.A.; Barzegar, M.; Gavlighi, H.A. Concentration of Omega-3 polyunsaturated fatty acids by polymeric membrane. Int. J. Food Sci. Technol. 2015, 50, 2411–2418. [Google Scholar] [CrossRef]
- Ghasemian, S.; Sahari, M.A.; Barzegar, M.; Ahmadi Gavlighi, H. Omega-3 Polyunsaturated Fatty Acids Concentration Using Synthesized Poly-Vinylidene Fluoride (PVDF) Asymmetric Membranes. J. Am. Oil Chem. Soc. 2016, 93, 1201–1210. [Google Scholar] [CrossRef]
- Ghasemian, S.; Sahari, M.A.; Barzegar, M.; Ahmadi Gavlighi, H. Omega-3 PUFA concentration by a novel PVDF nano-composite membrane filled with nano-porous silica particles. Food Chem. 2017, 230, 454–462. [Google Scholar] [CrossRef]
- Gilmer, C.M.; Bowden, N.B. Highly Cross-Linked Epoxy Nanofiltration Membranes for the Separation of Organic Chemicals and Fish Oil Ethyl Esters. ACS Appl. Mater. Interfaces 2016, 8, 24104–24111. [Google Scholar] [CrossRef]
- Shanmugam, K.; Donaldson, A.A. Extraction of EPA/DHA from 18/12EE Fish Oil Using AgNO3(aq): Composition, Yield, and Effects of Solvent Addition on Interfacial Tension and Flow Pattern in Mini-Fluidic Systems. Ind. Eng. Chem. Res. 2015, 54, 8295–8301. [Google Scholar] [CrossRef]
- Lembke, P. Production Techniques for Omega-3 Concentrates. In Omega-6/3 Fatty Acids: Functions, Sustainability Strategies and Perspectives; De Meester, F., Watson, R.R., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 353–364. [Google Scholar]
- Fagan, P.; Wijesundera, C. Rapid isolation of omega-3 long-chain polyunsaturated fatty acids using monolithic high performance liquid chromatography columns. J. Sep. Sci. 2013, 36, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Li, X.; Jin, Q.; Sun, Q. Preparation of highly purified ω-3 docosapentaenoic acid from seal oil via urea complexation combined with preparative high performance liquid chromatography. Sep. Sci. Technol. 2020, 56, 1769–1778. [Google Scholar] [CrossRef]
- Solaesa, A.G.; Sanz, M.T.; Falkeborg, M.; Beltran, S.; Guo, Z. Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chem. 2016, 190, 960–967. [Google Scholar] [CrossRef]
- Li, D.; Wang, W.; Qin, X.; Li, X.; Yang, B.; Wang, Y. A Novel Process for the Synthesis of Highly Pure n-3 Polyunsaturated Fatty Acid (PUFA)-Enriched Triglycerides by Combined Transesterification and Ethanolysis. J. Agric. Food Chem. 2016, 64, 6533–6538. [Google Scholar] [CrossRef]
- Magallanes, L.M.; Tarditto, L.V.; Grosso, N.R.; Pramparo, M.C.; Gayol, M.F. Highly concentrated omega-3 fatty acid ethyl esters by urea complexation and molecular distillation. J. Sci. Food Agric. 2019, 99, 877–884. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.K. Production of Omega-3 Fatty Acid Ethyl Esters from Menhaden Oil Using Proteus vulgaris Lipase-Mediated One-Step Transesterification and Urea Complexation. Appl. Biochem. Biotechnol. 2016, 179, 347–360. [Google Scholar] [CrossRef]
- Lei, Q.; Ba, S.; Zhang, H.; Wei, Y.; Lee, J.Y.; Li, T. Enrichment of omega-3 fatty acids in cod liver oil via alternate solvent winterization and enzymatic interesterification. Food Chem. 2016, 199, 364–371. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Shen, Q. Effect of purification methods on functional properties of sardine oil ethyl esters. J. Food Process. Preserv. 2021, 46, e16179. [Google Scholar] [CrossRef]
