Machine Learning Prediction of Mycobacterial Cell Wall Permeability of Drugs and Drug-like Compounds
Abstract
1. Introduction
2. Results and Discussion
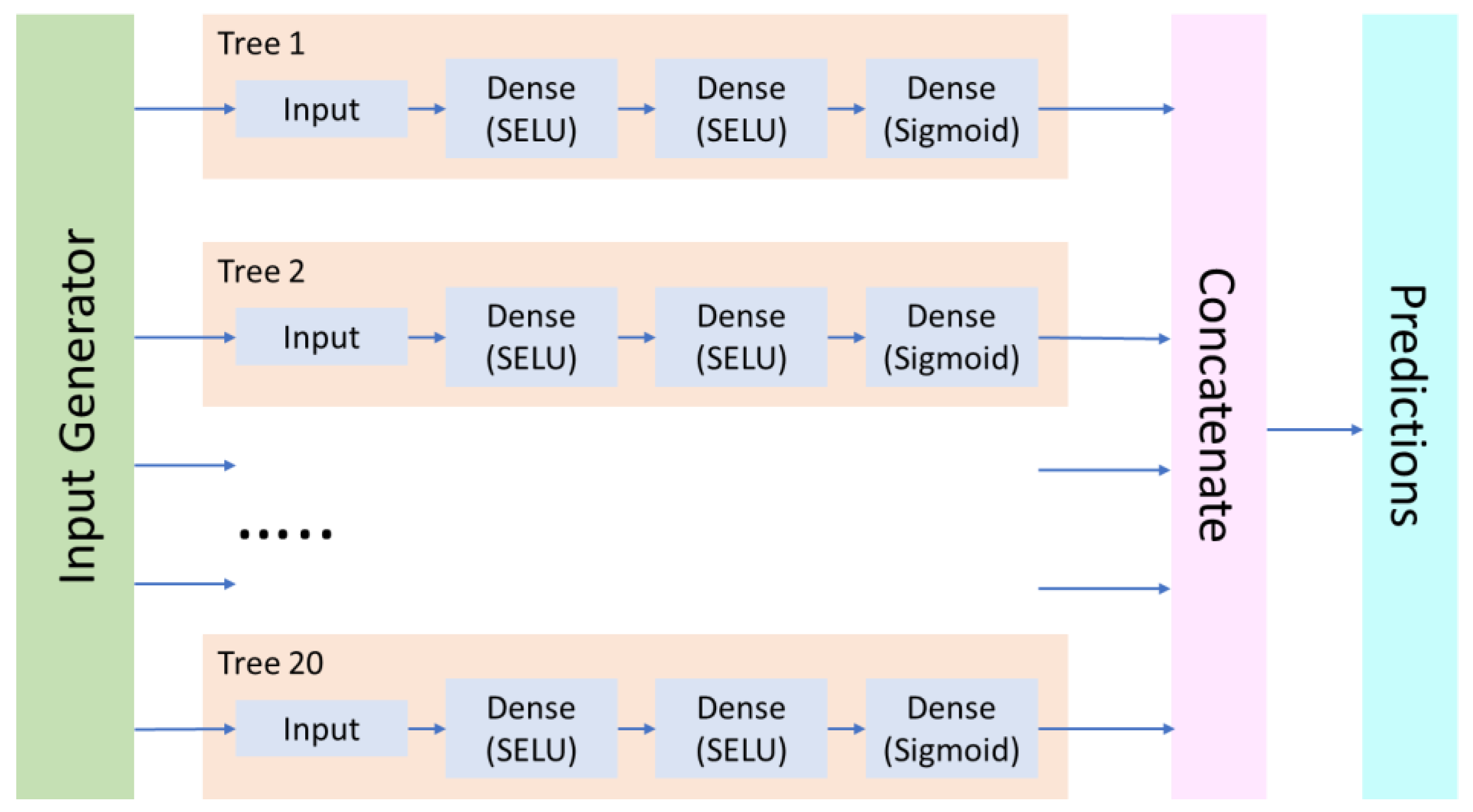
2.1. General Modeling Approach
2.2. Mycobacterium tuberculosis Inhibitor Permeability Dataset
2.3. Molecular Descriptors
2.4. Neural Network Modeling Procedure
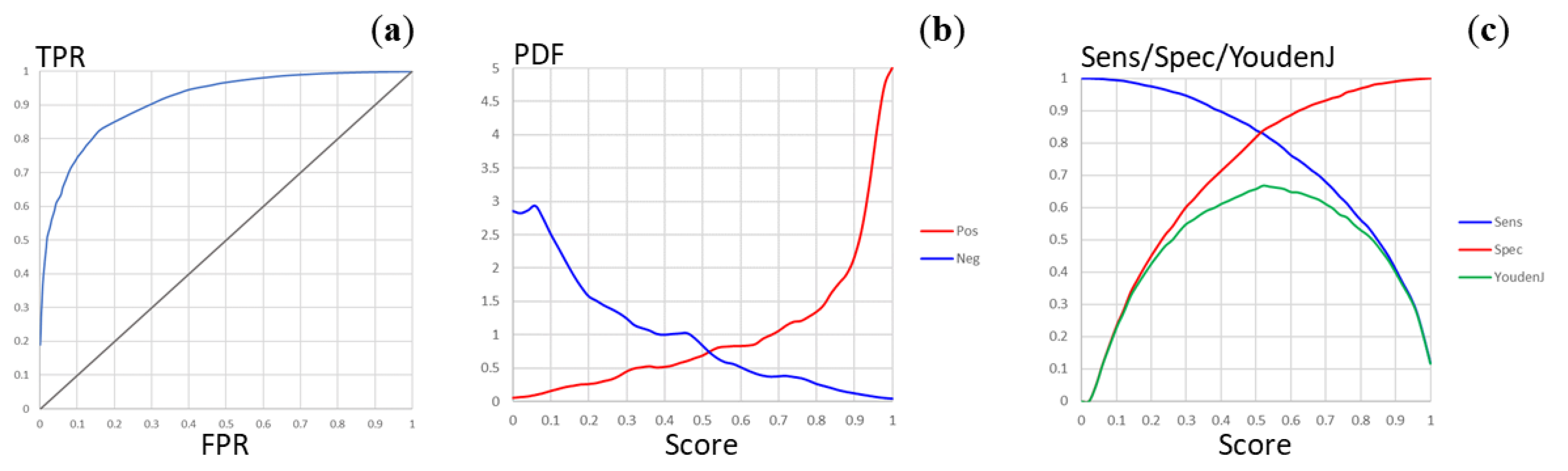
2.5. Predictive Model of Mycobacterium tuberculosis Permeability
3. Materials and Methods
3.1. Mycobacterium tuberculosis Inhibitor Permeability Dataset
3.2. Modeling Workflow
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Friedman, L.N.; Dedicoat, M.; Davies, P.D.O. (Eds.) Clinical Tuberculosis, 6th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; ISBN 978-1-351-24998-0. [Google Scholar]
- Sharma, S.K.; Mohan, A. Textbook of Tuberculosis and Nontuberculous Mycobacterial Diseases, 3rd ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2020; ISBN 978-93-89129-21-2. [Google Scholar]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An adaptable pathogen associated with multiple human diseases. Front. Cell. Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006172-9.
- Goossens, S.N.; Sampson, S.L.; Van Rie, A. Mechanisms of drug-induced tolerance in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2020, 34, e00141-20. [Google Scholar] [CrossRef]
- Liebenberg, D.; Gordhan, B.G.; Kana, B.D. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front. Cell. Infect. Microbiol. 2022, 12, 943545. [Google Scholar] [CrossRef]
- Li, H.; Yuan, J.; Duan, S.; Pang, Y. Resistance and tolerance of Mycobacterium tuberculosis to antimicrobial agents–How M. tuberculosis can escape antibiotics. WIREs Mech. Dis. 2022, 14, e1573. [Google Scholar] [CrossRef] [PubMed]
- Poulton, N.C.; Rock, J.M. Unraveling the mechanisms of intrinsic drug resistance in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2022, 12, 997283. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Peters, P.J.; Kumar, J. Tuberculosis: Past, present and future of the treatment and drug discovery research. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Kumar, M.; Kumar, A.; Kanchan, K. Comprehensive review on mechanism of action, resistance and evolution of antimycobacterial drugs. Life Sci. 2021, 274, 119301. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Sharma, R. Screening approaches and therapeutic targets: The two driving wheels of tuberculosis drug discovery. Biochem. Pharmacol. 2022, 197, 114906. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Lah, H.U.; Yousuf, S.K.; Ahmad, Z. Cell wall: A versatile fountain of drug targets in Mycobacterium tuberculosis. Biomed. Pharmacother. 2017, 95, 1520–1534. [Google Scholar] [CrossRef]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope–A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef]
- Abrahams, K.A.; Besra, G.S. Synthesis and recycling of the mycobacterial cell envelope. Curr. Opin. Microbiol. 2021, 60, 58–65. [Google Scholar] [CrossRef]
