Visible-Light-Enhanced Antibacterial Activity of Silver and Copper Co-Doped Titania Formed on Titanium via Chemical and Thermal Treatments
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Surface Structure Analysis
3.3. Evaluation of Apatite Formation Ability
3.4. Ag and Cu Ion Release Behavior
3.5. Evaluation of Antimicrobial Activity
3.6. Identification of Reactive Oxygen Species Induced by Visible-Light Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glockner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodont. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2021, 86, 231–240. [Google Scholar] [CrossRef]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and mechanisms of peri-implant diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef]
- Asensio, G.; Vizquez-Lasa, B.; Rojo, L. Achievements in the topographic design of commercial titanium dental implants: Towards anti-peri-implantitis surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial property and biocompatibility of silver, copper, and zinc in titanium dioxide layers incorporated by one-step micro-arc oxidation: A review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.W.; Chen, L.; Mou, Y.B. Surface modified techniques and emerging functional coating of dental implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2011, 176, 396–428. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin. Implant Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Effect of water treatment on the apatite-forming ability of NaOH-treated titanium metal. J. Biomed. Mater. Res. 2002, 63, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Nakamura, T.; Nishiguchi, S.; Tamura, L.; Uchida, M.; Kim, H.-M.; Kokubo, T. Bioactive titanium: Effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J. Biomed. Mater. Res. 2001, 56, 562–570. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, R.; Abe, S. Visible-light sensitization of TiO2 photocatalysts by wet-method N doping. Appl. Catal. A-Gen. 2005, 284, 131–137. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, X.; Wang, F.; Qin, X.; Zhang, C.; He, H. High-performance of Cu-TiO2 for photocatalytic oxidation of formaldehyde under visible light and the mechanism study. Chem. Eng. J. 2020, 390, 124481. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Kaur, R.; Pal, B. Plasmonic coinage metal-TiO2 hybrid nanocatalysts for highly efficient photocatalytic oxidation under sunlight irradiation. New J. Chem. 2015, 39, 5966–5976. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(II)-TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Tang, J. Charge transfer and photocatalytic activity in CuO/TiO2 nanoparticle heterojunctions synthesised through a rapid, one-pot, microwave solvothermal route. Chem. Cat. Chem. 2015, 7, 1659–1667. [Google Scholar]
- Huang, L.; Peng, F.; Wang, H.; Yu, H.; Li, Z. Preparation and characterization of Cu2O/TiO2 nano–nano heterostructure photocatalysts. Catal. Commun. 2009, 10, 1839–1843. [Google Scholar] [CrossRef]
- Liu, L.M.; Yang, W.Y.; Li, Q.; Gao, S.A.; Shang, J.K. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory. ACS Appl. Mater. Inter. 2014, 6, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, E.J.; Weon, K.-Y.; Lee, W.; Lee, S.H.; Choi, J.-S.; Park, G.H.; Lee, B.; Byun, M.R.; Baek, K.; et al. Non-invasive photodynamic therapy against-periodontitis-causing bacteria. Sci. Rep. 2019, 9, 8248. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, M.; Matsui, N.; Miyazaki, T.; Kanetaka, H. Effect of ammonia or nitric acid treatment on surface structure, in vitro apatite formation, and visible-light photocatalytic activity of bioactive titanium metal. Colloid Surf. B Biointerfaces 2013, 111, 503–508. [Google Scholar] [CrossRef]