- Dillon, J.T.; Aponte, J.C.; Tarozo, R.; Huang, Y. Purification of omega-3 polyunsaturated fatty acids from fish oil using silver-thiolate chromatographic material and high performance liquid chromatography. J. Chromatogr. A 2013, 1312, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Qilong, R.; Zongbi, B.; Min, L.; Yiwen, Y.; Huabin, X.; Baogen, S.; Qiwei, Y.; Zhiguo, Z. A Method for Preparing High-Purity EPA Ester and DHA Ester Monomer by Supercritical Chromatography. Patent No. CN104557542A, 27 April 2016. [Google Scholar]
- Kelliher, A.; Morrison, A.; Oroskar, A.; Rema, R.V.N.; Agarwal, A. Simulated Moving Bed Chromatographic Separation Process. Patent No. US9790162B2, 17 October 2017. [Google Scholar]
- Li, M.; Bao, Z.; Xing, H.; Yang, Q.; Yang, Y.; Ren, Q. Simulated moving bed chromatography for the separation of ethyl esters of eicosapentaenoic acid and docosahexaenoic acid under nonlinear conditions. J. Chromatogr. A 2015, 1425, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wang, S. Separation of eicosapentaenoic acid and docosahexaenoic acid by three-zone simulated moving bed chromatography. J. Chromatogr. A 2020, 1625, 461326. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Huang, X.; Zhang, L.; Li, J.; Zhang, J. Preparation of eicosapentaenoic acid ethyl ester from fish oil ethyl esters by continuous batch chromatography. J. Sep. Sci. 2019, 42, 3697–3702. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Cao, X.; Han, T.; Pei, H. Separation of high-purity eicosapentaenoic acid and docosahexaenoic acid from fish oil by pH-zone-refining countercurrent chromatography. J. Sep. Sci. 2019, 42, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wen, L.; Cao, X. A biphasic system based on guanidinium ionic liquid: Preparative separation of eicosapentaenoic acid ethyl ester and docosahexaenoic acid ethyl ester by countercurrent chromatography. J. Chromatogr. A 2020, 1618, 460872. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Martin, D.; Nieto-Fuentes, J.A.; Señoráns, F.J.; Reglero, G.; Soler-Rivas, C. Intestinal digestion of fish oils and ω-3 concentrates under in vitro conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 1315–1322. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R. Strategies to improve bioavailability of omega-3 fatty acids from ethyl ester concentrates. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 116–123. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Lopez-Toledano, M.A.; Saxena, V.; Legassie, J.D.; Liu, H.; Ghanta, A.; Riseman, S.; Cocilova, C.; Daak, A.; Thorsteinsson, T.; Rabinowicz, A.L.; et al. Advanced Lipid Technologies(R) (ALT(R)): A Proven Formulation Platform to Enhance the Bioavailability of Lipophilic Compounds. J. Drug Deliv. 2019, 2019, 1957360. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Toledano, M.A.; Thorsteinsson, T.; Daak, A.A.; Maki, K.C.; Johns, C.; Rabinowicz, A.L.; Sancilio, F.D. Minimal food effect for eicosapentaenoic acid and docosahexaenoic acid bioavailability from omega-3–acid ethyl esters with an Advanced Lipid TechnologiesTM (ALT®)–based formulation. J. Clin. Lipidol. 2017, 11, 394–405. [Google Scholar] [CrossRef]
- Qin, Y.; Nyheim, H.; Haram, E.M.; Moritz, J.M.; Hustvedt, S.O. A novel self-micro-emulsifying delivery system (SMEDS) formulation significantly improves the fasting absorption of EPA and DHA from a single dose of an omega-3 ethyl ester concentrate. Lipids Health Dis. 2017, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Bremmell, K.E.; Briskey, D.; Meola, T.R.; Mallard, A.; Prestidge, C.A.; Rao, A. A self-emulsifying Omega-3 ethyl ester formulation (AquaCelle) significantly improves eicosapentaenoic and docosahexaenoic acid bioavailability in healthy adults. Eur. J. Nutr. 2020, 59, 2729–2737. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Zhang, G.D.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and Non-Enzymatic Mechanisms Contribute to Lipid Oxidation During Seed Aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef]
