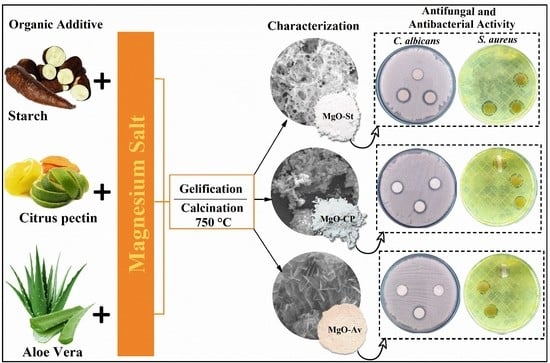
Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities
Abstract
1. Introduction
2. Results
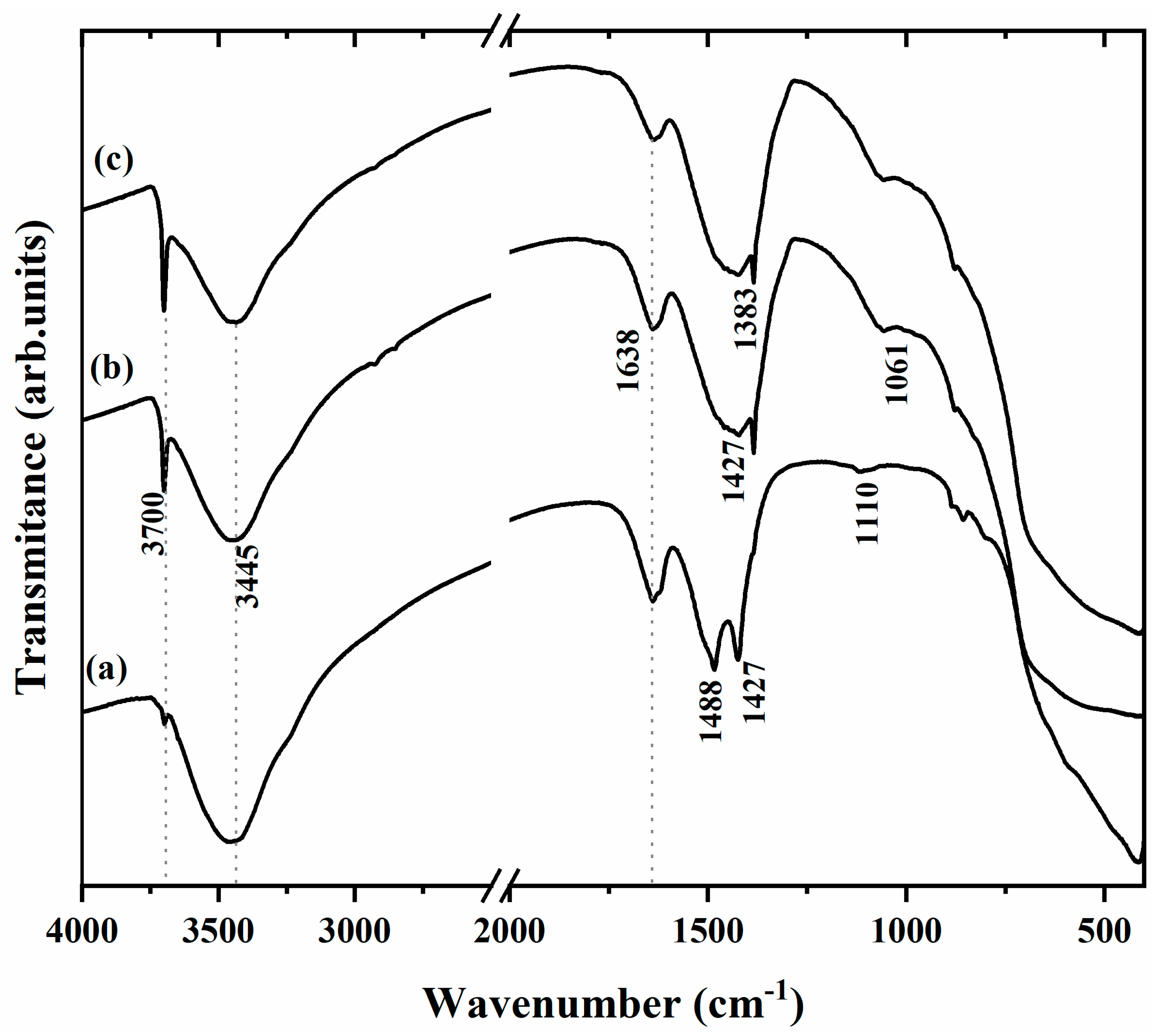
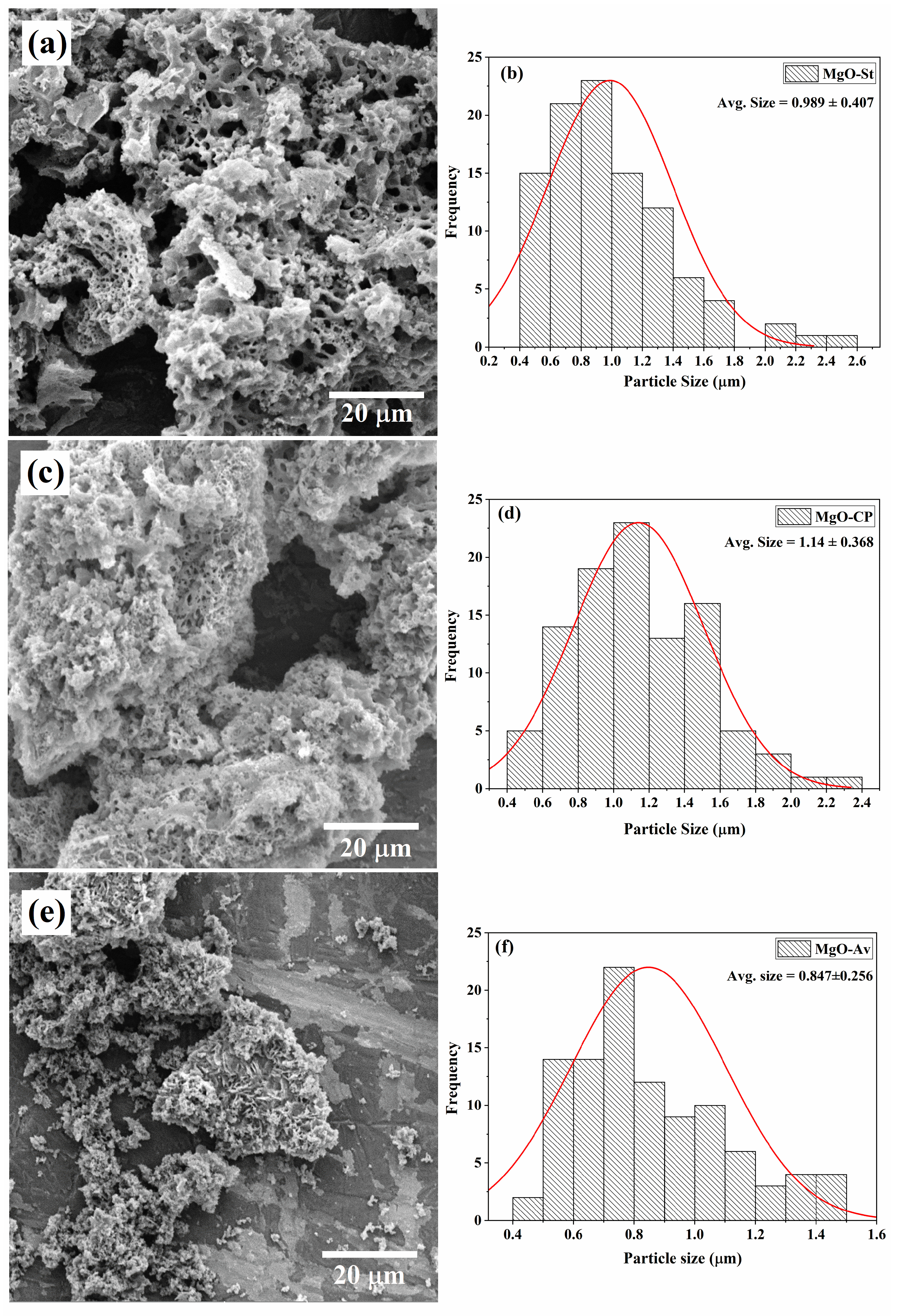
2.1. Characterization of Synthesized MgO Particles
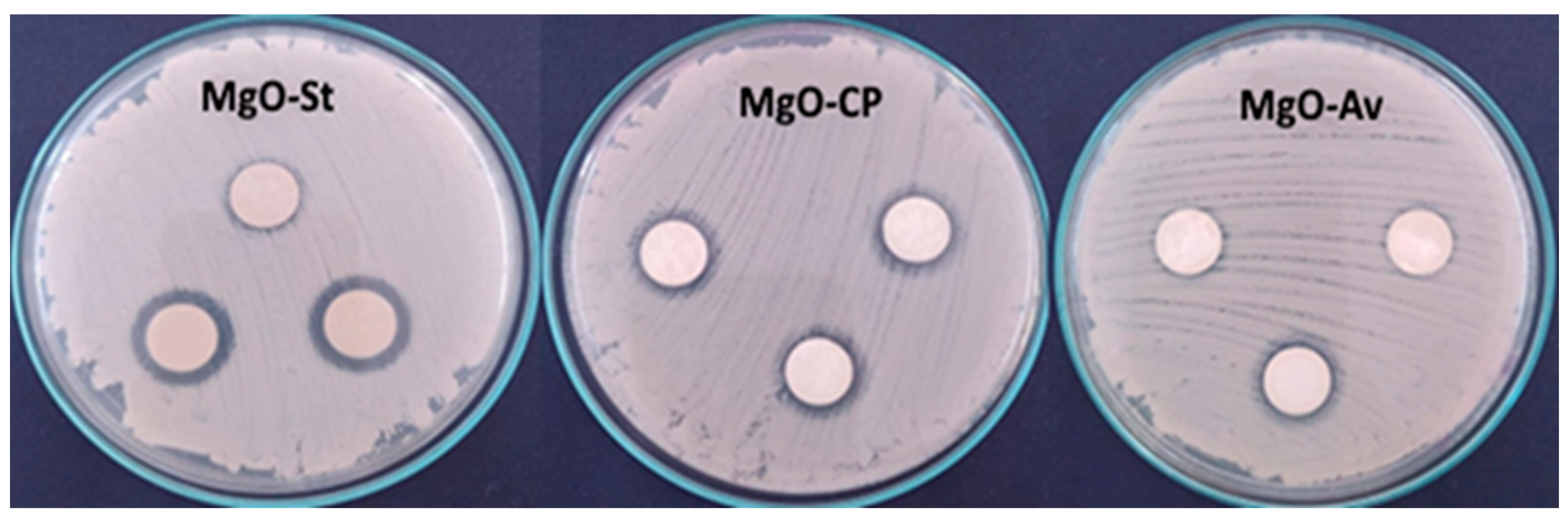
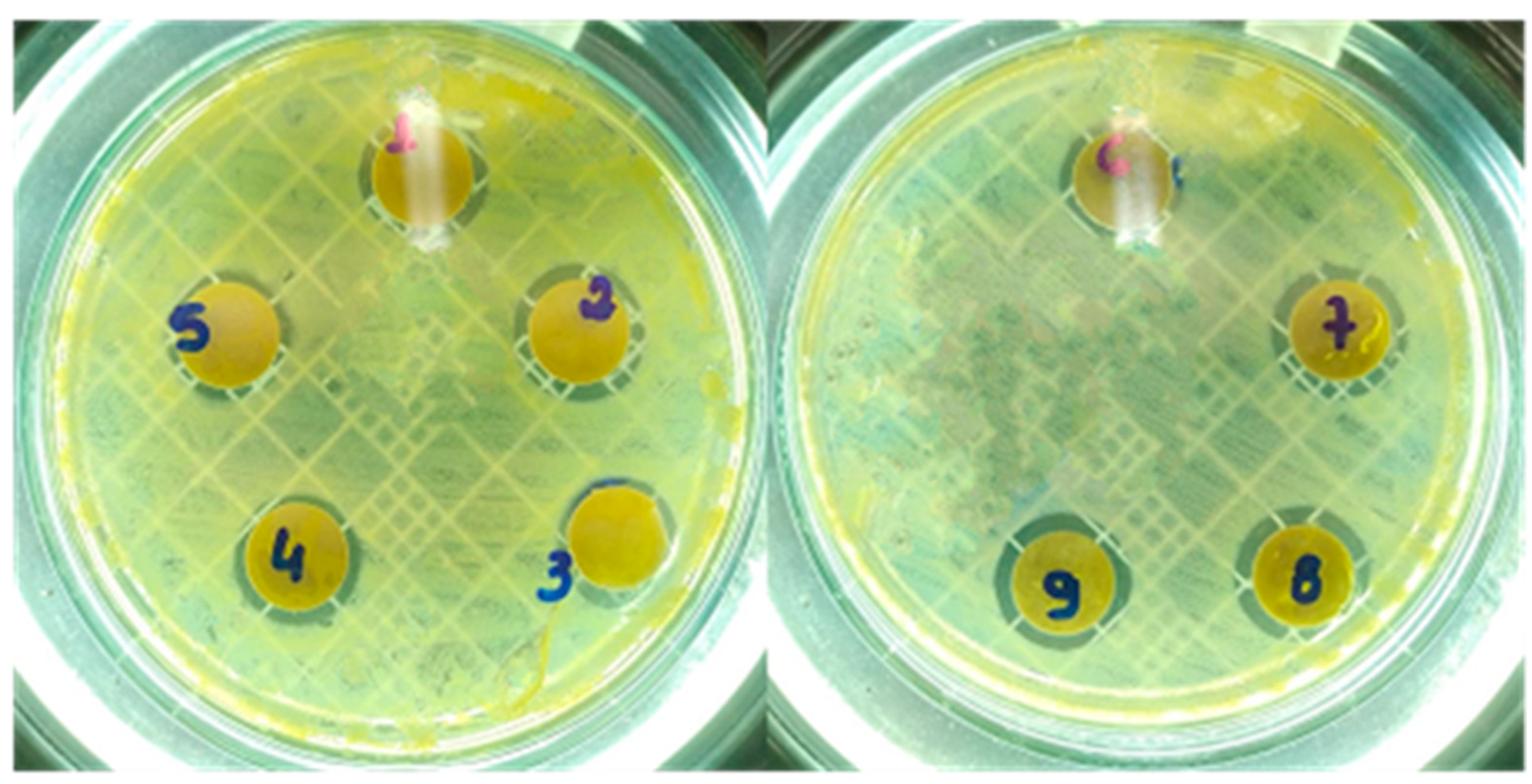
2.2. Evaluation of Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis Using Cassava Starch (Route I)
4.3. Synthesis Using Citrus Pectin (Route II)
4.4. Synthesis Using Aloe vera Barbadensis Miller (Route III)
4.5. Characterization Techniques
4.6. Antifungal and Antibacterial Tests
4.6.1. Disk Agar Diffusion Method
4.6.2. Minimum Inhibitory Concentration (MIC) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, C.; Yu, Z.; Yuan, K.; Jin, X.; Shi, S.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D.; Jiang, H. Improved Preparation of Electrospun MgO Ceramic Fibers with Mesoporous Structure and the Adsorption Properties for Lead and Cadmium. Ceram. Int. 2019, 45, 3743–3753. [Google Scholar] [CrossRef]
- Dhal, J.P.; Sethi, M.; Mishra, B.G.; Hota, G. MgO Nanomaterials with Different Morphologies and Their Sorption Capacity for Removal of Toxic Dyes. Mater. Lett. 2015, 141, 267–271. [Google Scholar] [CrossRef]
- Bassioni, G.; Farid, R.; Mohamed, M.; Hammouda, R.M.; Kühn, F.E. Effect of Different Parameters on Caustic Magnesia Hydration and Magnesium Hydroxide Rheology: A Review. Mater. Adv. 2021, 2, 6519–6531. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Prabu Kumar, S.; Gobinath, C.; Sivaramakrishnan, S. Microwave Assisted Green Synthesis of MgO Nanorods and Their Antibacterial and Anti-Breast Cancer Activities. Mater. Lett. 2017, 206, 217–220. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of MgO Nanostructures and Its Efficient Photocatalytic, Antibacterial and Anticancer Performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Seif, S.; Marofi, S.; Mahdavi, S. Removal of Cr3+ Ion from Aqueous Solutions Using MgO and Montmorillonite Nanoparticles. Environ. Earth Sci. 2019. [Google Scholar] [CrossRef]
- Ahmed, S.; Guo, Y.; Huang, R.; Li, D.; Tang, P.; Feng, Y. Hexamethylene Tetramine-Assisted Hydrothermal Synthesis of Porous Magnesium Oxide for High-Efficiency Removal of Phosphate in Aqueous Solution. J. Environ. Chem. Eng. 2017, 5, 4649–4655. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-Assisted Synthesis of Nanocrystalline MgO and Its Use as a Bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Wu, S.; Dong, Y.; Li, X.; Gong, M.; Zhao, R.; Gao, W.; Wu, H.; He, A.; Li, J.; Wang, X.; et al. Microstructure and Magnetic Properties of FeSiCr Soft Magnetic Powder Cores with a MgO Insulating Layer Prepared by the Sol-Gel Method. Ceram. Int. 2022, 48, 22237–22245. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal From Wastewater. Front. Chem. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araujo, E.M.; Melo, J.J.A. Biopolímeros, Polímeros Biodegradáveis e Polímeros Verdes, Revista Eletrônica de Materiais e Processos. Rev. Eletrônica Mater. e Process. 2011, 6, 127–139. [Google Scholar]
