Biologically Oriented Hybrids of Indole and Hydantoin Derivatives
Abstract
1. Introduction
2. Results and Discussion
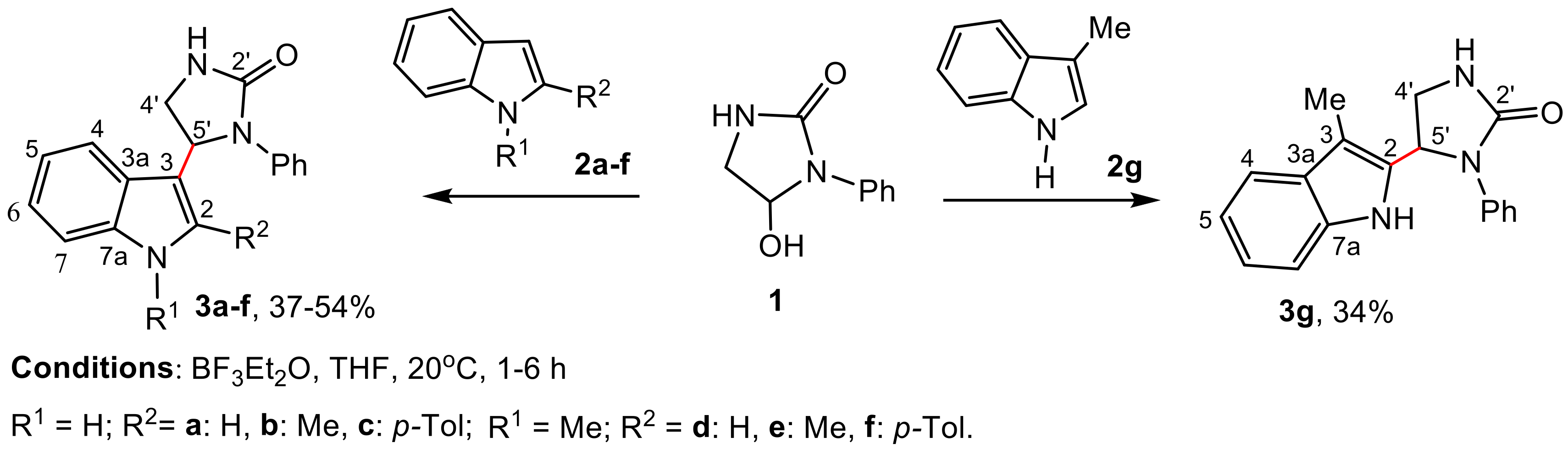
2.1. Synthesis
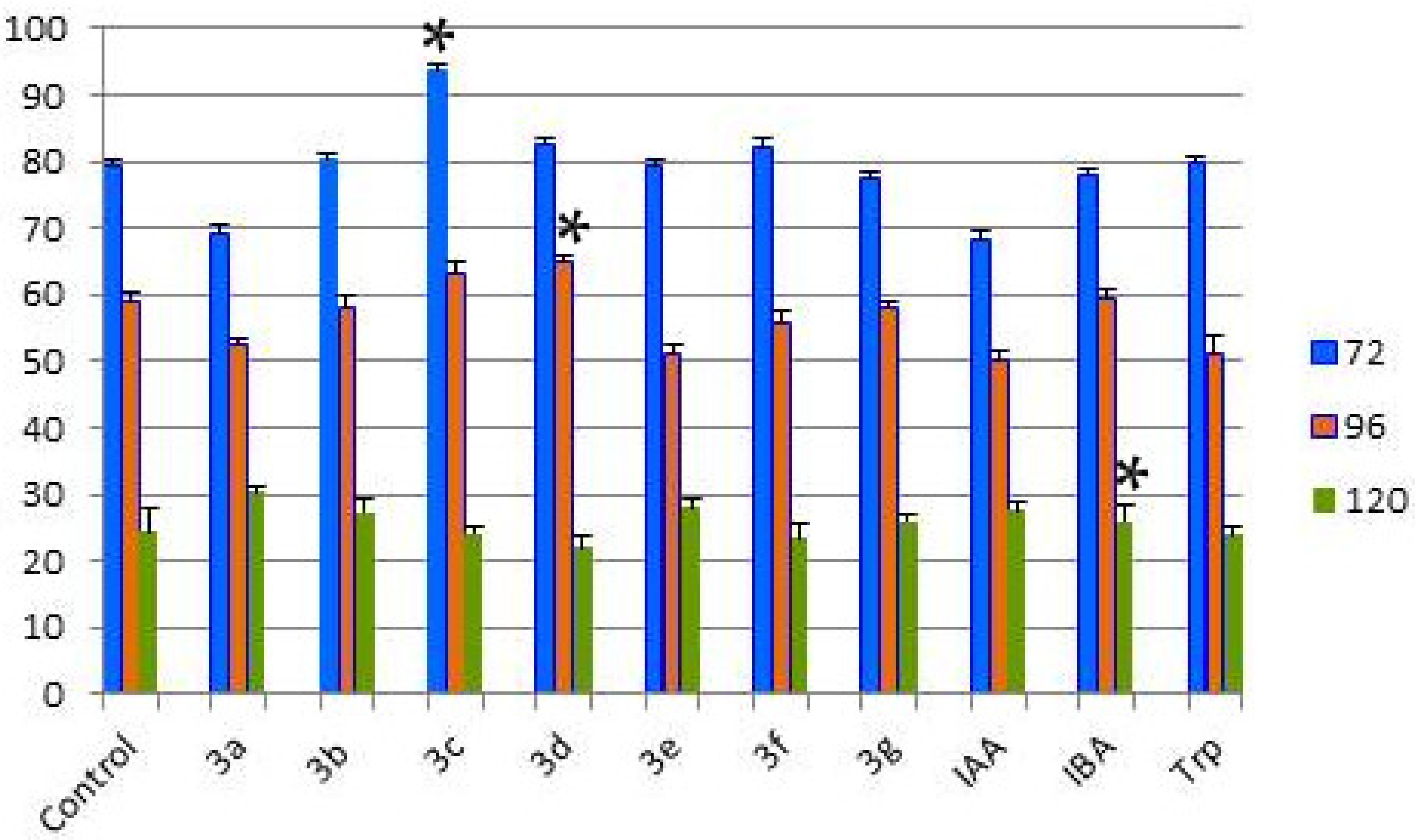
2.2. Investigation of the Influence of Hydantoin-Indole Hybrids on Wheat Seed Germination
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Reaction of Indoles 2 with Phenylimidazolidin-2-One, 1 (General Procedure)
3.2.1. 5-(1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3a)
3.2.2. 5-(2-Methyl-1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3b)
3.2.3. 5-(2-p-Tolyl-1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3c)
3.2.4. 5-(1-Methy-1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3d)
3.2.5. 5-(1,2-Dimethyl-1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3e)
3.2.6. 5-(1-Methyl-2-p-Tolyl-1H-Indol-3-yl)-1-Phenylimidazolidin-2-One (3f)
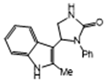
3.2.7. 5-(3-Methyl-1H-Indol-2-yl)-1-Phenylimidazolidin-2-One (3g)
3.3. Investigation of The Growth-Regulating Activity of Compounds 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent Advances in the Synthesis of Hydantoins: The State of the Art of a Valuable Scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Designed Synthesis of Diversely Substituted Hydantoins and Hydantoin-Based Hybrid Molecules: A Personal Account. Synlett 2021, 32, 1897–1910. [Google Scholar] [CrossRef]
- Gawas, P.P.; Ramakrishna, B.; Veeraiah, N.; Nutalapati, V. Multifunctional hydantoins: Recent advances in optoelectronics and medicinal drugs from Academia to the chemical industry. J. Mater. Chem. C. 2021, 9, 16341–16377. [Google Scholar] [CrossRef]
- Lee, T.H.; Khan, Z.; Kim, S.Y.; Lee, K.R. Thiohydantoin and Hydantoin Derivatives from the Roots of Armoracia rusticana and Their Neurotrophic and Antineuroinflammatory Activities. J. Nat. Prod. 2019, 82, 3020–3024. [Google Scholar] [CrossRef]
- Abida; Alam, M.T.; Asif, M. Study of Some Hyndantion Derivatives as Anticonvulsant Agents. Prog. Chem. Biochem. Res. 2020, 3, 93–104. [Google Scholar] [CrossRef]
- Su, M.; Xia, D.; Teng, P.; Nimmagadda, A.; Zhang, C.; Odom, T.; Cao, A.; Hu, Y.; Cai, J. Membrane-Active Hydantoin Derivatives as Antibiotic Agents. J. Med. Chem. 2017, 60, 8456–8465. [Google Scholar] [CrossRef]
- Fujisaki, F.; Toyofuku, K.; Egami, M.; Ishida, S.; Nakamoto, N.; Kashige, N.; Miake, F.; Sumoto, K. Antibacterial Activity of Some 5-Dialkylaminomethyl hydantoins and Related Derivatives. Chem. Pharm. Bull. 2013, 61, 1090–1093. [Google Scholar] [CrossRef]