- Kuang, W.; Zhang, H.; Wang, X.; Yang, P. Overcoming Mycobacterium tuberculosis through small molecule inhibitors to break down cell wall synthesis. Acta Pharm. Sin. B 2022, 12, 3201–3214. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kaushal, P.S. Protein synthesis in Mycobacterium tuberculosis as a potential target for therapeutic interventions. Mol. Aspects Med. 2021, 81, 101002. [Google Scholar] [CrossRef] [PubMed]
- Reiche, M.A.; Warner, D.F.; Mizrahi, V. Targeting DNA replication and repair for the development of novel therapeutics against tuberculosis. Front. Mol. Biosci. 2017, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Garg, T.; Srinivas, N.; Dasgupta, A.; Chopra, S. Targeting DNA gyrase to combat Mycobacterium tuberculosis: An update. Curr. Top. Med. Chem. 2019, 19, 579–593. [Google Scholar] [CrossRef]
- Miggiano, R.; Morrone, C.; Rossi, F.; Rizzi, M. Targeting genome integrity in Mycobacterium tuberculosis: From nucleotide synthesis to DNA replication and repair. Molecules 2020, 25, 1205. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, F.; Tambunan, U.S.F.; Siahaan, T.J. M. tuberculosis transcription machinery: A review on the mycobacterial RNA polymerase and drug discovery efforts. Life 2022, 12, 1774. [Google Scholar] [CrossRef]
- Roy, K.K.; Wani, M.A. Emerging opportunities of exploiting mycobacterial electron transport chain pathway for drug-resistant tuberculosis drug discovery. Expert Opin. Drug Discov. 2020, 15, 231–241. [Google Scholar] [CrossRef]
- Urban, M.; Šlachtová, V.; Brulíková, L. Small organic molecules targeting the energy metabolism of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2021, 212, 113139. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, E.J.; Wiggins, T.J.; Berney, M. Bioenergetic inhibitors: Antibiotic efficacy and mechanisms of action in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2020, 10, 611683. [Google Scholar] [CrossRef]
- Samuels, A.N.; Wang, E.R.; Harrison, G.A.; Valenta, J.C.; Stallings, C.L. Understanding the contribution of metabolism to Mycobacterium tuberculosis drug tolerance. Front. Cell. Infect. Microbiol. 2022, 12, 958555. [Google Scholar] [CrossRef] [PubMed]
- Yelamanchi, S.D.; Surolia, A. Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life 2021, 73, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Singh, A. Mycobacterial tuberculosis enzyme targets and their inhibitors. Curr. Top. Med. Chem. 2019, 19, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Huszár, S.; Chibale, K.; Singh, V. The quest for the holy grail: New antitubercular chemical entities, targets and strategies. Drug Discov. Today 2020, 25, 772–780. [Google Scholar] [CrossRef]
- Bahuguna, A.; Rawat, D.S. An overview of new antitubercular drugs, drug candidates, and their targets. Med. Res. Rev. 2020, 40, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl. Res. 2020, 220, 68–97. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Trifonov, L.; Yadav, V.D.; Barry, C.E.; Boshoff, H.I. Tuberculosis drug discovery: A decade of hit assessment for defined targets. Front. Cell. Infect. Microbiol. 2021, 11, 611304. [Google Scholar] [CrossRef]