- Dalpasquale, M.; Mariani, F.Q.; Müller, M.; Anaissi, F.J. Citrus Pectin as a Template for Synthesis of Colorful Aluminates. Dye. Pigment. 2016, 125, 124–131. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.H. A Review on the Relationship between Aloe Vera Components and Their Biologic Effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and Applications of Aloe Vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.P.L.; Melo, D.M.d.A.; Medeiros, R.L.B.d.A.; de Araújo, T.R.; Maziviero, F.V.; de Oliveira, Â.A.S. Green Synthesis with Aloe Vera of MgAl2O4 Substituted by Mn and without Calcination Treatment. Res. Soc. Dev. 2022, 11, e14411628873. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Davies, S.C.; Marcelin, J.R. Confronting Antimicrobial Resistance beyond the COVID-19 Pandemic and the 2020 US Election. Lancet 2020, 396, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Jordan, R.P.C.; Wei, X.-Q.; Alves, C.T.; Wise, M.P.; Wilson, M.J.; Lewis, M.A.O. Interactions of Candida Albicans with Host Epithelial Surfaces. J. Oral Microbiol. 2013, 5, 22434. [Google Scholar] [CrossRef]
- Aliprandini, E.; Tavares, J.; Panatieri, R.H.; Thiberge, S.; Yamamoto, M.M.; Silvie, O.; Ishino, T.; Yuda, M.; Dartevelle, S.; Traincard, F.; et al. Cytotoxic Anti-Circumsporozoite Antibodies Target Malaria Sporozoites in the Host Skin. Nat. Microbiol. 2018, 3, 1224–1233. [Google Scholar] [CrossRef]
- Hayat, S.; Muzammil, S.; Rasool, M.H.; Nisar, Z.; Hussain, S.Z.; Sabri, A.N.; Jamil, S. In Vitro Antibiofilm and Anti-Adhesion Effects of Magnesium Oxide Nanoparticles against Antibiotic Resistant Bacteria. Microbiol. Immunol. 2018, 62, 211–220. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Kavin Micheal, M.; Arasu, M.V.; Abdullah Al-Dhabi, N. Structural, Morphological and Optical Properties of MgO Nanoparticles for Antibacterial Applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial Resistance: The Example of Staphylococcus Aureus. J. Clin. Invest. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Anderson, J.B.; Kohn, L.M. Evolution of Drug Resistance in Candida Albicans. Annu. Rev. Microbiol. 2002, 56, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Masterson, K.; Meade, E.; Garvey, M.; Lynch, M.; Major, I.; Rowan, N.J. Development of a Low-Temperature Extrusion Process for Production of GRAS Bioactive-Polymer Loaded Compounds for Targeting Antimicrobial-Resistant (AMR) Bacteria. Sci. Total Environ. 2021, 800, 149545. [Google Scholar] [CrossRef] [PubMed]
- Shakir Shnain Al-Shammari, R.; Kareem Hammood Jaberi, A.; Lungu, A.; Brebenel, I.; Khaled Kaddem Al-Sudani, W.; Hafedh Mohammed Al-Saedi, J.; Pop, C.E.; Mernea, M.; Stoian, G.; Mihailescu, D.F. The Antifungal Activity of Zein Nanoparticles Loaded With Transition Metal Ions. Rev. Roum. Chim. 2021, 66, 829–834. [Google Scholar] [CrossRef]
- Umaralikhan, L.; Jamal Mohamed Jaffar, M. Green Synthesis of MgO Nanoparticles and It Antibacterial Activity. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 477–485. [Google Scholar] [CrossRef]
- Mutis González, N.; Pineda Gómez, P.; Rodríguez García, M.E. Effect of the Addition of Potassium and Magnesium Ions on the Thermal, Pasting, and Functional Properties of Plantain Starch (Musa Paradisiaca). Int. J. Biol. Macromol. 2019, 124, 41–49. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ramakrishna, S.; Esmaeili, H.; Bahrani, S.; Koosha, M.; Babapoor, A. Green Synthesis of Supermagnetic Fe3O4–MgO Nanoparticles via Nutmeg Essential Oil toward Superior Anti-Bacterial and Anti-Fungal Performance. J. Drug Deliv. Sci. Technol. 2019, 54, 101352. [Google Scholar] [CrossRef]