- Lin, X.; Tago, K.; Okazaki, N.; So, T.; Takahashi, K.; Mashino, T.; Tamura, H.; Funakoshi-Tago, M. The indole-hydantoin derivative exhibits anti-inflammatory activity by preventing the transactivation of NF-κB through the inhibition of NF-κBp65 phosphorylation at Ser276. Int. Immunopharmacol. 2021, 100, 108092. [Google Scholar] [CrossRef]
- Gazizov, A.S.; Smolobochkin, A.V.; Kuznetsova, E.A.; Abdullaeva, D.S.; Burilov, A.R.; Pudovik, M.A.; Voloshina, A.D.; Syakaev, V.V.; Lyubina, A.P.; Amerhanova, S.K.; et al. The Highly Regioselective Synthesis of Novel Imidazolidin-2-Ones via the Intramolecular Cyclization/Electrophilic Substitution of Urea Derivatives and the Evaluation of Their Anticancer Activity. Molecules 2021, 26, 4432. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Hemimycalins C–E; Cytotoxic and Antimicrobial Alkaloids with Hydantoin and 2-Iminoimidazolidin-4-one Backbones from the Red Sea Marine Sponge Hemimycale sp. Mar. Drugs. 2021, 19, 691. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.N.U.; Gnanamani, S.; Shanmugam, P.; Venugopal, S.; Murthy, S.; Ramasamy, B. Synthesis, antioxidant, and antimicrobial activity of 3-(1H-indole-3-carbonyl)- 2H-chromen-2-ones. J. Heterocycl. Chem. 2021, 58, 2000–2008. [Google Scholar] [CrossRef]
- Tiwari, S.; Kirar, S.; Banerjee, U.C.; Neerupudi, K.B.; Singh, S.; Wani, A.A.; Bharatam, P.V.; Singh, I.P. Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents. Bioorg. Chem. 2020, 99, 103787. [Google Scholar] [CrossRef] [PubMed]
- Konus, M.; Çetin, D.; Kızılkan, N.D.; Yılmaz, C.; Fidan, C.; Algso, M.; Kavak, E.; Kivrak, A.; Kurt-Kızıldogan, A.; Otur, Ç.; et al. Synthesis and biological activity of new indole based derivatives as potent anticancer, antioxidant and antimicrobial agents. J. Mol. Structure. 2022, 1263, 133168. [Google Scholar] [CrossRef]
- Bonvicini, F.; Locatelli, A.; Morigi, R.; Leoni, A.; Gentilomi, G.A. Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity. Molecules 2022, 27, 5781. [Google Scholar] [CrossRef]
- Champciaux, B.; Raynaud, C.; Viljoen, A.; Chene, L.; Thibonnet, J.; Vincent, S.P.; Kremer, L.; Thiery, E. Synthesis and biological evaluation of 3,4-dihydro-1H-[1,4] oxazepino [6,5,4-hi]indol-1-ones and 4,6-dihydrooxepino [5,4,3-cd]indol-1(3H)-ones as Mycobacterium tuberculosis inhibitors. Bioorg. Med. Chem. 2021, 43, 116248. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic Prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Stec, J.; Onajole, O.K.; Lun, S.; Guo, H.; Merenbloom, B.; Vistoli, G.; Bishai, W.R.; Kozikowski, A.P. Indole-2-carboxamide-based MmpL3 Inhibitors Show Exceptional Antitubercular Activity in an Animal Model of Tuberculosis Infection. J. Med. Chem. 2016, 59, 6232–6247. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef]