- Lu, J.; Langton, M.; Sampels, S.; Pickova, J. Lipolysis and Oxidation in Ultra-High Temperature Milk Depend on Sampling Month, Storage Duration, and Temperature. J. Food Sci. 2019, 84, 1045–1053. [Google Scholar] [CrossRef]
- Johnson, D.R.; Inchingolo, R.; Decker, E.A. The ability of oxygen scavenging packaging to inhibit vitamin degradation and lipid oxidation in fish oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2018, 47, 467–475. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Dey, T.K.; Maiti, I.; Chakraborty, S.; Ghosh, M.; Dhar, P. Enzymatic synthesis of lipophilic lutein-PUFA esters and assessment of their stabilization potential in EPA-DHA rich fish oil matrix. J. Food Sci. Technol. 2019, 56, 2345–2354. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhu, Y.M.; Liu, N.; Fan, D.M.; Wang, M.F.; Zhao, Y.L. Antioxidative Properties and Chemical Changes of Quercetin in Fish Oil: Quercetin Reacts with Free Fatty Acids to Form Its Ester Derivatives. J. Agr. Food Chem. 2021, 69, 1057–1067. [Google Scholar] [CrossRef]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 41–54. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Ha, H.K.; Lee, M.R.; Kim, J.W.; Kim, H.J.; Lee, W.J. Physicochemical Property and Oxidative Stability of Whey Protein Concentrate Multiple Nanoemulsion Containing Fish Oil. J. Food Sci. 2017, 82, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Ruktanonchai, U.; Min, S.-G.; Chun, J.-Y.; Soottitantawat, A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion–diffusion method. Food Chem. 2010, 119, 1694–1703. [Google Scholar] [CrossRef]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent advances in the spray drying encapsulation of essential fatty acids and functional oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harslof, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Amiri-Jami, M.; LaPointe, G.; Griffiths, M.W. Engineering of EPA/DHA omega-3 fatty acid production by Lactococcus lactis subsp cremoris MG1363. Appl. Microbiol. Biotechnol. 2014, 98, 3071–3080. [Google Scholar] [CrossRef]
- Jakhwal, P.; Kumar Biswas, J.; Tiwari, A.; Kwon, E.E.; Bhatnagar, A. Genetic and non-genetic tailoring of microalgae for the enhanced production of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Bioresour. Technol. 2022, 344, 126250. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Wei, Y.; Zhang, Y.; Liang, M. Lipid contents in farmed fish are influenced by dietary DHA/EPA ratio: A study with the marine flatfish, tongue sole (Cynoglossus semilaevis). Aquaculture 2018, 485, 183–190. [Google Scholar] [CrossRef]
- Venslauskas, K.; Navickas, K.; Nappa, M.; Kangas, P.; Mozuraityte, R.; Slizyte, R.; Zuperka, V. Energetic and Economic Evaluation of Zero-Waste Fish Co-Stream Processing. Int. J. Environ. Res. Public Health 2021, 18, 2358. [Google Scholar] [CrossRef]
| Extraction Method | Principle | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Microwave-Assisted Extraction (MAE) | Microwave energy is used to rapidly heat a solid sample in contact with a solvent | High extraction rates; reduced extraction time and solvent consumption | High power consumption; difficulty in mass production; risk of oxidation | [39,40,41,42] |