- Moulavi, M.H.; Kale, B.B.; Bankar, D.; Amalnerkar, D.P.; Vinu, A.; Kanade, K.G. Green Synthetic Methodology: An Evaluative Study for Impact of Surface Basicity of MnO2 Doped MgO Nanocomposites in Wittig Reaction. J. Solid State Chem. 2019, 269, 167–174. [Google Scholar] [CrossRef]
- Li, N.; Dang, H.; Chang, Z.; Zhao, X.; Zhang, M.; Li, W.; Zhou, H.; Sun, C. Synthesis of Uniformly Distributed Magnesium Oxide Micro-/Nanostructured Materials with Deep Eutectic Solvent for Dye Adsorption. J. Alloys Compd. 2019, 808. [Google Scholar] [CrossRef]
- Siddqui, N.; Sarkar, B.; Pendem, C.; Khatun, R.; Sivakumar Konthala, L.N.; Sasaki, T.; Bordoloi, A.; Bal, R. Highly Selective Transfer Hydrogenation of α,β-Unsaturated Carbonyl Compounds Using Cu-Based Nanocatalysts. Catal. Sci. Technol. 2017, 7, 2828–2837. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Rahman, M.M. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)2 Mixed with Polyethylene Glycol. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.K.; Thuy Chau, N.T.; Viet, P.H. Preparation and Characterization of a Chitosan/MgO Composite for the Effective Removal of Reactive Blue 19 Dye from Aqueous Solution. J. Sci. Adv. Mater. Devices 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. Defect and Adsorbate Induced Infrared Modes in Sol-Gel Derived Magnesium Oxide Nano-Crystallites. Solid State Commun. 2008, 147, 405–408. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-Free Synthesis of MgO Nanoparticles for Effective Photocatalytic Applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- Ananda, A.; Ramakrishnappa, T.; Archana, S.; Yadav, L.S.R.; Shilpa, B.M.; Nagaraju, G.; Jayanna, B.K. Materials Today: Proceedings Green Synthesis of MgO Nanoparticles Using Phyllanthus Emblica for Evans Blue Degradation and Antibacterial Activity. Mater Today Proc. 2021, 49, 801–810. [Google Scholar] [CrossRef]
- Mastuli, M.S.; Kamarulzaman, N.; Nawawi, M.A.; Mahat, A.M.; Rusdi, R.; Kamarudin, N. Growth Mechanisms of MgO Nanocrystals via a Sol-Gel Synthesis Using Different Complexing Agents. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Guo, L.; Lei, R.; Zhang, T.C.; Du, D.; Zhan, W. Insight into the Role and Mechanism of Polysaccharide in Polymorphous Magnesium Oxide Nanoparticle Synthesis for Arsenate Removal. Chemosphere 2022, 296, 133878. [Google Scholar] [CrossRef]
- Sejas, L.M.; Silbert, S.; Reis, A.O.; Sader, H.S. Avaliação Da Qualidade Dos Discos Com Antimicrobianos Para Testes de Disco-Difusão Disponíveis Comercialmente No Brasil. J. Bras. Patol. e Med. Lab. 2003, 39. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Sridevi, C.; Rajavel, R.; Karthikeyan, P. Journal of Science: Advanced Materials and Devices Smart Fl Ower like MgO / Tb, Eu-Substituted Hydroxyapatite Dual Layer Coating on 316L SS for Enhanced Corrosion Resistance, Antibacterial Activity and Osteocompatibility. J. Sci. Adv. Mater. Devices 2020, 10. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Arzate-Cardenas, M.A.; Mojarro-Olmos, A.; Romo-López, M.A. Synthesis, Characterization, Toxicological and Antibacterial Activity Evaluation of Cu@ZnO Nanocomposites. Ceram. Int. 2019, 45, 17476–17488. [Google Scholar] [CrossRef]