- Kawashita, M.; Yokohama, Y.; Cui, X.Y.; Miyazaki, T.; Kanetaka, H. In vitro apatite formation and visible-light photocatalytic activity of Ti metal subjected to chemical and thermal treatments. Ceram. Int. 2014, 40, 12629–12636. [Google Scholar] [CrossRef]
- Kawashita, M.; Endo, E.; Watanabe, T.; Miyazaki, T.; Furuya, M.; Yokota, K.; Abiko, Y.; Kanetaka, H.; Takahashi, N. Formation of bioactive N-doped TiO2 on Ti with visible light-induced antibacterial activity using NaOH, hot water, and subsequent ammonia atmospheric heat treatment. Colloid Surf. B Biointerfaces 2016, 145, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Iwatsu, M.; Kanetaka, H.; Mokudai, T.; Ogawa, T.; Kawashita, M.; Sasaki, K. Visible light-induced photocatalytic and antibacterial activity of N-doped TiO2. J. Biomed. Mater. Res. Part B 2020, 108, 451–459. [Google Scholar] [CrossRef]
- Suzuki, K.; Yokoi, T.; Iwatsu, M.; Mokudai, T.; Kanetaka, H.; Kawashita, M. Antibacterial properties of Cu-doped TiO2 prepared by chemical and heat treatment of Ti metal. J. Asian Ceram. Soc. 2021, 9, 1448–1456. [Google Scholar] [CrossRef]
- Kawashita, M.; Iwabuchi, Y.; Suzuki, K.; Furuya, M.; Yokota, K.; Kanetaka, H. Surface structure and in vitro apatite-forming ability of titanium doped with various metals. Colloid Surf. A Physicochem. Eng. Asp. 2018, 555, 558–564. [Google Scholar] [CrossRef]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Antibacterial and bioactive calcium titanate layers formed on Ti metal and its alloys. J. Mater. Sci. Mater. Med. 2014, 25, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Pattanayak, D.K. Silver incorporated antibacterial, cell compatible and bioactive titania layer on Ti metal for biomedical applications. RSC Adv. 2014, 4, 61444–61455. [Google Scholar] [CrossRef]