- Pecnard, S.N.; Hamze, A.L.; Bignon, J.M.; Prost, B.T.; Deroussent, A.; Gallego-Yerga, L.; Pelaez, R.; Paik, J.; Diederich, M.; Alami, M.; et al. Anticancer properties of indole derivatives as Iso Combretastatin A-4 analogues. Eur. J. Med. Chem. 2021, 223, 113656. [Google Scholar] [CrossRef]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg. Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Sharma, R.; Singh, R.K. Target-based anticancer indole derivatives and insight into structure-activity relationship: A mechanistic review update (2018–2021). Acta Pharm. Sin. B. 2022, 12, 3006–3027. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Sharma, V.; Verma, A.; Venugopal, S.; Mittal, A.; Singh, G.; Kaur, B. Indole Derived Anticancer Agents. Chem. Sel. 2022, 7, e202202361. [Google Scholar] [CrossRef]
- Sevinçli, Z.; Duran, G.N.; Özbil, M.; Karalı, N. Synthesis, molecular modeling and antiviral activity of novel 5-fluoro-1H-indole-2,3-dione 3-thiosemicarbazones. Bioorg. Chem. 2020, 104, 104202. [Google Scholar] [CrossRef]
- Garg, V.; Maurya, R.K.; Thanikachalam, P.V.; Monga, V. An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem. 2019, 180, 562–612. [Google Scholar] [CrossRef]
- Li, T.; Xu, H. Recent Progress of Bioactivities, Mechanisms of Action, Total Synthesis, Structural Modifications and Structure-activity Relationships of Indole Derivatives: A Review. Mini-Rev. Med. Chem. 2022, 22, 2702–2725. [Google Scholar] [CrossRef]
- Nisha Singh, S.; Sharma, N.; Chandra, R. The indole nucleus as a selective COX-2 inhibitor and anti-inflammatory agent (2011–2022). Org. Chem. Front. 2022, 9, 3624–3639. [Google Scholar] [CrossRef]
- Wang, C.; Fan, L.; Pan, Z.; Fan, S.; Shi, L.; Li, X.; Zhao, J.; Wu, L.; Yang, G.; Xu, C. Synthesis of Novel Indole Schiff Base Compounds and Their Antifungal Activities. Molecules 2022, 27, 6858. [Google Scholar] [CrossRef]
- Pagniez, F.; Lebouvier, N.; Na, Y.M.; Ourliac-Garnier, I.; Picot, C.; Le Borgne, M. Le Pape, P. Biological exploration of a novel 1,2,4-triazole-indole hybrid molecule as antifungal agent. J. Enzym. Inhib. Med. Chem. 2020, 35, 398–403. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Jia, C.-Y.; Gu, Y.-C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.-H.; Yang, G.-F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef]