- EUCAST Definitive Document. Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents.Terminol. Clin.Microbiol Infect 1998, 4, 291–296. [Google Scholar] [CrossRef]
- Almeida, E.; De Bona, M.; Gisele, F.; Fruet, T.K.; Cristina, T.; Jorge, M.; De Moura, A.C. Comparação de Métodos Para Avaliação Da Atividade Antimicrobiana e Determinação Da Concentração Inibitória Mínima (Cim) de Extratos Vegetais Aquosos e Etanólicos. Arq. Inst. Biol. 2014, 81, 218–225. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2016, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- El-Shaer, A.; Abdelfatah, M.; Mahmoud, K.R.; Momay, S.; Eraky, M.R. Correlation between Photoluminescence and Positron Annihilation Lifetime Spectroscopy to Characterize Defects in Calcined MgO Nanoparticles as a First Step to Explain Antibacterial Activity. J. Alloys Compd. 2020, 817, 152799. [Google Scholar] [CrossRef]
- Almontasser, A.; Parveen, A.; Azam, A. Synthesis, Characterization and Antibacterial Activity of Magnesium Oxide (MgO) Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Borth, K.W.; Peron, D.C.; Teixeira, V.d.C.; Galante, D.; Bittencourt, C.; Anaissi, F.J. Synthesis of Green Cool Pigments (CoxZn1-XO) for Application in NIR Radiation Reflectance. J. Alloys Compd. 2019, 780, 17–24. [Google Scholar] [CrossRef]
- Khort, A.; Hedberg, J.; Mei, N.; Romanovski, V.; Blomberg, E.; Odnevall, I. Corrosion and Transformation of Solution Combustion Synthesized Co, Ni and CoNi Nanoparticles in Synthetic Freshwater with and without Natural Organic Matter. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; Volume 32.