- Angula, K.T.; Legoabe, L.J.; Beteck, R.M. Chemical classes presenting novel antituberculosis agents currently in different phases of drug development: A 2010–2020 review. Pharmaceuticals 2021, 14, 461. [Google Scholar] [CrossRef]
- Yang, L.; Hu, X.; Chai, X.; Ye, Q.; Pang, J.; Li, D.; Hou, T. Opportunities for overcoming tuberculosis: Emerging targets and their inhibitors. Drug Discov. Today 2022, 27, 326–336. [Google Scholar] [CrossRef]
- Mi, J.; Gong, W.; Wu, X. Advances in key drug target identification and new drug development for tuberculosis. Biomed. Res. Int. 2022, 2022, 5099312. [Google Scholar] [CrossRef]
- Singh, V.; Chibale, K. Strategies to combat multi-drug resistance in tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef]
- Torfs, E.; Piller, T.; Cos, P.; Cappoen, D. Opportunities for overcoming Mycobacterium tuberculosis drug resistance: Emerging mycobacterial targets and host-directed therapy. Int. J. Mol. Sci. 2019, 20, 2868. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, F.; Saragih, M.; Tambunan, U.S.F. Recent progress and challenges for drug-resistant tuberculosis treatment. Pharmaceutics 2021, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Modak, B.; Girkar, S.; Narayan, R.; Kapoor, S. Mycobacterial membranes as actionable targets for lipid-centric therapy in tuberculosis. J. Med. Chem. 2022, 65, 3046–3065. [Google Scholar] [CrossRef] [PubMed]
- Fullam, E.; Young, R.J. Physicochemical properties and Mycobacterium tuberculosis transporters: Keys to efficacious antitubercular drugs? RSC Med. Chem. 2021, 12, 43–56. [Google Scholar] [CrossRef]
- de Oliveira, M.C.B.; Balan, A. The ATP-Binding Cassette (ABC) transport systems in Mycobacterium tuberculosis: Structure, function, and possible targets for therapeutics. Biology 2020, 9, 443. [Google Scholar] [CrossRef]
- Stelitano, G.; Sammartino, J.C.; Chiarelli, L.R. Multitargeting compounds: A promising strategy to overcome multi-drug resistant tuberculosis. Molecules 2020, 25, 1239. [Google Scholar] [CrossRef]
- Jeong, E.-K.; Lee, H.-J.; Jung, Y.-J. Host-directed therapies for tuberculosis. Pathogens 2022, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.-H.; Lowrie, D.B.; Fan, X.-Y. Research advances for virus-vectored tuberculosis vaccines and latest findings on tuberculosis vaccine development. Front. Immunol. 2022, 13, 895020. [Google Scholar] [CrossRef]
- Flores-Valdez, M.A.; Kupz, A.; Subbian, S. Recent developments in mycobacteria-based live attenuated vaccine candidates for tuberculosis. Biomedicines 2022, 10, 2749. [Google Scholar] [CrossRef]
- Bouzeyen, R.; Javid, B. Therapeutic vaccines for tuberculosis: An overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Mandlik, S.; Pokharkar, V. Nanocarrier-based approaches for the efficient delivery of anti-tubercular drugs and vaccines for management of tuberculosis. Front. Pharmacol. 2021, 12, 749945. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, D.C.D.S.; Cavalcanti, I.D.L.; Medeiros, S.M.D.F.R.D.S.; de Souza, J.B.; Lira Nogueira, M.C.D.B.; Cavalcanti, I.M.F. Nanotechnology and tuberculosis: An old disease with new treatment strategies. Tuberculosis 2022, 135, 102208. [Google Scholar] [CrossRef] [PubMed]