- Chauhan, M.; Saxena, A.; Saha, B. An insight in anti-malarial potential of indole scaffold: A review. Eur. J. Med. Chem. 2021, 218, 113400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Jawaid Akhtar, M.; Al Balushi, K.A.; Khan, S.A. Rational drug design strategies for the development of promising multi-target directed indole hybrids as Anti-Alzheimer agents. Bioorg. Chem. 2022, 127, 105941. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-L.; Liu, J.; Fang, W.-Y.; Ravindar, L.; Rakesh, K. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 194, 112245. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef]
- Korth, C.; Klingenstein, R.; Müller-Schiffmann, A. Hybrid Molecules Synergistically Acting Against Protein Aggregation Diseases. Curr. Top. Med. Chem. 2013, 13, 2484–2490. [Google Scholar] [CrossRef]
- Reader, J.C.; Matthews, T.P.; Klair, S.; Cheung, K.M.; Scanlon, J.; Proisy, N.; Addison, G.; Ellard, J.; Piton, N.; Taylor, S.; et al. Structure-Guided Evolution of Potent and Selective CHK1 Inhibitors through Scaffold Morphing. J. Med. Chem. 2011, 54, 8328–8342. [Google Scholar] [CrossRef]
- Lewis, R.; Bagnall, A.M.; Leitner, M. Sertindole for schizophrenia. Cochrane Database Syst. Rev. 2005, 3, CD001715. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.; Ranganathan, R.; Dinesh, M.; Arul, P.; Ponnuswamy, A.; Kalaiselvi, P.; Chellammal, S.; Subramanian, G. Design, Synthesis, and Antibacterial Studies of Potent Pyrazolinyltriazoles. Res. Chem. Intermed. 2017, 43, 2471–2490. [Google Scholar] [CrossRef]
- Bhatia, R.; Vyas, A.; El-Bahy, S.M.; Hessien, M.M.; Mersal, G.A.M.; Ibrahim, M.M.; Dogra, R.; Kumar, B. Rationale Design, Synthesis, Pharmacological and In-silico Investigation of Indole-Functionalized Isoxazoles as Anti-inflammatory Agents. Chem. Select 2022, 7, e202200800. [Google Scholar] [CrossRef]
- Husain, A.; Alasmari, A.F.; Azmi, S.N.H.; Ali, N.; Sarker, M.M.R.; Alharbi, M.; Ishtikhar, M.; Khan, S.A. Rational drug design, synthesis, and in vivo biological activity of new indolyl-imidazolone hybrids as potential and safer non-steroidal anti-inflammatory agents. J. King Saud Univers. Sci. 2022, 34, 102023. [Google Scholar] [CrossRef]
- Nazir, M.; Abbasi, M.A.; Aziz-ur-Rehman; Siddiqui, S.Z.; Khan, K.M.; Kanwal; Salar, U.; Shahid, M.; Ashraf, M.; Lodhi, M.A.; et al. New indole based hybrid oxadiazole scaffolds with N-substituted acetamides: As potent anti-diabetic agents. Bioorg. Chem. 2018, 81, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Lal, K.; Kumar, A.; Kumar, A.; Kumar, D. Indole-chalcone linked 1,2,3-triazole hybrids: Facile synthesis, antimicrobial evaluation and docking studies as potential antimicrobial agents. J. Mol. Struct. 