- Rajendran, A.; Pattanayak, D.K. Mechanistic studies of biomineralisation on silver incorporated anatase TiO2. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110558. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Manaka, T.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Ashida, M.; Nagai, A.; Hanawa, T. Corrosion behavior and bacterial viability on different surface states of copper. Mater. Trans. 2020, 61, 1143–1148. [Google Scholar] [CrossRef]
- Takadama, H.; Kim, H.-M.; Kokubo, T.; Nakamura, T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J. Biomed. Mater. Res. 2001, 57, 441–448. [Google Scholar] [CrossRef]
- Wang, X.-X.; Hayakawa, S.; Tsuru, K.; Osaka, A. Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 2002, 23, 1353–1357. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.-M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Characteristic and in vitro bioactivity of a microarc-oxidized TiO2-based coating after chemical treatment. Acta Biomater. 2007, 3, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. Part A 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, M.; Toda, S.; Kim, H.-M.; Kokubo, T.; Masuda, M. Preparation of antibacterial silver-doped glass microspheres. J. Biomed. Mater. Res. Part A 2003, 66, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Bajpai, M.; Sharma, L. Copper nanoparticles loaded alginate-impregnated cotton fabric with antibacterial properties. J. Appl. Polym. Sci. 2012, 126, E319–E326. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 1986, 166, 519–527. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simon, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Takadama, H.; Kokubo, T. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar]

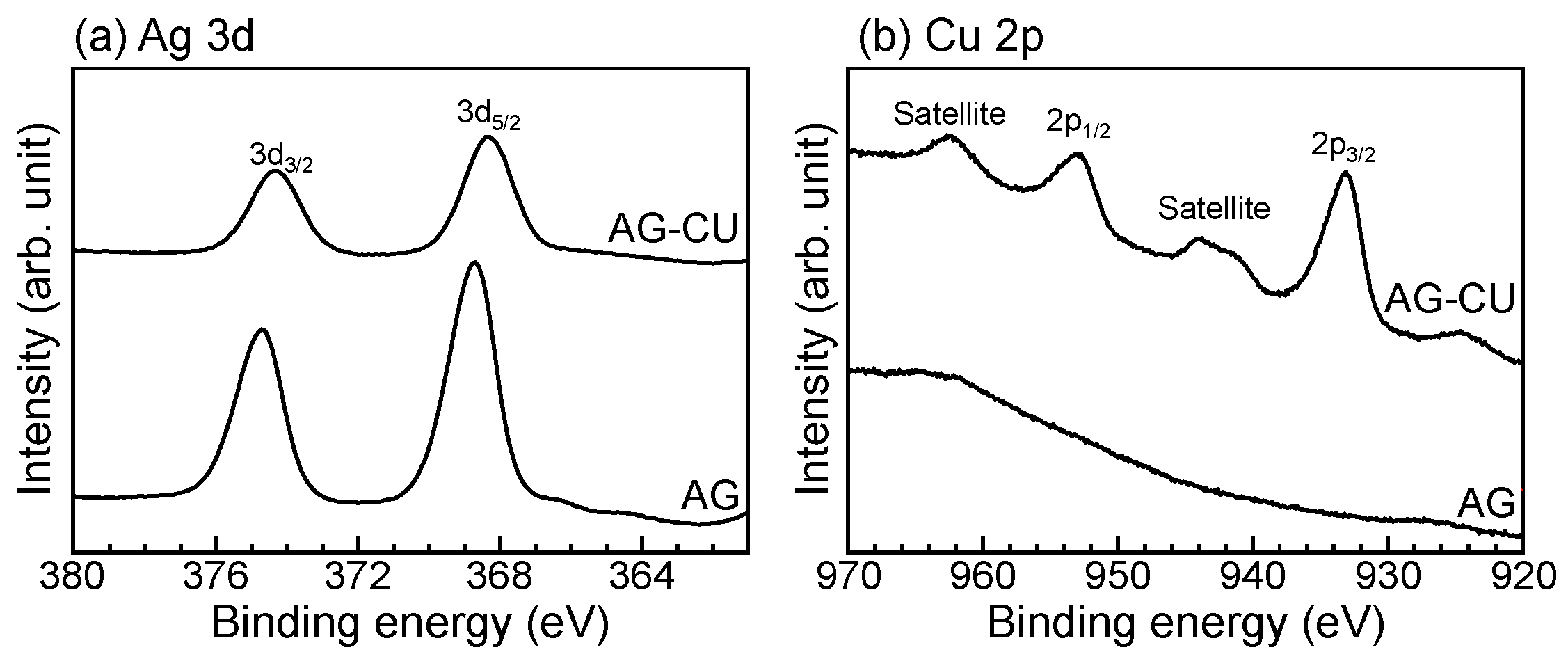
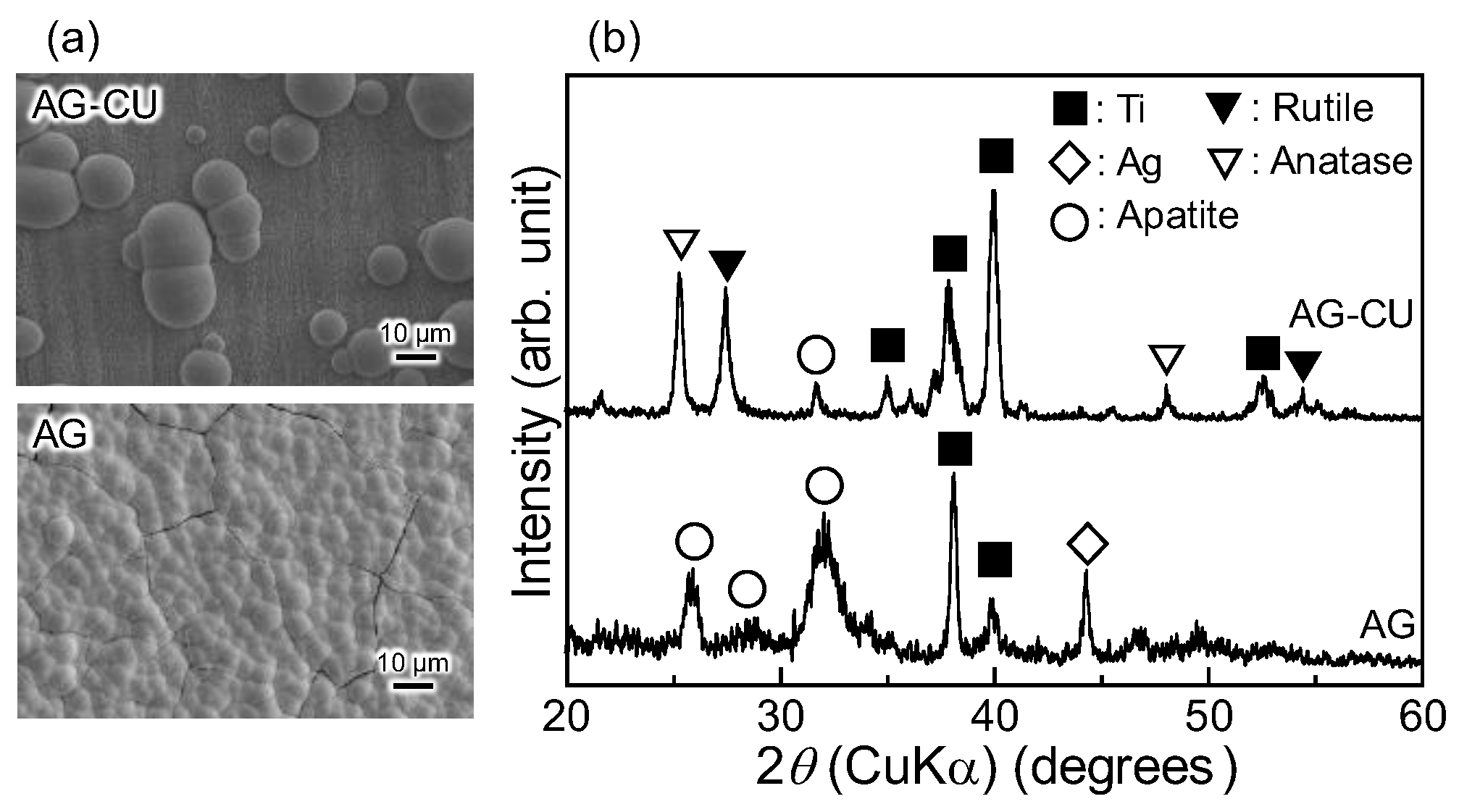
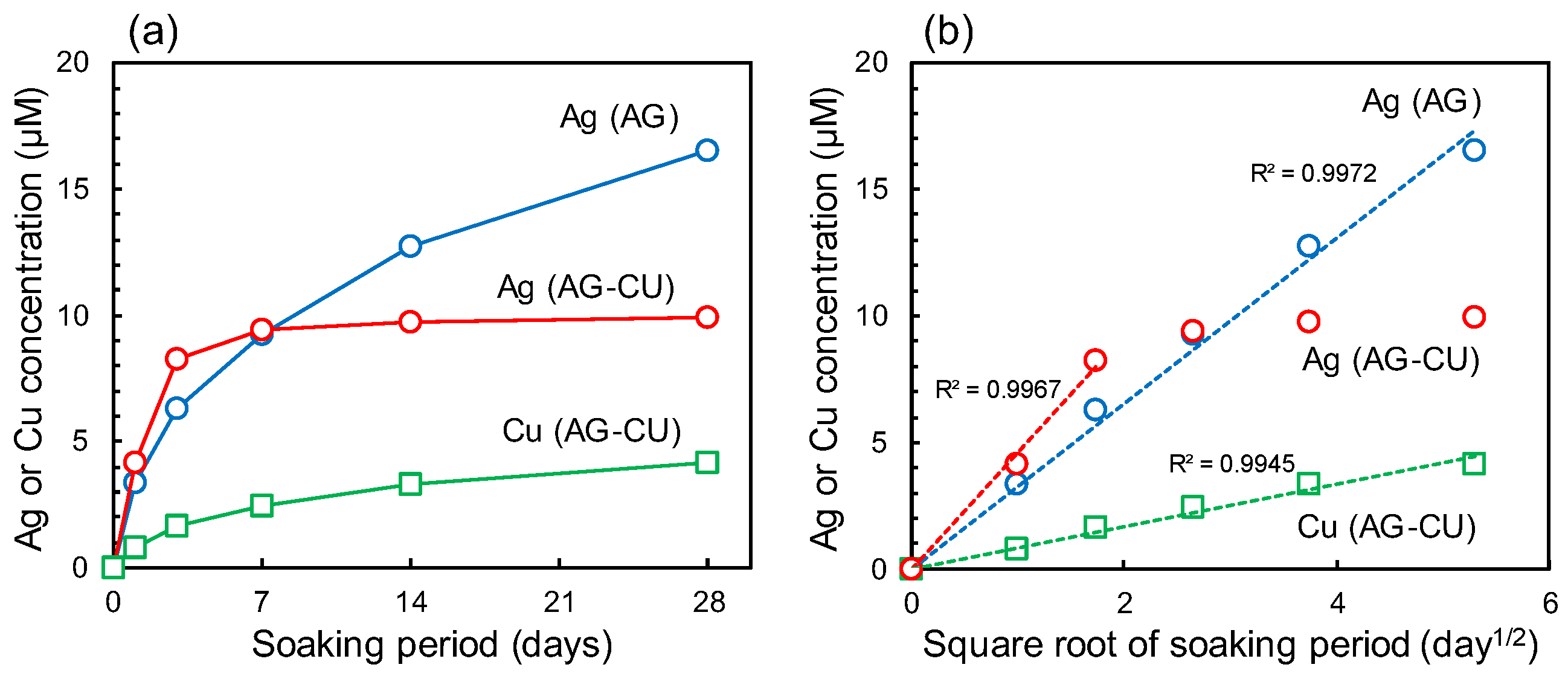

| Sample | Composition (at.%) | ||||
|---|---|---|---|---|---|
| O | Ti | Ag | Cu | C | |