- Dalberto, P.F.; de Souza, E.V.; Abbadi, B.L.; Neves, C.E.; Rambo, R.S.; Ramos, A.S.; Macchi, F.S.; Machado, P.; Bizarro, C.V.; Basso, L.A. Handling the hurdles on the way to anti-tuberculosis drug development. Front. Chem. 2020, 8, 586294. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Besra, G.S. Mycobacterial drug discovery. RSC Med. Chem. 2020, 11, 1354–1365. [Google Scholar] [CrossRef]
- Craggs, P.D.; de Carvalho, L.P.S. Bottlenecks and opportunities in antibiotic discovery against Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2022, 69, 102191. [Google Scholar] [CrossRef] [PubMed]
- Macalino, S.J.Y.; Billones, J.B.; Organo, V.G.; Carrillo, M.C.O. In silico strategies in tuberculosis drug discovery. Molecules 2020, 25, 665. [Google Scholar] [CrossRef]
- Machado, D.; Girardini, M.; Viveiros, M.; Pieroni, M. Challenging the drug-likeness dogma for new drug discovery in tuberculosis. Front. Microbiol. 2018, 9, 1367. [Google Scholar] [CrossRef]
- Tanner, L.; Denti, P.; Wiesner, L.; Warner, D.F. Drug permeation and metabolism in Mycobacterium tuberculosis: Prioritising local exposure as essential criterion in new TB drug development. IUBMB Life 2018, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Jarlier, V.; Nikaido, H. Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. J. Bacteriol. 1990, 172, 1418–1423. [Google Scholar] [CrossRef]
- Trias, J.; Benz, R. Permeability of the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 1994, 14, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, M.; Kim, P.; Myung, P.K. 3D-QSAR and cell wall permeability of antitubercular nitroimidazoles against Mycobacterium tuberculosis. Molecules 2013, 18, 13870–13885. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Hopfinger, A.J. Molecular modeling and simulation of Mycobacterium tuberculosis cell wall permeability. Biomacromolecules 2004, 5, 1066–1077. [Google Scholar] [CrossRef]
- Merget, B.; Zilian, D.; Müller, T.; Sotriffer, C.A. MycPermCheck: The Mycobacterium tuberculosis permeability prediction tool for small molecules. Bioinformatics 2013, 29, 62–68. [Google Scholar] [CrossRef]
- Ekins, S.; Bradford, J.; Dole, K.; Spektor, A.; Gregory, K.; Blondeau, D.; Hohman, M.; Bunin, B.A. A collaborative database and computational models for tuberculosis drug discovery. Mol. Biosyst. 2010, 6, 840–851. [Google Scholar] [CrossRef]
- Irwin, J.J.; Shoichet, B.K. ZINC–A free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef]
- Janardhan, S.; Ram Vivek, M.; Sastry, G.N. Modeling the permeability of drug-like molecules through the cell wall of Mycobacterium tuberculosis: An analogue based approach. Mol. Biosyst. 2016, 12, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Nagamani, S.; Sastry, G.N. Mycobacterium tuberculosis cell wall permeability model generation using chemoinformatics and machine learning approaches. ACS Omega 2021, 6, 17472–17482. [Google Scholar] [CrossRef] [PubMed]
- Zefirov, N.S.; Palyulin, V.A. Fragmental approach in QSPR. J. Chem. Inf. Comput. Sci. 2002, 42, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, N.V.; Baskin, I.I.; Palyulin, V.A.; Zefirov, N.S. Artificial neural network and fragmental approach in prediction of physicochemical properties of organic compounds. Russ. Chem. Bull. 2003, 52, 20–29. [Google Scholar] [CrossRef]