2022, 1261, 132867. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, Synthesis, Anticancer Evaluation, Enzymatic Assays, and a Molecular Modeling Study of Novel Pyrazole–Indole Hybrids. ACS Omega. 2021, 6, 12361–12374. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn-Dylevycha, A.; Radkob, L.; Finiukc, N.; Garazd, M.; Kashchak, N.; Posyniak, A.; Niemczuk, K.; Stoika, R.; Lesyk, R. Synthesis of novel indole-thiazolidinone hybrid structures as promising scaffold with anticancer potential. Bioorg. Med. Chem. 2021, 50, 116453. [Google Scholar] [CrossRef]
- Hawash, M.; Kahraman, D.C.; Ergun, S.G.; Cetin-Atalay, R.; Baytas, S.N. Synthesis of novel indole-isoxazole hybrids and evaluation of their cytotoxic activities on hepatocellular carcinoma cell lines. BMC Chem. 2021, 15, 66–79. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Li, G.; Zhang, Z.; Ma, L.; Cheng, B.; Chen, J. Discovery of Novel Benzimidazole and Indazole Analogues as Tubulin Polymerization Inhibitors with Potent Anticancer Activities. J. Med. Chem. 2021, 64, 4498–4515. [Google Scholar] [CrossRef]
- Al-Hussain, S.A.; Farghaly, T.A.; Zaki, M.E.A.; Abdulwahab, H.G.; Al-Qurashi, N.T.; Muhammad, Z.A. Discovery of novel indolyl-1,2,4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity. Bioorg. Chem. 2020, 105, 104330. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, J.K.; Guo, W.; Yuan, T. Strangulation and IBA treatments as an effective method to propagate tree peonies, Paeonia suffruticosa. Sci. Hortic. 2023, 307, 111487. [Google Scholar] [CrossRef]
- Shaistul, I.; Firoz, M. Plant growth regulators modulate photosynthetic efficiency, antioxidant system, root cell viability and nutrient acquisition to promote growth, yield and quality of Indian mustard. Acta Physiologiae Plant. 2022, 44, 132. [Google Scholar] [CrossRef]
- Šípošová, K.; Labancová, E.; Kučerová, D.; Kollárová, K.; Vivodová, Z. Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium. Plants. 2021, 10, 2503. [Google Scholar] [CrossRef] [PubMed]
- Arif, U.; Hussain, K.; Nawaz, K. Improvement of wheat (Triticum aestivum l.) Productivity with the applications of plant growth regulators. Pak. J. Bot. 2022, 54, 1–11. [Google Scholar] [CrossRef]