- Radaelli, M.; da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; da Costa, L.A.M.A.; Ethur, E.M. Antimicrobial Activities of Six Essential Oils Commonly Used as Condiments in Brazil against Clostridium Perfringens. Brazilian J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]


| Sample | Elements (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Ca | Zn | Cu | K | Al | S | P | Others | |
| Mg(NO3)2.6H2O | 98.0 | 0.8 | - | 0.1 | - | - | 1.0 | - | 0.1 |
| Starch | 6.6 | 26.0 | 4.0 | 4.4 | 22.5 | 15.2 | 11.6 | 4.9 | 4.8 |
| MgO-St | 86.6 | 2.7 | 0.7 | 0.4 | 1.0 | 6.3 | - | 1.7 | 0.6 |
| Aloe vera | 0.1 | 20.9 | 1.0 | 3.4 | 0.5 | - | 1.2 | - | 72.9 |
| MgO-Av | 64.1 | 8.8 | 0.4 | 0.7 | 0.4 | - | 1.1 | 0.4 | 24.1 |
| Citric pectin | 1.7 | 21.8 | 2.2 | 2.1 | 52.6 | 7.7 | 4.9 | 1.4 | 5.6 |
| MgO-CP | 76.2 | 8.1 | 1.6 | 0.5 | 1.3 | 7.3 | 2.6 | 0.5 | 1.9 |
| Microorganism | Disk Diffusion Method | ||
|---|---|---|---|
| MgO-St | MgO-CP | MgO-Av | |
| Staphylococcus aureus | 2.86 ± 0.23 a | 2.43 ± 0.51 b | 2.20 ± 0.34 b |
| Escherichia coli | 0 | 0 | 0 |
| Candida albicans | 3.0 ± 0 a | 1.36 ± 0.11 b | 0.16 ± 0.28 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaba, N.; Jaerger, S.; Horsth, D.F.L.; Primo, J.d.O.; Correa, J.d.S.; Bittencourt, C.; Zanette, C.M.; Anaissi, F.J. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules 2023, 28, 142. https://doi.org/10.3390/molecules28010142
Balaba N, Jaerger S, Horsth DFL, Primo JdO, Correa JdS, Bittencourt C, Zanette CM, Anaissi FJ. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules. 2023; 28(1):142. https://doi.org/10.3390/molecules28010142
Chicago/Turabian StyleBalaba, Nayara, Silvia Jaerger, Dienifer F. L. Horsth, Julia de O. Primo, Jamille de S. Correa, Carla Bittencourt, Cristina M. Zanette, and Fauze J. Anaissi. 2023. "Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities" Molecules 28, no. 1: 142. https://doi.org/10.3390/molecules28010142
APA StyleBalaba, N., Jaerger, S., Horsth, D. F. L., Primo, J. d. O., Correa, J. d. S., Bittencourt, C., Zanette, C. M., & Anaissi, F. J. (2023). Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules, 28(1), 142. https://doi.org/10.3390/molecules28010142