- Artemenko, N.V.; Baskin, I.I.; Palyulin, V.A.; Zefirov, N.S. Prediction of physical properties of organic compounds using artificial neural networks within the substructure approach. Dokl. Chem. 2001, 381, 317–320. [Google Scholar] [CrossRef]
- Radchenko, E.V.; Dyabina, A.S.; Palyulin, V.A.; Zefirov, N.S. Prediction of human intestinal absorption of drug compounds. Russ. Chem. Bull. 2016, 65, 576–580. [Google Scholar] [CrossRef]
- Dyabina, A.S.; Radchenko, E.V.; Palyulin, V.A.; Zefirov, N.S. Prediction of blood-brain barrier permeability of organic compounds. Dokl. Biochem. Biophys. 2016, 470, 371–374. [Google Scholar] [CrossRef]
- Radchenko, E.V.; Dyabina, A.S.; Palyulin, V.A. Towards deep neural network models for the prediction of the blood-brain barrier permeability for diverse organic compounds. Molecules 2020, 25, 5901. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, E.V.; Rulev, Y.A.; Safanyaev, A.Y.; Palyulin, V.A.; Zefirov, N.S. Computer-aided estimation of the hERG-mediated cardiotoxicity risk of potential drug components. Dokl. Biochem. Biophys. 2017, 473, 128–131. [Google Scholar] [CrossRef]
- Artemenko, N.V.; Palyulin, V.A.; Zefirov, N.S. Neural-network model of the lipophilicity of organic compounds based on fragment descriptors. Dokl. Chem. 2002, 383, 114–116. [Google Scholar] [CrossRef]
- Berishvili, V.P.; Kuimov, A.N.; Voronkov, A.E.; Radchenko, E.V.; Kumar, P.; Choonara, Y.E.; Pillay, V.; Kamal, A.; Palyulin, V.A. Discovery of novel tankyrase inhibitors through molecular docking-based virtual screening and molecular dynamics simulation studies. Molecules 2020, 25, 3171. [Google Scholar] [CrossRef]
- Vasilenko, D.A.; Sadovnikov, K.S.; Sedenkova, K.N.; Karlov, D.S.; Radchenko, E.V.; Grishin, Y.K.; Rybakov, V.B.; Kuznetsova, T.S.; Zamoyski, V.L.; Grigoriev, V.V.; et al. A facile approach to bis(isoxazoles), promising ligands of the AMPA receptor. Molecules 2021, 26, 6411. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Lushchekina, S.V.; Astakhova, T.Y.; Serkov, I.V.; Proshin, A.N.; Radchenko, E.V.; Palyulin, V.A.; et al. Bis-amiridines as acetylcholinesterase and butyrylcholinesterase inhibitors: N-Functionalization determines the multitarget anti-Alzheimer’s activity profile. Molecules 2022, 27, 1060. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Zverev, D.V.; Nazarova, A.A.; Lavrov, M.I.; Radchenko, E.V.; Grishin, Y.K.; Gabrel’yan, A.V.; Zamoyski, V.L.; Grigoriev, V.V.; Averina, E.B.; et al. Novel nanomolar allosteric modulators of AMPA receptor of bis(pyrimidine) series: Synthesis, biotesting and SAR analysis. Molecules 2022, 27, 8252. [Google Scholar] [CrossRef]