- Tahoun, A.M.M.A.; El-Enin, M.M.A.; Mancy, A.G.; Sheta, M.H.; Shaaban, A. Integrative Soil Application of Humic Acid and Foliar Plant Growth Stimulants Improves Soil Properties and Wheat Yield and Quality in Nutrient-Poor Sandy Soil of a Semiarid Region. J. Soil Sci. Plant Nutr. 2022, 22, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Bowden, A.T.; Knight, P.R.; Ryals, J.B.; Coker, C.E.H.; Langlois, S.A.; Broderick, S.R.; Blythe, E.K.; Sakhanokho, H.F.; Babiker, E.M. Evaluation of One-Time Applications of Foliar Applied Auxin Co-Applied with Surfactant for Use in Commercial Cutting Propagation. Agronomy 2022, 12, 2243. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 Expression Is Required for Auxin-Induced Adventitious Root Formation via MxSPL26 Independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Shu, W.; Zhou, H.; Jiang, C.; Zhao, S.; Wang, L.; Li, Q.; Yang, Z.; Groover, A.; Lu, M.Z. The auxin receptor TIR1 homolog (PagFBL 1) regulates adventitious rooting through interactions with Aux/IAA28 in Populus. Plant Biotechnol. J. 2019, 17, 338–349. [Google Scholar] [CrossRef]
- Abotlasha, J.J.; Issa, F.H.; Al-Burki, F.R. Effect of Auxin Spraying and Plant Extracts on Two Cultivars of Bean (Vicia Faba L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 923, 012009. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of Auxin in the Growth, Development, and Stress Tolerance of Horticultural. Plants Cells 2022, 11, 2761. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Kong, X.; Chen, Y.; Li, J. Exogenous tryptophan application improves cadmium tolerance and inhibits cadmium upward transport in broccoli (Brassica oleraceavar. italica). Front. Plant Sci. 2022, 13, 969675. [Google Scholar] [CrossRef]
- Li, K.; Shi, D.Q. Synthesis and herbicidal activity of 3-aryl-1-[2-(aryloxy)propanoyl]imidazolidine-2,4-diones. J. Heterocycl. Chem. 2009, 46, 544–547. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Z.; Song, H.; Liu, Y.; Wang, L.; Wang, Q. Design, Synthesis, and Biological Activity of β-Carboline Analogues Containing Hydantoin, Thiohydantoin, and Urea Moieties. J. Agric. Food Chem. 2018, 66, 8253–8261. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Hao, Y.H.; Song, H.S.; Liu, Y.; Li, Y.; Zhang, J.; Wang, Q. Design, synthesis, characterization, and biological activities of novel spirooxindole analogues containing hydantoin, thiohydantoin, urea, and thiourea moieties. J. Agric. Food Chem. 2020, 68, 10618–1062530. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.S. Pesticidal effects of some imidazolidine and oxazolone derivatives. World J. Agric. Sci. 2009, 5, 105–113. [Google Scholar]
- Liu, W.; Zhang, S.; Xiao, L.; Wan, Y.; He, L.; Wang, K.; Qi, Z.; Li, X. Synthesis and biological activity of novel hydantoin cyclohexyl sulfonamide derivatives as potential antimicrobial agents in agriculture. Pest. Manag. Sci. 2022, 78, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Sviridova, L.A.; Protopopova, P.S.; Akimov, M.G.; Dudina, P.V.; Melnikova, E.K.; Kochetkov, K.A. Synthesis of new physiologically active (2-oxoimidazolidin-5-yl)indoles. Mendeleev Commun. 2020, 30, 347–349. [Google Scholar] [CrossRef]