| Ultrasound-Assisted Extraction (UAE) | Ultrasound is used to penetrate the solvent in contact with the lipid and thus enhance solvent penetration | High extraction rates; reduced extraction time and solvent consumption | Difficult to scale up; high power consumption | [43,44,45,46] |
| Enzymatic Methods | Enzymatic specificity | Requires no organic solvent; low energy consumption | High price; difficult to mass-produce; reduction in enzyme activity and enzyme recovery | [47,48,49] |
| Supercritical Fluid Extraction (SFE) | SC-CO2 is used as the solvent | Fast, efficient, and highly productive; no organic solvent needed; high purity; low-temperature operation | Requires expensive and complex equipment; high power consumption | [50,51,52] |
| Substance | Molecular Weight | Melting Point | Flash Point | Boiling Point | Intensity | Refractive Index | 1-Octanol/Water Partition Coefficient (log P) | References |
|---|---|---|---|---|---|---|---|---|
| EPA-EE | 330.50 | - | 103.1 ± 24.0 | 417.0 ± 34.0 | 0.909 ± 0.06 | - | 7.642 ± 0.362 | [93] |
| DHA-EE | 356.54 | - | 102.1 ± 21.2 | 443.5 ± 24.0 | 0.914 ± 0.06 | - | 8.154 ± 0.375 |
| Urea Complexation | Molecular Distillation | Low-Temperature Crystallization | Enzymatic Purification | Membrane Technology | Liquid Chromatography | Supercritical Fluid Chromatography | Supercritical Fluid Fractionation | |
|---|---|---|---|---|---|---|---|---|
| Enrichment Mechanism | Degree of saturation | Boiling point | Melting point | Enzyme selection specificity | Pore size and chemical affinity | Chain length and degree of unsaturation | Chain length and degree of unsaturation | Chain length |
| Conditions | −10–90 °C, 1 bar | 140–220 °C, 0.001 mbar | −70–0 °C, 1 bar | 25–65 °C, 1 bar | 25–40 °C, 3–6 bar | 20–50 °C, 1 bar | 35–50 °C, >140 bar | 35–50 °C, >140 bar |
| Omega-3 purity | 60–99% | 65–75% | 60–90% | 46–99% | 35–54% | >90% | >90% | 75–85% |
| Operation Mode | Batch | Continuous | Batch | Batch or semi-batch | Batch | Semi-batch | Continuous | Continuous |
| Risk of Oxidation | Possible | Low | Possible | Low | Low | Possible | Low | Low |
| Capital Investment | Low | Moderate | Moderate | Moderate | Moderate | High | High | High |
| Reference | [95,96,99] | [94,112] | [106,107,108,109] | [114,116,117,118,119] | [122,123,124,125] | [129] | [80] | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, M.; You, Y.; Zhang, Y.; Wu, G.; Karrar, E.; Zhang, L.; Zhang, H.; Jin, Q.; Wang, X. Highly Valuable Fish Oil: Formation Process, Enrichment, Subsequent Utilization, and Storage of Eicosapentaenoic Acid Ethyl Esters. Molecules 2023, 28, 672. https://doi.org/10.3390/molecules28020672
Yi M, You Y, Zhang Y, Wu G, Karrar E, Zhang L, Zhang H, Jin Q, Wang X. Highly Valuable Fish Oil: Formation Process, Enrichment, Subsequent Utilization, and Storage of Eicosapentaenoic Acid Ethyl Esters. Molecules. 2023; 28(2):672. https://doi.org/10.3390/molecules28020672
Chicago/Turabian StyleYi, Mengyuan, Yue You, Yiren Zhang, Gangcheng Wu, Emad Karrar, Le Zhang, Hui Zhang, Qingzhe Jin, and Xingguo Wang. 2023. "Highly Valuable Fish Oil: Formation Process, Enrichment, Subsequent Utilization, and Storage of Eicosapentaenoic Acid Ethyl Esters" Molecules 28, no. 2: 672. https://doi.org/10.3390/molecules28020672
APA StyleYi, M., You, Y., Zhang, Y., Wu, G., Karrar, E., Zhang, L., Zhang, H., Jin, Q., & Wang, X. (2023). Highly Valuable Fish Oil: Formation Process, Enrichment, Subsequent Utilization, and Storage of Eicosapentaenoic Acid Ethyl Esters. Molecules, 28(2), 672. https://doi.org/10.3390/molecules28020672