- Elkina, N.A.; Grishchenko, M.V.; Shchegolkov, E.V.; Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Radchenko, E.V.; et al. New multifunctional agents for potential Alzheimer’s disease treatment based on tacrine conjugates with 2-arylhydrazinylidene-1,3-diketones. Biomolecules 2022, 12, 1551. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Tetko, I.V.; Engkvist, O.; Koch, U.; Reymond, J.-L.; Chen, H. BIGCHEM: Challenges and opportunities for Big Data analysis in chemistry. Mol. Inform. 2016, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but verify: On the importance of chemical structure curation in cheminformatics and QSAR modeling research. J. Chem. Inf. Model. 2010, 50, 1189–1204. [Google Scholar] [CrossRef]
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but verify II: A practical guide to chemogenomics data curation. J. Chem. Inf. Model. 2016, 56, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Baskin, I.I.; Halberstam, N.M.; Artemenko, N.V.; Palyulin, V.A.; Zefirov, N.S. NASAWIN–A universal software for QSPR/QSAR studies. In EuroQSAR 2002 Designing Drugs and Crop Protectants: Processes, Problems and Solutions; Ford, M., Livingstone, D., Dearden, J., van de Waterbeemd, H., Eds.; Blackwell Science Inc.: New York, NY, USA, 2003; pp. 260–263. ISBN 978-1-4051-2516-1. [Google Scholar]
- Filzmoser, P.; Liebmann, B.; Varmuza, K. Repeated double cross validation. J. Chemom. 2009, 23, 160–171. [Google Scholar] [CrossRef]
- Klambauer, G.; Unterthiner, T.; Mayr, A.; Hochreiter, S. Self-normalizing neural networks. In Proceedings of the 31st International Conference on Neural Information Processing Systems: NIPS’17, Long Beach, CA, USA, 4–9 December 2017; Curran Associates Inc.: Red Hook, NY, USA, 2017; pp. 972–981, ISBN 978-1-5108-6096-4. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A Next-Generation Hyperparameter Optimization Framework. Available online: http://arxiv.org/abs/1907.10902 (accessed on 18 December 2022).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]

| Dataset | MtbPen8242 | MtbPen5371ad | |||
|---|---|---|---|---|---|
| Predicted | Predicted | ||||
| Positive | Negative | Positive | Negative | ||
| Observed | Positive | 1435 | 1236 | 2214 | 457 |
| Negative | 528 | 5043 | 437 | 2263 | |
| AID 1 | ID | Type | Activity/Compound Count 2 | Description | Activity Condition 3 |
|---|---|---|---|---|---|
| 1332 | C01 | Cell | 1118 | High throughput screen to identify inhibitors of Mycobacterium tuberculosis H37Rv | Inh30 |
| 1626 | C02 | Cell | 215,397 | High throughput screen to identify inhibitors of Mycobacterium tuberculosis H37Rv | Inh30 |
| 1949 | C03 | Cell | 100,697 | High throughput screen of 100,000 compound library to identify inhibitors of Mycobacterium tuberculosis H37Rv | Inh30 |
| 2842 | C04 | Cell | 23,823 | High throughput screen of a putative kinase compound library to identify inhibitors of Mycobacterium tuberculosis H37Rv | Inh30 |
| 449762 | C05 | Cell | 327,669 | High throughput screening assay used to identify novel compounds that inhibit Mycobacterium tuberculosis in 7H9 media | Inh30 |
| 1259343 | C06 | Cell | 6225 | High throughput screening of small molecules that kill Mycobacterium tuberculosis | Inh30 |
| 1259417 | C07 | Cell | 1105 | High throughput whole cell screen to identify inhibitors of Mycobacterium tuberculosis | Inh30 |
| 1671161 | C08 | Cell | 96,022/86,588 | Phenotypic growth assay for Mycobacterium tuberculosis grown for 4 days on DPPC, cholesterol, tyloxapol-based media | Inh30 |