- Karabelas, K.; Lepisto, M.; Sjo, P. WO 9932483. Chem. Abstr. 1999, 131, 58823. [Google Scholar]
- Gorunova, O.N. Modification of Heterocycles by Amidoalkylation. Ineos Open 2021, 4, 90–102. [Google Scholar] [CrossRef]
- Cortes, S.; Kohn, H. Selective Reductions of 3-Substituted Hydantoins to 4-Hydroxy- 2-imidazolidinones and Vicinal Diamines. J. Org. Chem. 1983, 48, 2246–2254. [Google Scholar] [CrossRef]
- Sviridova, L.A.; Aganas’eva, S.V.; Golubeva, G.A.; Terent’ev, P.B.; Bundel, Y.G. Introduction of the Pyrazolidine Ring in to the Pyrrole Ring of Indole. Chem. Heterocycl. Comp. 1990, 26, 1008–1012. [Google Scholar] [CrossRef]
- Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N.A. 5-Hydroxy-1-phenylimidazolidine-2-thione as a new amidoalkylating agent for heterocyclic compounds. Russ. Chem. Bull 2022, 71, 587–590. [Google Scholar] [CrossRef]
- Gorunova, O.N.; Bystrova, N.A.; Kochetkov, K.A. Synthesis of Bis-Heterocyclic Derivatives of Thiohydantoin. Ineos Open 2021, 4, 140–143. [Google Scholar] [CrossRef]
- Morales-Rios, M.S.; Espiieira, J.; Joseph-Nathan, P. 13C NMR Spectroscopy of Indole Derivatives. Magn. Reson. Chem. 1987, 25, 377–395. [Google Scholar] [CrossRef]
- Morales-Rios, M.S.; del Rio, R.E.; Joseph-Nathan, P. Unambiguous Assignment of the 13C NMR Spectra of Methylindole. Magn. Reson. Chem. 1988, 26, 552–558. [Google Scholar] [CrossRef]
- Safina, G.F.; Filipenko, G.I. Effect of heteroauxin and amber acid on germinating ability of wheat seeds after their durable storage. Biology 2018, 11, 143–147. [Google Scholar] [CrossRef]
- State Register of Selection Achievements Approved for Use. V.1. “Varieties of Plants” (official edition). FGBNU “Rosinformagrotech”. Moscow 2021, 1, 719. [Google Scholar]
- Dutta, P.; Bandopadhyay, P.; Bera, A. Identification of Leaf Based Physiological Markersfor Drought Susceptibility during Early Seedling Development of Mungbean. Amer. J. Plant Sci. 2016, 7, 1921–1936. [Google Scholar] [CrossRef]
- ISTA. Chapter 1: International Rules for Seed Testing. Certificates 2022, 1, 1–14. [Google Scholar]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Wang, R.; Zhao, Y. Recent advances in auxin research in rice and their implications for crop improvement. J. Exp. Bot. 2018, 69, 255–263. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf relative water content in Physiological Breeding II: A Field Guide to Wheat Phenotyping. In The International Maize and Wheat Improvement Center; Chapter 5; Pask, A., Pietragalla, J., Mullan, D., Reynolds, M., Eds.; CIMMYT: México-Veracruz, Mexico, 2012; Volume 25, ISBN 978-970-648-182-5. [Google Scholar]