| AG-CU | 59.2 ± 0.6 | 31.0 ± 0.8 | 2.5 ± 0.2 | 4.9 ± 0.6 | 2.3 ± 0.2 |
| AG | 59.0 ± 1.2 | 31.7 ± 0.7 | 6.3 ± 1.8 | − | 3.1 ± 0.3 |
| Sample | Element | EB (eV) | α′ (eV) |
|---|---|---|---|
| AG-CU | Ag | 368.4 ± 0.2 | 723.5 ± 0.4 |
| Cu | 933.0 ± 0.2 | 1850.4 ± 0.2 | |
| AG | Ag | 368.6 ± 0.3 | 723.3 ± 0.1 |
| Sample | Concentration (µM) | |
|---|---|---|
| H2O2 | •OH | |
| AG-CU | 8.0 × 10−2 | 2.5 |
| AG | 8.9 × 10−2 | 1.3 |
| Control (untreated Ti) | 8.9 × 10−2 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Iwatsu, M.; Mokudai, T.; Furuya, M.; Yokota, K.; Kanetaka, H.; Shimabukuro, M.; Yokoi, T.; Kawashita, M. Visible-Light-Enhanced Antibacterial Activity of Silver and Copper Co-Doped Titania Formed on Titanium via Chemical and Thermal Treatments. Molecules 2023, 28, 650. https://doi.org/10.3390/molecules28020650
Suzuki K, Iwatsu M, Mokudai T, Furuya M, Yokota K, Kanetaka H, Shimabukuro M, Yokoi T, Kawashita M. Visible-Light-Enhanced Antibacterial Activity of Silver and Copper Co-Doped Titania Formed on Titanium via Chemical and Thermal Treatments. Molecules. 2023; 28(2):650. https://doi.org/10.3390/molecules28020650
Chicago/Turabian StyleSuzuki, Kanae, Misato Iwatsu, Takayuki Mokudai, Maiko Furuya, Kotone Yokota, Hiroyasu Kanetaka, Masaya Shimabukuro, Taishi Yokoi, and Masakazu Kawashita. 2023. "Visible-Light-Enhanced Antibacterial Activity of Silver and Copper Co-Doped Titania Formed on Titanium via Chemical and Thermal Treatments" Molecules 28, no. 2: 650. https://doi.org/10.3390/molecules28020650
APA StyleSuzuki, K., Iwatsu, M., Mokudai, T., Furuya, M., Yokota, K., Kanetaka, H., Shimabukuro, M., Yokoi, T., & Kawashita, M. (2023). Visible-Light-Enhanced Antibacterial Activity of Silver and Copper Co-Doped Titania Formed on Titanium via Chemical and Thermal Treatments. Molecules, 28(2), 650. https://doi.org/10.3390/molecules28020650