| 1671162 | C09 | Cell | 103,984/86,574 | Phenotypic growth assay for Mycobacterium tuberculosis grown for 3 days on 7H9, glucose tyloxapol-based media | Inh30 |
| 1671174 | C10 | Cell | 53,171/53,165 | Phenotypic assay to identify agents that inhibit growth of Mycobacterium tuberculosis | Inh30 |
| 488890 | C11 | Cell | 324,545 | Elucidation of physiology of non-replicating, drug-tolerant Mycobacterium tuberculosis | Inh30 |
| 375 | T01 | Target | 10,011/10,009 | Mycobacterium tuberculosis pantothenate synthetase assay | Outcome |
| 1376 | T02 | Target | 216,162/215,860 | Inhibitors of mycobacterial glucosamine-1-phosphate acetyl transferase (GlmU) | Outcome |
| 2606 | T03 | Target | 324,858/324,747 | Primary biochemical high throughput screening assay to identify inhibitors of the membrane-associated serine protease Rv3671c in M. tuberculosis | Outcome |
| 504406 | T04 | Target | 324,148/324,048 | High throughput screening of inhibitors of Mycobacterium tuberculosis UDP-galactopyranose mutase (UGM) enzyme | Outcome |
| 540299 | T05 | Target | 103,205/102,628 | A screen for compounds that inhibit the MenB enzyme of Mycobacterium tuberculosis | Outcome |
| 588335 | T06 | Target | 356,407/356,160 | Counterscreen for inhibitors of the fructose-bisphosphate aldolase (FBA) of M. tuberculosis | Outcome |
| 602481 | T07 | Target | 356,486/353,572 | Mycobacterium tuberculosis BioA enzyme inhibitor | Outcome |
| 1159583 | T08 | Target | 301,203/300,060 | High throughput screen for small molecule inhibitors of a hypoxia-regulated fluorescent biosensor in Mycobacterium tuberculosis | Outcome |
| 1671160 | T09 | Target | 8874/8841 | Assay for Asp RNA synthetase-1 from Mycobacterium tuberculosis | Inh30 |
| 1671178 | T10 | Target | 67,199/66,591 | Mycobacterium tuberculosis polyketide synthase 13 thioesterase (PKS13) | Inh30 |
| 2221 | T11 | Target | 293,466/293,376 | Cell-free homogenous primary high throughput screen to identify inhibitors of RecA intein splicing activity | Outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radchenko, E.V.; Antonyan, G.V.; Ignatov, S.K.; Palyulin, V.A. Machine Learning Prediction of Mycobacterial Cell Wall Permeability of Drugs and Drug-like Compounds. Molecules 2023, 28, 633. https://doi.org/10.3390/molecules28020633
Radchenko EV, Antonyan GV, Ignatov SK, Palyulin VA. Machine Learning Prediction of Mycobacterial Cell Wall Permeability of Drugs and Drug-like Compounds. Molecules. 2023; 28(2):633. https://doi.org/10.3390/molecules28020633
Chicago/Turabian StyleRadchenko, Eugene V., Grigory V. Antonyan, Stanislav K. Ignatov, and Vladimir A. Palyulin. 2023. "Machine Learning Prediction of Mycobacterial Cell Wall Permeability of Drugs and Drug-like Compounds" Molecules 28, no. 2: 633. https://doi.org/10.3390/molecules28020633
APA StyleRadchenko, E. V., Antonyan, G. V., Ignatov, S. K., & Palyulin, V. A. (2023). Machine Learning Prediction of Mycobacterial Cell Wall Permeability of Drugs and Drug-like Compounds. Molecules, 28(2), 633. https://doi.org/10.3390/molecules28020633