- Sviridova, L.A.; Golubeva, G.A.; Tavtorkin, A.N.; Nelyubina, Y.V.; Kochetkov, K.A. Diastereoselective Reductive Amination of Pyrazolidinyl Alkyl Ketones. Chem. Heterocycl. Comp. 2008, 44, 542–548. [Google Scholar] [CrossRef]
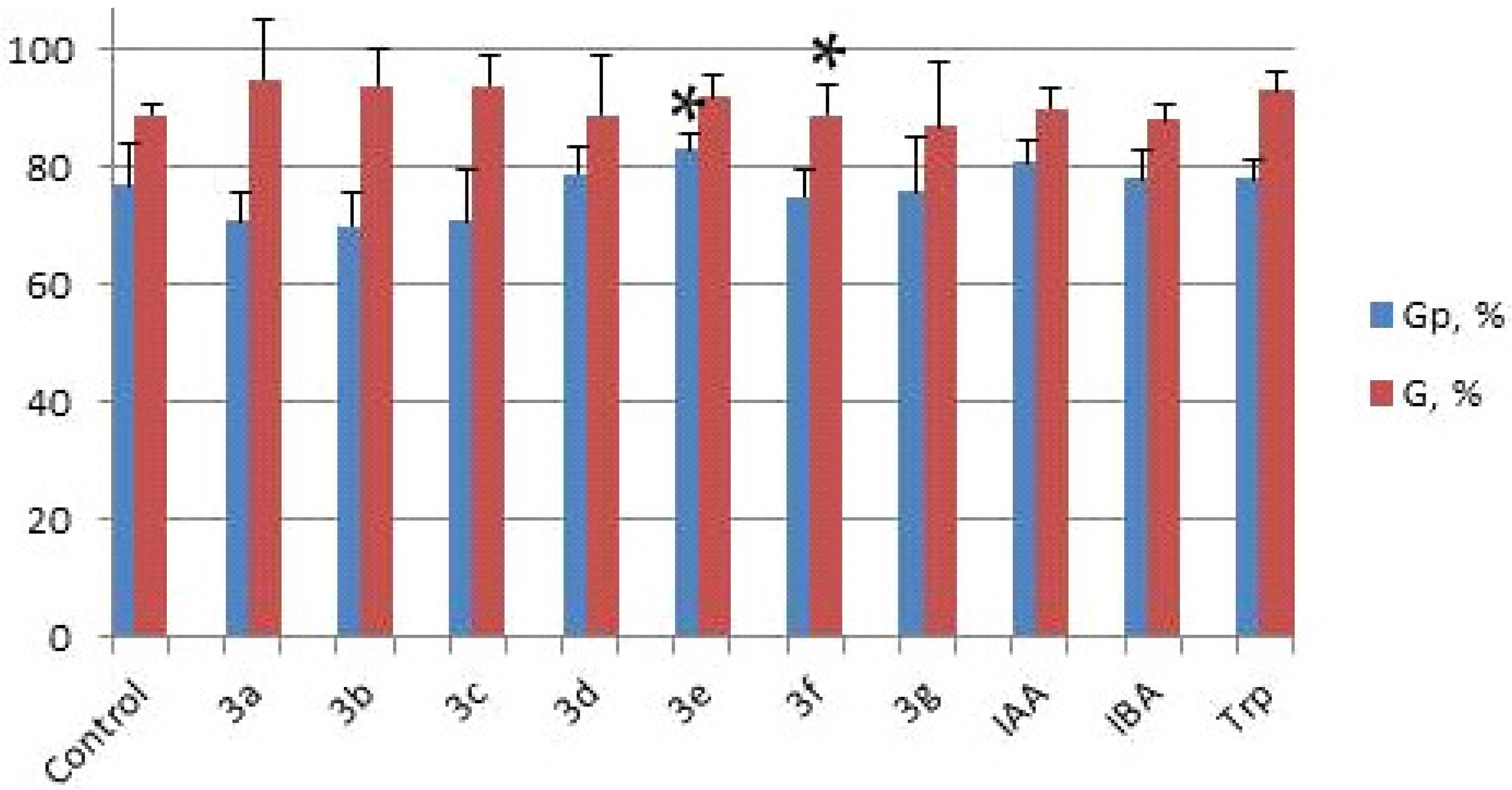
| Formula | Compound № | Germination Potential Gp, % | Germination G, % | Primary Root Length, cm | Roots Number | Lateral Roots Length, cm |
|---|---|---|---|---|---|---|
| - | Control | 77 | 89 | - | 4 | 5.4 |
 | 3a | 71 | 95 | 10.7 * | 6 | 7.1 * |
 | 3b | 70 | 94 | 10.0 | 6 | 6.8 |
 | 3c | 71 | 94 | 9.6 | 6 | 6.7 |
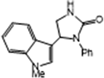 | 3d | 79 | 89 | 8.1 | 6 | 6.3 |
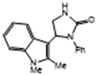 | 3e | 83 * | 92 | 9.9 | 6 | 6.5 |
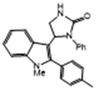 | 3f | 75 | 89 * | 10.1 | 6 | 6.5 * |
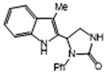 | 3g | 76 | 87 | 9.5 * | 6 | 6.8 |
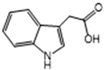 | IAA | 81 | 90 | 9.8 | 6 | 6.7 |
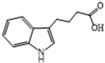 | IBA | 78 | 88 | 8.8 | 6 | 6.4 |
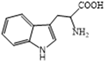 | Trp | 78 | 93 | 8.0 | 5 | 5.2 |
| Hours After the Watering | Control | 3a | 3b | 3c | 3d | 3e | 3f | 3g | IAA | IBA | Trp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 | 79.39 | 69.34 | 80.10 | 93.75 * | 82.48 | 79.39 | 82.29 | 77.39 | 68.42 | 77.87 | 79.56 |
| 96 | 58.93 | 52.57 | 58.15 | 63.19 | 64.78 * | 50.88 | 55.71 | 57.79 | 50.34 | 59.31 | 50.88 |
| 120 | 24.38 | 30.31 | 27.41 | 24.09 | 22.07 | 27.94 | 23.51 | 25.96 | 27.63 | 25.89 * | 23.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N.A. Biologically Oriented Hybrids of Indole and Hydantoin Derivatives. Molecules 2023, 28, 602. https://doi.org/10.3390/molecules28020602
Kochetkov KA, Gorunova ON, Bystrova NA. Biologically Oriented Hybrids of Indole and Hydantoin Derivatives. Molecules. 2023; 28(2):602. https://doi.org/10.3390/molecules28020602
Chicago/Turabian StyleKochetkov, Konstantin A., Olga N. Gorunova, and Natalia A. Bystrova. 2023. "Biologically Oriented Hybrids of Indole and Hydantoin Derivatives" Molecules 28, no. 2: 602. https://doi.org/10.3390/molecules28020602
APA StyleKochetkov, K. A., Gorunova, O. N., & Bystrova, N. A. (2023). Biologically Oriented Hybrids of Indole and Hydantoin Derivatives. Molecules, 28(2), 602. https://doi.org/10.3390/molecules28020602







